Drug Detail:Seglentis (Celecoxib and tramadol hydrochloride)
Drug Class: Narcotic analgesic combinations
Highlights of Prescribing Information
SEGLENTIS (celecoxib and tramadol hydrochloride) tablets, for oral use, C-IV
Initial U.S. Approval: 2021
WARNING: ADDICTION, ABUSE, AND MISUSE; RISK EVALUATION AND MITIGATION STRATEGY (REMS); LIFE-THREATENING RESPIRATORY DEPRESSION; ACCIDENTAL INGESTION; CARDIOVASCULAR THROMBOTIC EVENTS; GASTROINTESTINAL BLEEDING, ULCERATION, AND PERFORATION; ULTRA-RAPID METABOLISM OF TRAMADOL AND OTHER RISK FACTORS FOR LIFE-THREATENING RESPIRATORY DEPRESSION IN CHILDREN; NEONATAL OPIOID WITHDRAWAL SYNDROME; INTERACTIONS WITH DRUGS AFFECTING CYTOCHROME P450 ISOENZYMES; RISKS FROM CONCOMITANT USE WITH BENZODIAZEPINES OR OTHER CNS DEPRESSANTS
See full prescribing information for complete boxed warning.
- SEGLENTIS exposes users to the risks of addiction, abuse, and misuse, which can lead to overdose and death. Assess each patient's risk prior to prescribing SEGLENTIS and monitor regularly for these behaviors or conditions. (5.1)
- To ensure that the benefits of opioid analgesics outweigh the risks of addiction, abuse, and misuse, the Food and Drug Administration (FDA) has required a Risk Evaluation and Mitigation Strategy (REMS) for these products. (5.2)
- Serious, life-threatening, or fatal respiratory depression may occur. Monitor closely, especially during initiation or following a dose increase. (5.3)
- Accidental ingestion of SEGLENTIS, especially by children, can result in a fatal overdose of tramadol. (5.3)
- Nonsteroidal anti-inflammatory drugs (NSAIDs) cause an increased risk of serious cardiovascular thrombotic events, including myocardial infarction and stroke, which can be fatal. This risk may occur early in the treatment and may increase with duration of use. (5.4)
- SEGLENTIS is contraindicated in the setting of coronary artery bypass graft (CABG) surgery. (4, 5.4)
- NSAIDs cause an increased risk of serious gastrointestinal (GI) adverse events including bleeding, ulceration, and perforation of the stomach or intestines, which can be fatal. These events can occur at any time during use and without warning symptoms. Elderly patients and patients with a prior history of peptic ulcer disease and/or GI bleeding are at greater risk for serious GI events. (5.5)
- Life-threatening respiratory depression and death have occurred in children who received tramadol. Some of the reported cases followed tonsillectomy and/or adenoidectomy; in at least one case, the child had evidence of being an ultra-rapid metabolizer of tramadol due to a CYP2D6 polymorphism (5.6)
- SEGLENTIS is contraindicated in children younger than 12 years of age and in children younger than 18 years of age following tonsillectomy and/or adenoidectomy (4). Avoid the use of SEGLENTIS in adolescents 12 to 18 years of age who have other risk factors that may increase their sensitivity to the respiratory depressant effects of tramadol. (5.6)
- Prolonged use of SEGLENTIS during pregnancy can result in neonatal opioid withdrawal syndrome, which may be life threatening if not recognized and treated. If prolonged opioid use is required in a pregnant woman, advise the patient of the risk of neonatal opioid withdrawal syndrome and ensure that appropriate treatment will be available. (5.7)
- The effects of concomitant use or discontinuation of cytochrome P450 3A4 inducers, 3A4 inhibitors, or 2D6 inhibitors with tramadol are complex. Use of cytochrome P450 3A4 inducers, 3A4 inhibitors, or 2D6 inhibitors with SEGLENTIS requires careful consideration of the effects on the parent drug, tramadol, and the active metabolite, M1. (5.8, 7)
- Concomitant use of opioids with benzodiazepines or other central nervous system (CNS) depressants, including alcohol, may result in profound sedation, respiratory depression, coma, and death. Reserve concomitant prescribing for use in patients for whom alternative treatment options are inadequate; limit dosages and durations to the minimum required; and follow patients for signs and symptoms of respiratory depression and sedation. (5.9, 7)
Indications and Usage for Seglentis
SEGLENTIS contains tramadol hydrochloride, an opioid agonist, and celecoxib, a nonsteroidal anti-inflammatory drug, and is indicated for the management of acute pain in adults that is severe enough to require an opioid analgesic and for which alternative treatments are inadequate (1).
Limitations of Use (1):
Because of the risks of addiction, abuse, and misuse with opioids, even at recommended doses (5.1), reserve SEGLENTIS for use in patients for whom alternative treatment options [e.g., non-opioid analgesics]:
- Have not been tolerated, or are not expected to be tolerated
- Have not provided adequate analgesia, or are not expected to provide adequate analgesia.
Seglentis Dosage and Administration
- Use SEGLENTIS for the shortest duration consistent with individual patient treatment goals (2.1).
- Initiate treatment of SEGLENTIS with two tablets every 12 hours as needed for pain relief (2.3).
- When initiating treatment with SEGLENTIS, take into account the patient's severity of pain, patient response, prior analgesic treatment experience, and risk factors for addiction, abuse, and misuse (2.1).
- Discuss availability of naloxone with the patient and assess each patient's need for access to naloxone, both when initiating and renewing treatment with SEGLENTIS. Consider prescribing naloxone based on the patient's risk factors for overdose (2.2, 5.1, 5.3, 5.9).
- Monitor patients closely for respiratory depression, especially within the first 24-72 hours of initiating therapy with SEGLENTIS (2.1).
- Do not abruptly discontinue SEGLENTIS in a physically dependent patient (2.4, 5.28).
- Do not use with other celecoxib- or tramadol-containing products (2.1).
Dosage Forms and Strengths
Tablets: celecoxib 56 mg and tramadol hydrochloride 44 mg (3).
Contraindications
- Children younger than 12 years of age (4).
- Postoperative management in children younger than 18 years of age following tonsillectomy and/or adenoidectomy (4).
- Significant respiratory depression (4).
- In the setting of CABG surgery (4).
- Acute or severe bronchial asthma in an unmonitored setting or in absence of resuscitative equipment (4).
- Known or suspected gastrointestinal obstruction, including paralytic ileus (4).
- Hypersensitivity to tramadol, celecoxib, any other component of this product, or sulfonamides, or opioids (4).
- Concurrent use of monoamine oxidase inhibitors (MAOIs) or use of MAOIs within the last 14 days (4).
- History of asthma, urticaria, or other allergic-type reactions after taking aspirin or other NSAIDs (4).
Warnings and Precautions
Serotonin Syndrome: May be life-threatening. Can occur with use of tramadol alone, with concomitant use of serotonergic drugs, with drugs that impair metabolism of serotonin or tramadol (5.10).
Risk of Seizure: Can occur at the recommended dose of tramadol. Concomitant use with other drugs may increase seizure risk. Risk may increase in patients with epilepsy, a history of seizures, and in patients with a recognized risk for seizures (5.11).
Risk of Suicide: Do not prescribe for suicidal or addiction-prone patients (5.12).
Adrenal Insufficiency: If diagnosed, treat with physiologic replacement of corticosteroids, and wean patient off the opioid (5.13).
Life-Threatening Respiratory Depression in Patients with Chronic Pulmonary Disease or in Elderly, Cachectic, or Debilitated Patients: Monitor closely, particularly during initiation and titration (5.14).
Severe Hypotension with Tramadol: Monitor during dosage initiation. Avoid use of SEGLENTIS in patients with circulatory shock (5.15).
Risk of Use in Patients with Increased Intracranial Pressure, Brain Tumors, Head Injury, or Impaired Consciousness: Monitor for sedation and respiratory depression. Avoid use of SEGLENTIS in patients with impaired consciousness or coma (5.16).
Anaphylactic Reactions: Seek emergency help if an anaphylactic reaction occurs (5.18).
Hepatotoxicity: Inform patients of warning signs and symptoms of hepatotoxicity. Discontinue if abnormal liver tests persist or worsen or if clinical signs and symptoms of liver disease develop (5.19).
Hypertension: Patients taking some antihypertensive medications may have impaired response to these therapies when taking NSAIDs. Monitor blood pressure (5.20, 7).
Heart Failure and Edema: Avoid use of SEGLENTIS in patients with severe heart failure unless benefits are expected to outweigh risk of worsening heart failure (5.21).
Renal Toxicity: Monitor renal function in patients with renal or hepatic impairment, heart failure, dehydration, or hypovolemia. Avoid use of SEGLENTIS in patients with advanced renal disease unless benefits are expected to outweigh risk of worsening renal function (5.22).
Exacerbation of Asthma Related to Aspirin Sensitivity: SEGLENTIS is contraindicated in patients with aspirin-sensitive asthma. Monitor patients with preexisting asthma (without aspirin sensitivity) (5.23).
Serious Skin Reactions: Discontinue SEGLENTIS at first appearance of skin rash or other signs of hypersensitivity (5.24).
Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS): Discontinue and evaluate clinically (5.25).
Fetal Toxicity: Limit use of NSAIDs, including SEGLENTIS, between about 20 to 30 weeks in pregnancy due to the risk of oligohydramnios/fetal renal dysfunction. Avoid use of NSAIDs in women at about 30 weeks gestation and later in pregnancy due to the risks of oligohydramnios/fetal renal dysfunction and premature closure of the fetal ductus arteriosus (5.26, 8.1).
Hematologic Toxicity: Monitor hemoglobin or hematocrit in patients with any signs or symptoms of anemia (5.27, 7)
Adverse Reactions/Side Effects
Most common adverse reactions (incidence > 5% and > placebo) are nausea, vomiting, dizziness, headache, somnolence (6.1).
To report SUSPECTED ADVERSE REACTIONS, contact Kowa Pharmaceuticals America, Inc., at toll-free phone 1-888-SEGLENTIS or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
Mixed Agonist/Antagonist and Partial Agonist Opioid Analgesics: Avoid use with SEGLENTIS because they may reduce analgesic effect of SEGLENTIS or precipitate withdrawal symptoms (7).
Drugs that Interfere with Hemostasis (e.g., warfarin, aspirin, selective serotonin reuptake inhibitors [SSRIs]/serotonin norepinephrine reuptake inhibitors [SNRIs]): Monitor patients for bleeding who are concomitantly taking SEGLENTIS with drugs that interfere with hemostasis. Concomitant use of SEGLENTIS and analgesic doses of aspirin is not generally recommended (7).
Angiotensin Converting Enzyme (ACE) Inhibitors, Angiotensin Receptor Blockers (ARB), or Beta-Blockers: Concomitant use with SEGLENTIS may diminish the antihypertensive effect of these drugs. Monitor blood pressure (7).
ACE Inhibitors and ARBs: Concomitant use with SEGLENTIS in elderly, volume depleted, or those with renal impairment may result in deterioration of renal function. In such high-risk patients, monitor for signs of worsening renal function (7).
Diuretics: NSAIDs can reduce natriuretic effect of furosemide and thiazide diuretics. Monitor patients to assure diuretic efficacy including antihypertensive effects (7).
Digoxin: Concomitant use with SEGLENTIS can increase serum concentration and prolong half-life of digoxin. Monitor serum digoxin levels (7).
Use In Specific Populations
Pregnancy: May cause fetal harm (8.1)
Lactation: Breastfeeding not recommended (8.2).
Infertility: NSAIDs are associated with reversible infertility. Consider withdrawal of SEGLENTIS in women who have difficulties conceiving (8.3).
Severe Renal Impairment: Use not recommended (8.6).
Moderate and Severe Hepatic Impairment: Use not recommended (8.7).
Poor Metabolizers of CYP2C9: Due to the inability to start SEGLENTIS at a lower dose, use of SEGLENTIS is not recommended (8.8).
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 10/2021
Related/similar drugs
aspirin, acetaminophen, tramadol, naproxen, oxycodone, TylenolFull Prescribing Information
WARNING: ADDICTION, ABUSE, AND MISUSE; RISK EVALUATION AND MITIGATION STRATEGY (REMS); LIFE-THREATENING RESPIRATORY DEPRESSION; ACCIDENTAL INGESTION; CARDIOVASCULAR THROMBOTIC EVENTS; GASTROINTESTINAL BLEEDING, ULCERATION, AND PERFORATION; ULTRA-RAPID METABOLISM OF TRAMADOL AND OTHER RISK FACTORS FOR LIFE-THREATENING RESPIRATORY DEPRESSION IN CHILDREN; NEONATAL OPIOID WITHDRAWAL SYNDROME; INTERACTIONS WITH DRUGS AFFECTING CYTOCHROME P450 ISOENZYMES; RISKS FROM CONCOMITANT USE WITH BENZODIAZEPINES OR OTHER CNS DEPRESSANTS
ADDICTION, ABUSE, AND MISUSE
SEGLENTIS exposes patients and other users to the risks of opioid addiction, abuse, and misuse, which can lead to overdose and death. Assess each patient's risk prior to prescribing SEGLENTIS and monitor all patients regularly for the development of these behaviors and conditions [see Warnings and Precautions (5.1)].
OPIOID ANALGESIC RISK EVALUATION AND MITIGATION STRATEGY (REMS)
To ensure that the benefits of opioid analgesics outweigh the risks of addiction, abuse, and misuse, the Food and Drug Administration (FDA) has required a REMS for these products [see Warnings and Precautions (5.2)]. Under the requirements of the REMS, drug companies with approved opioid analgesic products must make REMS-compliant education programs available to healthcare providers. Healthcare providers are strongly encouraged to
- complete a REMS-compliant education program,
- counsel patients and/or their caregivers, with every prescription, on safe use, serious risks, storage, and disposal of these products,
- emphasize to patients and their caregivers the importance of reading the Medication Guide every time it is provided by their pharmacist, and
- consider other tools to improve patient, household, and community safety.
LIFE-THREATENING RESPIRATORY DEPRESSION
Serious, life-threatening, or fatal respiratory depression may occur with use of SEGLENTIS. Monitor for respiratory depression, especially during initiation of SEGLENTIS [see Warnings and Precautions (5.3)].
ACCIDENTAL INGESTION
Accidental ingestion of even one dose of SEGLENTIS, especially by children, can be fatal [see Warnings and Precautions (5.3)].
CARDIOVASCULAR THROMBOTIC EVENTS
- Nonsteroidal anti-inflammatory drugs (NSAIDs) cause an increased risk of serious cardiovascular thrombotic events, including myocardial infarction, and stroke, which can be fatal. This risk may occur early in the treatment and may increase with duration of use [see Warnings and Precautions (5.4)].
- SEGLENTIS is contraindicated in the setting of coronary artery bypass graft (CABG) surgery [see Contraindications (4) and Warnings and Precautions (5.4)].
GASTROINTESTINAL BLEEDING, ULCERATION, AND PERFORATION
NSAIDs cause an increased risk of serious gastrointestinal (GI) adverse events including bleeding, ulceration, and perforation of the stomach or intestines, which can be fatal. These events can occur at any time during use and without warning symptoms. Elderly patients and patients with a prior history of peptic ulcer disease and/or GI bleeding are at greater risk for serious (GI) events [see Warnings and Precautions (5.5)].
ULTRA-RAPID METABOLISM OF TRAMADOL AND OTHER RISK FACTORS FOR LIFE-THREATENING RESPIRATORY DEPRESSION IN CHILDREN
Life-threatening respiratory depression and death have occurred in children who received tramadol. Some of the reported cases followed tonsillectomy and/or adenoidectomy; in at least one case, the child had evidence of being an ultra-rapid metabolizer of tramadol due to a CYP2D6 polymorphism [see Warnings and Precautions (5.6)]. SEGLENTIS is contraindicated in children younger than 12 years of age and in children younger than 18 years of age following tonsillectomy and/or adenoidectomy [see Contraindications (4)]. Avoid the use of SEGLENTIS in adolescents 12 to 18 years of age who have other risk factors that may increase their sensitivity to the respiratory depressant effects of tramadol [see Warnings and Precautions (5.6)].
NEONATAL OPIOID WITHDRAWAL SYNDROME
Prolonged use of SEGLENTIS during pregnancy can result in neonatal opioid withdrawal syndrome, which may be life-threatening if not recognized and treated, and requires management according to protocols developed by neonatology experts. If opioid use is required for a prolonged period in a pregnant woman, advise the patient of the risk of neonatal opioid withdrawal syndrome and ensure that appropriate treatment will be available [see Warnings and Precautions (5.7)].
INTERACTIONS WITH DRUGS AFFECTING CYTOCHROME P450 ISOENZYMES
The effects of concomitant use or discontinuation of cytochrome P450 3A4 inducers, 3A4 inhibitors, or 2D6 inhibitors with tramadol are complex. Use of cytochrome P450 3A4 inducers, 3A4 inhibitors, or 2D6 inhibitors with SEGLENTIS requires careful consideration of the effects on the parent drug, tramadol, and the active metabolite, M1 [see Warnings and Precautions (5.8), Drug Interactions (7)].
RISKS FROM CONCOMITANT USE WITH BENZODIAZEPINES OR OTHER CNS DEPRESSANTS
Concomitant use of opioids with benzodiazepines or other central nervous system (CNS) depressants, including alcohol, may result in profound sedation, respiratory depression, coma, and death [see Warnings and Precautions (5.9) and Drug Interactions (7)].
- Reserve concomitant prescribing of SEGLENTIS and benzodiazepines or other CNS depressants for use in patients for whom alternative treatment options are inadequate.
- Limit treatment to the minimum duration.
- Follow patients for signs and symptoms of respiratory depression and sedation.
1. Indications and Usage for Seglentis
SEGLENTIS is indicated for the management of acute pain in adults that is severe enough to require an opioid analgesic and for which alternative treatments are inadequate.
2. Seglentis Dosage and Administration
2.1 Important Dosage and Administration Instructions
- Do not exceed the recommended dose of SEGLENTIS.
- Do not co-administer SEGLENTIS with other tramadol or celecoxib containing products.
- Use SEGLENTIS for the shortest duration consistent with individual patient treatment goals [see Warnings and Precautions (5.1)].
- When initiating treatment with SEGLENTIS, take into account the patient's severity of pain, patient response, prior analgesic treatment experience, and risk factors for addiction, abuse, and misuse [see Warnings and Precautions (5.1)].
- Monitor patients closely for respiratory depression, especially within the first 24-72 hours of initiating therapy [see Warnings and Precautions (5.3)].
2.2 Patient Access to Naloxone for the Emergency Treatment of Opioid Overdose
Discuss the availability of naloxone for the emergency treatment of opioid overdose with the patient and caregiver and assess the potential need for access to naloxone, both when initiating and renewing treatment with SEGLENTIS [see Warnings and Precautions (5.3)].
Inform patients and caregivers about the various ways to obtain naloxone as permitted by individual state naloxone dispensing and prescribing requirements or guidelines (e.g., by prescription, directly from a pharmacist, or as part of a community-based program).
Consider prescribing naloxone, based on the patient's risk factors for overdose, such as concomitant use of CNS depressants, a history of opioid use disorder, or prior opioid overdose. However, the presence of risk factors for overdose should not prevent the proper management of pain in any given patient [see Warnings and Precautions (5.1, 5.3, 5.9)].
Consider prescribing naloxone if the patient has household members (including children) or other close contacts at risk for accidental exposure or overdose.
2.3 Recommended Dosage
The dose of SEGLENTIS 56 mg/44 mg is 2 tablets every 12 hours as needed for pain.
2.4 Safe Reduction or Discontinuation of SEGLENTIS
Do not abruptly discontinue SEGLENTIS in patients who may be physically dependent on opioids. Rapid discontinuation of opioid analgesics in patients who are physically dependent on opioids has resulted in serious withdrawal symptoms, uncontrolled pain, and suicide. Rapid discontinuation has also been associated with attempts to find other sources of opioid analgesics, which may be confused with drug-seeking for abuse. Patients may also attempt to treat their pain or withdrawal symptoms with illicit opioids, such as heroin, and other substances.
When a decision has been made to decrease the dose or discontinue therapy in an opioid-dependent patient taking SEGLENTIS, there are a variety of factors that should be considered, including the total daily dose of opioid (including SEGLENTIS) the patient has been taking, the duration of treatment, the type of pain being treated, and the physical and psychological attributes of the patient. It is important to ensure ongoing care of the patient and to agree on an appropriate tapering schedule and follow-up plan so that patient and provider goals and expectations are clear and realistic. When opioid analgesics are being discontinued due to a suspected substance use disorder, evaluate and treat the patient, or refer for evaluation and treatment of the substance use disorder. Treatment should include evidence-based approaches, such as medication assisted treatment of opioid use disorder. Complex patients with co-morbid pain and substance use disorders may benefit from referral to a specialist.
There are no standard opioid tapering schedules that are suitable for all patients. Good clinical practice dictates a patient-specific plan to taper the dose of the opioid gradually. For patients on SEGLENTIS who are physically opioid-dependent, initiate the taper by a small enough increment (e.g., no greater than 10% to 25% of the total daily opioid dose) to avoid withdrawal symptoms, and proceed with dose-lowering at an interval of every 2 to 4 weeks. Patients who have been taking opioids for briefer periods of time may tolerate a more rapid taper.
It may be necessary to provide the patient with a reduced dosing schedule of SEGLENTIS to accomplish a successful taper. Reassess the patient frequently to manage pain and withdrawal symptoms, should they emerge. Common withdrawal symptoms include restlessness, lacrimation, rhinorrhea, yawning, perspiration, chills, myalgia, and mydriasis. Other signs and symptoms also may develop, including irritability, anxiety, backache, joint pain, weakness, abdominal cramps, insomnia, nausea, anorexia, vomiting, diarrhea, or increased blood pressure, respiratory rate, or heart rate. If withdrawal symptoms arise, it may be necessary to pause the taper for a period of time or raise the dose of the opioid analgesic to the previous dose, and then proceed with a slower taper. In addition, monitor patients for any changes in mood, emergence of suicidal thoughts, or use of other substances.
When managing patients taking opioid analgesics, particularly those who have been treated for a long duration and/or with high doses for chronic pain, ensure that a multimodal approach to pain management, including mental health support (if needed), is in place prior to initiating an opioid analgesic taper. A multimodal approach to pain management may optimize the treatment of chronic pain, as well as assist with the successful tapering of the opioid analgesic [see Warnings and Precautions (5.28), Drug Abuse and Dependence (9.2 and 9.3)].
3. Dosage Forms and Strengths
SEGLENTIS coated tablets contain 56 mg celecoxib and 44 mg tramadol hydrochloride (equivalent to 39 mg tramadol). The tablets are white to off-white elongated coated tablets debossed with "100" on one side and "CTC" on the other.
4. Contraindications
SEGLENTIS is contraindicated in:
- All patients younger than 12 years of age [see Warnings and Precautions (5.6)].
- Post-operative management in children younger than 18 years of age following tonsillectomy and/or adenoidectomy [see Warnings and Precautions (5.6)].
5. Warnings and Precautions
5.4 Cardiovascular Thrombotic Events
5.5 Gastrointestinal Bleeding, Ulceration, and Perforation
5.6 Ultra-Rapid Metabolism of Tramadol and Other Risk Factors for Life-threatening Respiratory Depression in Children
5.8 Risks of Interactions with Drugs Affecting Cytochrome P450 Isoenzymes
5.14 Life-Threatening Respiratory Depression in Patients with Chronic Pulmonary Disease or in Elderly, Cachectic, or Debilitated Patients
5.16 Risk of Use in Patients with Increased Intracranial Pressure, Brain Tumors, Head Injury, or Impaired Consciousness
5.19 Hepatotoxicity
As tramadol and celecoxib are both extensively metabolized by the liver, the use of SEGLENTIS in patients with moderate and severe hepatic impairment is not recommended [see Use in Specific Populations (8.7), Clinical Pharmacology (12.3)].
5.22 Renal Toxicity and Hyperkalemia
5.25 Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS)
Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) has been reported in patients taking NSAIDs such as SEGLENTIS. Some of these events have been fatal or life-threatening. DRESS typically, although not exclusively, presents with fever, rash, lymphadenopathy, and/or facial swelling. Other clinical manifestations may include hepatitis, nephritis, hematological abnormalities, myocarditis, or myositis. Sometimes symptoms of DRESS may resemble an acute viral infection. Eosinophilia is often present. Because this disorder is variable in its presentation, other organ systems not noted here may be involved. It is important to note that early manifestations of hypersensitivity, such as fever or lymphadenopathy, may be present even though rash is not evident. If such signs or symptoms are present, discontinue SEGLENTIS and evaluate the patient immediately.
5.31 Hyponatremia
Hyponatremia (serum sodium < 135 mmol/L) has been reported with the use of tramadol, a component of SEGLENTIS, and many cases are severe (sodium level < 120 mmol/L). Most cases of hyponatremia occurred in females over the age of 65 and within the first week of therapy. In some reports, hyponatremia resulted from the syndrome of inappropriate antidiuretic hormone secretion (SIADH). Monitor for signs and symptoms of hyponatremia (e.g., confusion, disorientation), during treatment with SEGLENTIS, especially during initiation of therapy. If signs and symptoms of hyponatremia are present, initiate appropriate treatment (e.g., fluid restriction) and discontinue SEGLENTIS [see Dosage and Administration (2.4)].
5.32 Hypoglycemia
Cases of tramadol-associated hypoglycemia have been reported, some resulting in hospitalization. In most cases, patients had predisposing risk factors (e.g., diabetes). If hypoglycemia is suspected, monitor blood glucose levels and consider drug discontinuation as appropriate [see Dosage and Administration (2.4)].
6. Adverse Reactions/Side Effects
The following serious adverse reactions are discussed, or described in greater detail, in other sections:
- Addiction, Abuse, and Misuse [see Warnings and Precautions (5.1)]
- Life-Threatening Respiratory Depression [see Warnings and Precautions (5.3)]
- Cardiovascular Thrombotic Events [see Warnings and Precautions (5.4)]
- GI Bleeding, Ulceration and Perforation [see Warnings and Precautions (5.5)]
- Ultra-Rapid Metabolism of Tramadol and Other Risk Factors for Life-threatening Respiratory Depression in Children [see Warnings and Precautions (5.6)]
- Neonatal Opioid Withdrawal Syndrome [see Warnings and Precautions (5.7)]
- Interactions with Benzodiazepines or Other CNS Depressants [see Warnings and Precautions (5.9)]
- Serotonin Syndrome [see Warnings and Precautions (5.10)]
- Seizures [see Warnings and Precautions (5.11)]
- Suicide [see Warnings and Precautions (5.12)]
- Adrenal Insufficiency [see Warnings and Precautions (5.13)]
- Severe Hypotension [see Warnings and Precautions (5.15)]
- Gastrointestinal Adverse Reactions [see Warnings and Precautions (5.17)]
- Anaphylaxis and Other Hypersensitivity Reactions [see Warnings and Precautions (5.18)]
- Hepatotoxicity [see Warnings and Precautions (5.19)]
- Hypertension [see Warnings and Precautions (5.20)]
- Heart Failure and Edema [see Warnings and Precautions (5.21)]
- Renal Toxicity and Hyperkalemia [see Warnings and Precautions (5.22)]
- Serious Skin Reactions [see Warnings and Precautions (5.24)]
- Hematologic Toxicity [see Warnings and Precautions (5.27)]
- Withdrawal [see Warnings and Precautions (5.28)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
A total of 550 subjects in 7 clinical studies, from Phase 1 to Phase 3, were exposed to SEGLENTIS during the clinical development program, including 385 subjects exposed to 200 mg of SEGLENTIS, either single or multiple administration.
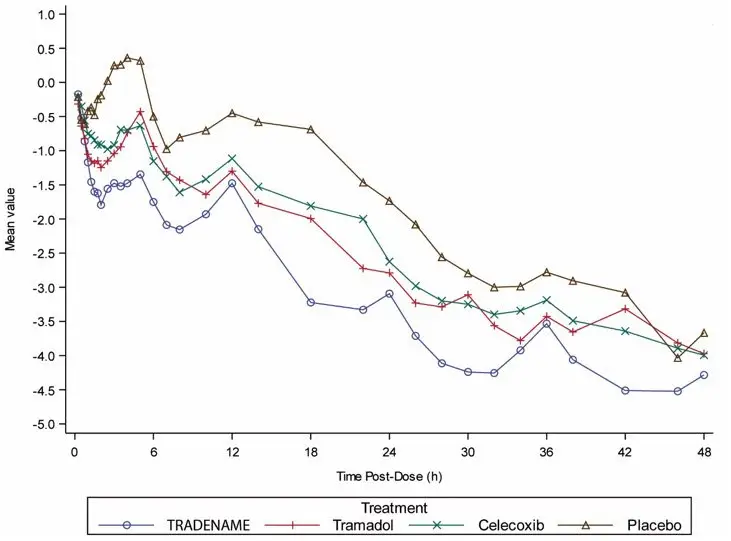
In a placebo-controlled post-bunionectomy acute pain trial, 637 patients received 200 mg of SEGLENTIS every 12 hours or 50 mg tramadol every 6 hours or 100 mg celecoxib every 12 hours or placebo, orally for 48 hours (blinded period) [see Clinical Studies (14)] and followed up to 7 days post-dose. Table 1 lists the adverse reactions reported by > 5% of patients in any treatment group and greater in SEGLENTIS than placebo. Discontinuation due to adverse events occurred in 1.6% of SEGLENTIS-treated patients (3 out of 183), 1.6% of tramadol-treated patients (3 out of 183), no celecoxib-treated patients, and no placebo-treated patients. The adverse reactions that led to discontinuation of study drug were nausea (1.1%) and pruritus/rash (0.5%) in the SEGLENTIS group, and vomiting (1.1%) and supraventricular tachycardia (0.5%) in the tramadol group.
| System Organ Class Preferred Term | SEGLENTIS (N = 183) n (%) | Tramadol (N = 183) n (%) | Celecoxib (N = 182) n (%) | Placebo (N = 89) n (%) |
|---|---|---|---|---|
| Total daily dose: 400 mg of SEGLENTIS (200 mg twice a day); 200 mg of tramadol (50 mg four times a day); 200 mg of celecoxib (100 mg twice a day); or placebo. Note: Acetaminophen 1 g IV and oxycodone hydrochloride 5 mg Immediate Release (IR) tablets were permitted as rescue medication. |
||||
| Gastrointestinal disorders | ||||
| Nausea | 55 (30.1) | 69 (37.7) | 30 (16.5) | 17 (19.1) |
| Vomiting | 29 (15.8) | 30 (16.4) | 4 (2.2) | 2 (2.2) |
| Nervous system disorders | ||||
| Dizziness | 31 (16.9) | 34 (18.6) | 9 (4.9) | 13 (14.6) |
| Headache | 21 (11.5) | 33 (18.0) | 20 (11.0) | 6 (6.7) |
| Somnolence | 15 (8.2) | 10 (5.5) | 4 (2.2) | 3 (3.4) |
| Metabolism and nutritional disorders | ||||
| Decreased appetite | 6 (3.3) | 11 (6.0) | 1 (0.5) | 0 |
6.2 Postmarketing Experience
The following adverse reactions have been identified during post approval use of either tramadol or celecoxib-containing products. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Serotonin Syndrome
Cases of serotonin syndrome, a potentially life-threatening condition, have been reported during concomitant use of opioids with serotonergic drugs.
Adrenal Insufficiency
Cases of adrenal insufficiency have been reported with opioid use, more often following greater than one month of use.
Androgen Deficiency
Cases of androgen deficiency have occurred with chronic use of opioids [see Clinical Pharmacology (12.2)].
QT Prolongation/Torsade De Pointes
Cases of QT prolongation and/or torsade de pointes have been reported with tramadol use. Many of these cases were reported in patients taking another drug labeled for QT prolongation, in patients with a risk factor for QT prolongation (e.g., hypokalemia), or in the overdose setting.
Eye Disorders
Miosis, mydriasis.
Metabolism and Nutrition Disorders
Cases of hypoglycemia have been reported very rarely in patients taking tramadol. Most reports were in patients with predisposing risk factors, including diabetes or renal insufficiency, or in elderly patients.
Hyponatremia
Cases of severe hyponatremia and/or SIADH have been reported in patients taking tramadol, most often in females over the age of 65, and within the first week of therapy [see Warnings and Precautions (5.31)].
Hypoglycemia
Cases of hypoglycemia have been reported in patients taking tramadol. Most reports were in patients with predisposing risk factors, including diabetes or renal insufficiency, or in elderly patients [see Warnings and Precautions (5.32)].
Nervous System Disorders
Movement disorder, speech disorder.
Psychiatric Disorders
Delirium.
Cardiovascular
Vasculitis, deep venous thrombosis.
General
Anaphylactoid reaction, angioedema.
Liver and Biliary
Liver necrosis, hepatitis, jaundice, hepatic failure.
Hemic and Lymphatic
Agranulocytosis, aplastic anemia, pancytopenia, leucopenia.
Metabolic
Hypoglycemia, hyponatremia.
Nervous
Aseptic meningitis, ageusia, anosmia, fatal intracranial hemorrhage.
Renal
Interstitial nephritis.
7. Drug Interactions
| Inhibitors of CYP2D6 | |
| Clinical Impact: | The concomitant use of SEGLENTIS and CYP2D6 inhibitors may result in an increase in the plasma concentration of tramadol and a decrease in the plasma concentration of M1. Since M1 is a more potent µ-opioid agonist, decreased M1 exposure could result in decreased therapeutic effects, and may result in signs and symptoms of opioid withdrawal in patients who had developed physical dependence to tramadol. Increased tramadol exposure can result in increased or prolonged therapeutic effects and increased risk for serious adverse events including seizures and serotonin syndrome. After stopping a CYP2D6 inhibitor, as the effects of the inhibitor decline, the tramadol plasma concentration will decrease and the M1 plasma concentration will increase which could increase or prolong therapeutic effects but also increase adverse reactions related to opioid toxicity and may cause potentially fatal respiratory depression [see Clinical Pharmacology (12.3)]. |
| Intervention: | If concomitant use of a CYP2D6 inhibitor is necessary, follow patients closely for adverse reactions including opioid withdrawal, seizures, and serotonin syndrome. If a CYP2D6 inhibitor is discontinued follow patients closely for adverse events including respiratory depression and sedation. |
| Examples: | Quinidine, fluoxetine, paroxetine, and bupropion. |
| CYP2D6 Substrates | |
| Clinical Impact: | In vitro studies indicate that celecoxib, although not a substrate, is an inhibitor of CYP2D6. Therefore, there is a potential for an in vivo drug interaction with drugs that are metabolized by CYP2D6 (e.g., atomoxetine), and celecoxib, which may enhance the exposure and toxicity of CYP2D6 substrate drugs. |
| Intervention: | If concomitant use of a CYP2D6 substrate drug is necessary, follow patients closely for adverse events of that CYP2D6 substrate drug. Evaluate each patient's medical history when consideration is given to prescribing SEGLENTIS [see Clinical Pharmacology (12.3)]. |
| Inhibitors of CYP3A4 | |
| Clinical Impact: | The concomitant use of SEGLENTIS and CYP3A4 inhibitors can increase the plasma concentration of tramadol and may result in a greater amount of metabolism via CYP2D6 and greater levels of M1. Follow patients closely for increased risk of serious adverse events including seizures and serotonin syndrome, and adverse reactions related to opioid toxicity including potentially fatal respiratory depression. After stopping a CYP3A4 inhibitor, as the effects of the inhibitor decline, the tramadol plasma concentration will decrease [see Clinical Pharmacology (12.3)], resulting in decreased opioid efficacy and possibly signs and symptoms of opioid withdrawal in patients who had developed physical dependence to tramadol. |
| Intervention: | If concomitant use is necessary, follow patients closely for seizures and serotonin syndrome, and signs of respiratory depression and sedation at frequent intervals. If a CYP3A4 inhibitor is discontinued, follow patients for efficacy maintenance and for signs and symptoms of opioid withdrawal. |
| Examples: | Macrolide antibiotics (e.g., erythromycin), azole-antifungal agents (e.g., ketoconazole), protease inhibitors (e.g., ritonavir). |
| CYP3A4 Inducers | |
| Clinical Impact: | The concomitant use of SEGLENTIS and CYP3A4 inducers can decrease the plasma concentration of tramadol [see Clinical Pharmacology (12.3)], resulting in decreased efficacy or onset of a withdrawal syndrome in patients who have developed physical dependence to tramadol. After stopping a CYP3A4 inducer, as the effects of the inducer decline, the tramadol plasma concentration will increase [see Clinical Pharmacology (12.3)], which could increase or prolong both the therapeutic effects and adverse reactions, and may cause seizures and serotonin syndrome, and potentially fatal respiratory depression. |
| Intervention: | If concomitant use is necessary, follow patients for efficacy maintenance and for signs of opioid withdrawal. If a CYP3A4 inducer is discontinued, monitor for seizures and serotonin syndrome, and signs of sedation and respiratory depression. Patients taking carbamazepine, a CYP3A4 inducer, may have a significantly reduced analgesic effect of tramadol. Because carbamazepine increases tramadol metabolism and because of the seizure risk associated with tramadol, concomitant administration of SEGLENTIS and carbamazepine is not recommended. |
| Examples: | Rifampin, carbamazepine, phenytoin. |
| CYP2C9 Inhibitors or inducers | |
| Clinical Impact: | Celecoxib metabolism is predominantly mediated via CYP2C9 in the liver. Coadministration of celecoxib with drugs that are known to inhibit CYP2C9 (e.g., fluconazole) may enhance the exposure and toxicity of celecoxib whereas co-administration with CYP2C9 inducers (e.g., rifampin) may lead to compromised efficacy of celecoxib. |
| Intervention: | If concomitant use with CYP2C9 inhibitor drugs is necessary, follow patients for adverse events of celecoxib from SEGLENTIS. If concomitant use with CYP2C9 inducer drugs is necessary, follow patients for efficacy maintenance of SEGLENTIS. Evaluate each patient's medical history when consideration is given to prescribing SEGLENTIS. |
| Drugs That Interfere with Hemostasis | |
| Clinical Impact: | Celecoxib and anticoagulants such as warfarin have a synergistic effect on bleeding. The concomitant use of celecoxib and anticoagulants have an increased risk of serious bleeding compared to the use of either drug alone. Serotonin release by platelets plays an important role in hemostasis. Case-control and cohort epidemiological studies showed that concomitant use of drugs that interfere with serotonin reuptake and an NSAID may potentiate the risk of bleeding more than an NSAID alone. |
| Intervention: | Monitor patients with concomitant use of SEGLENTIS with anticoagulants (e.g., warfarin), antiplatelet agents (e.g., aspirin), selective serotonin reuptake inhibitors (SSRIs), and serotonin norepinephrine reuptake inhibitors (SNRIs) for signs of bleeding [see Warnings and Precautions (5.27)]. |
| Aspirin | |
| Clinical Impact: | Controlled clinical studies showed that the concomitant use of NSAIDs and analgesic doses of aspirin does not produce any greater therapeutic effect than the use of NSAIDs alone. In a clinical study, the concomitant use of an NSAID and aspirin was associated with a significantly increased incidence of GI adverse reactions as compared to use of the NSAID alone [see Warnings and Precautions (5.5)]. In two studies in healthy volunteers, and in patients with osteoarthritis and established heart disease respectively, celecoxib (200-400 mg daily) has demonstrated a lack of interference with the cardioprotective antiplatelet effect of aspirin (100-325 mg). |
| Intervention: | Concomitant use of SEGLENTIS and analgesic doses of aspirin is not generally recommended because of the increased risk of bleeding [see Warnings and Precautions (5.27)]. SEGLENTIS is not a substitute for low dose aspirin for cardiovascular protection. |
| NSAIDs and Salicylates | |
| Clinical Impact: | Concomitant use of celecoxib with other NSAIDs or salicylates (e.g., diflunisal, salsalate) increases the risk of GI toxicity, with little or no increase in efficacy [see Warnings and Precautions (5.5)]. |
| Intervention: | The concomitant use of SEGLENTIS with other NSAIDs or salicylates is not recommended. |
| Benzodiazepines and Other Central Nervous System (CNS) Depressants | |
| Clinical Impact: | Due to additive pharmacologic effect, the concomitant use of benzodiazepines or other CNS depressants, including alcohol, can increase the risk of respiratory depression, profound sedation, coma, and death. |
| Intervention: | Reserve concomitant prescribing of these drugs for use in patients for whom alternative treatment options are inadequate. Limit dosages and durations to the minimum required. Follow patients closely for signs of respiratory depression and sedation [see Warnings and Precautions (5.9)]. |
| Examples: | Benzodiazepines and other sedatives/hypnotics, anxiolytics, tranquilizers, muscle relaxants, general anesthetics, antipsychotics, other opioids, alcohol. |
| Serotonergic Drugs | |
| Clinical Impact: | The concomitant use of opioids with other drugs that affect the serotonergic neurotransmitter system has resulted in serotonin syndrome. |
| Intervention: | If concomitant use is warranted, carefully observe the patient, particularly during treatment initiation. Discontinue SEGLENTIS if serotonin syndrome is suspected. |
| Examples: | Selective serotonin reuptake inhibitors (SSRIs), serotonin and norepinephrine reuptake inhibitors (SNRIs), tricyclic antidepressants (TCAs), triptans, 5-HT3 receptor antagonists, drugs that affect the serotonin neurotransmitter system (e.g., mirtazapine, trazodone, tramadol), monoamine oxidase (MAO) inhibitors (those intended to treat psychiatric disorders and also others, such as linezolid and intravenous methylene blue). |
| Monoamine Oxidase Inhibitors (MAOIs) | |
| Clinical Impact: | MAOI interactions with opioids may manifest as serotonin syndrome [see Warnings and Precautions (5.10)] or opioid toxicity (e.g., respiratory depression, coma) [see Warnings and Precautions (5.3)]. |
| Intervention: | Do not use SEGLENTIS in patients taking MAOIs or within 14 days of stopping such treatment. |
| Examples | Phenelzine, tranylcypromine, linezolid. |
| ACE Inhibitors, Angiotensin Receptor Blockers, and Beta-Blockers | |
| Clinical Impact: | NSAIDs may diminish the antihypertensive effect of angiotensin converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs), or beta-blockers (including propranolol). In patients who are elderly, volume-depleted (including those on diuretic therapy), or have renal impairment, co-administration of an NSAID with ACE inhibitors or ARBs may result in deterioration of renal function, including possible acute renal failure. These effects are usually reversible. |
| Intervention: | During concomitant use of SEGLENTIS and ACE-inhibitors, ARBs, or beta-blockers, monitor blood pressure to ensure that the desired blood pressure is obtained. During concomitant use of SEGLENTIS and ACE-inhibitors or ARBs in patients who are elderly, volume-depleted, or have impaired renal function, monitor for signs of worsening renal function [see Warnings and Precautions (5.22)]. When these drugs are administered concomitantly, patients should be adequately hydrated. Assess renal function at the beginning of the concomitant treatment and periodically thereafter. |
| Mixed Agonist/Antagonist and Partial Agonist Opioid Analgesics | |
| Clinical Impact: | May reduce the analgesic effect of SEGLENTIS and/or precipitate withdrawal symptoms. |
| Intervention: | Avoid concomitant use. |
| Examples: | Butorphanol, nalbuphine, pentazocine, buprenorphine. |
| Muscle Relaxants | |
| Clinical Impact: | Tramadol may enhance the neuromuscular blocking action of skeletal muscle relaxants and produce an increased degree of respiratory depression. |
| Intervention: | Monitor patients for signs of respiratory depression that may be greater than otherwise expected and decrease the dosage of the muscle relaxant as necessary. |
| Diuretics | |
| Clinical Impact: | Opioids can reduce the efficacy of diuretics by inducing the release of antidiuretic hormone. Clinical studies, as well as post-marketing observations, showed that NSAIDs reduced the natriuretic effect of loop diuretics (e.g., furosemide) and thiazide diuretics in some patients. This effect has been attributed to the NSAID inhibition of renal prostaglandin synthesis. |
| Intervention: | Monitor patients for signs of diminished diuresis and/or effects on blood pressure and increase the dosage of the diuretic as needed. During concomitant use of SEGLENTIS with diuretics, observe patients for signs of worsening renal function, in addition to assuring diuretic efficacy including antihypertensive effects [see Warnings and Precautions (5.22)]. |
| Digoxin | |
| Clinical Impact: | Post-marketing surveillance of tramadol has revealed rare reports of digoxin toxicity. The concomitant use of celecoxib with digoxin has been reported to increase the serum concentration and prolong the half-life of digoxin. |
| Intervention: | During concomitant use of SEGLENTIS and digoxin, monitor serum digoxin levels. Follow patients for signs of digoxin toxicity and adjust the dosage of digoxin as needed. |
| Anticholinergic Drugs | |
| Clinical Impact: | The concomitant use of anticholinergic drugs may increase risk of urinary retention and/or severe constipation, which may lead to paralytic ileus. |
| Intervention: | Monitor patients for signs of urinary retention or reduced gastric motility when SEGLENTIS is used concomitantly with anticholinergic drugs. |
| Lithium | |
| Clinical Impact: | NSAIDs have produced elevations in plasma lithium levels and reductions in renal lithium clearance. The mean minimum lithium concentration increased 15%, and the renal clearance decreased by approximately 20%. This effect has been attributed to NSAID inhibition of renal prostaglandin synthesis. |
| Intervention: | During concomitant use of SEGLENTIS and lithium, monitor patients for signs of lithium toxicity. |
| Warfarin | |
| Clinical Impact: | Post-marketing surveillance of tramadol has revealed rare reports of alteration of warfarin effect, including elevation of prothrombin times. |
| Intervention: | Monitor the prothrombin time of patients on warfarin for signs of an interaction and adjust the dosage of warfarin as needed. |
| Methotrexate | |
| Clinical Impact: | Concomitant use of NSAIDs and methotrexate may increase the risk for methotrexate toxicity (e.g., neutropenia, thrombocytopenia, renal dysfunction). Celecoxib has no effect on methotrexate pharmacokinetics. |
| Intervention: | During concomitant use of SEGLENTIS and methotrexate, monitor patients for methotrexate toxicity. |
| Cyclosporine | |
| Clinical Impact: | Concomitant use of celecoxib and cyclosporine may increase cyclosporine's nephrotoxicity. |
| Intervention: | During concomitant use of SEGLENTIS and cyclosporine, monitor patients for signs of worsening renal function. |
| Pemetrexed | |
| Clinical Impact: | Concomitant use of celecoxib and pemetrexed may increase the risk of pemetrexed-associated myelosuppression, renal, and GI toxicity (see the pemetrexed prescribing information). |
| Intervention: | During concomitant use of SEGLENTIS and pemetrexed, in patients with renal impairment whose creatinine clearance ranges from 45 to 79 mL/min, monitor for myelosuppression, renal and GI toxicity. NSAIDs with short elimination half-lives (e.g., diclofenac, indomethacin) should be avoided for a period of two days before, the day of, and two days following administration of pemetrexed. In the absence of data regarding potential interaction between pemetrexed and NSAIDs with longer half-lives (e.g., meloxicam, nabumetone), patients taking these NSAIDs should interrupt dosing for at least five days before, the day of, and two days following pemetrexed administration. |
| Corticosteroids | |
| Clinical Impact: | Concomitant use of corticosteroids with celecoxib may increase the risk of GI ulceration or bleeding. |
| Intervention: | Monitor patients with concomitant use of SEGLENTIS with corticosteroids for signs of bleeding [see Warnings and Precautions (5.5)]. |
8. Use In Specific Populations
8.1 Pregnancy
Risk Summary
Based on animal data, advise pregnant women of the potential risk to fetus. Prolonged use of opioid analgesics during pregnancy may cause neonatal opioid withdrawal syndrome (see Clinical Considerations).
Use of NSAIDs, including SEGLENTIS, can cause premature closure of the fetal ductus arteriosus and fetal renal dysfunction leading to oligohydramnios and, in some cases, neonatal renal impairment. Because of these risks, limit dose and duration of SEGLENTIS use between about 20 and 30 weeks of gestation and avoid SEGLENTIS use at about 30 weeks of gestation and later in pregnancy (see Clinical Considerations, Data).
There are no available data on use of SEGLENTIS in pregnant women. In an animal reproduction study, oral administration of celecoxib and tramadol co-crystal to pregnant rabbits during the period of organogenesis, resulted in embryo-fetal deaths and an increase of incidence of vertebral defects at approximately 4.7 and 0.11 times the dose of celecoxib and tramadol, respectively, at the maximum recommended human dose (MRHD) of SEGLENTIS at 400 mg/day (224 mg celecoxib/176 mg tramadol) (see Data).
Data
Human Data
8.2 Lactation
Clinical Considerations
If infants are exposed to SEGLENTIS through breast milk, they should be monitored for excess sedation and respiratory depression. Withdrawal symptoms can occur in breastfed infants when maternal administration of an opioid analgesic is stopped, or when breastfeeding is stopped.
8.4 Pediatric Use
The safety and effectiveness of SEGLENTIS in pediatric patients have not been established.
8.5 Geriatric Use
In the randomized, double-blind, active- and placebo-controlled, parallel group study comparing SEGLENTIS to tramadol, celecoxib, and placebo in patients with acute post-operative pain following unilateral first metatarsal osteotomy with internal fixation, 9.1% of patients were ≥65 years of age. Age subgroup examination was planned by protocol and it revealed a similar trend in efficacy compared to younger patients and no untoward or unexpected adverse reactions were seen in the elderly patients who received SEGLENTIS.
No dose adjustments are required for elderly patients.
8.6 Renal Impairment
Because SEGLENTIS contains celecoxib, the use of SEGLENTIS in patients with severe renal impairment is not recommended [see Warnings and Precautions (5.22) and Clinical Pharmacology (12.3)].
The pharmacokinetics and tolerability of SEGLENTIS in patients with renal impairment has not been studied.
8.7 Hepatic Impairment
As tramadol and celecoxib are both extensively metabolized by the liver, the use of SEGLENTIS in patients with moderate and severe hepatic impairment is not recommended [see Warnings and Precautions (5.19), Clinical Pharmacology (12.3)].
The pharmacokinetics and tolerability of SEGLENTIS in patients with impaired hepatic function have not been studied.
9. Drug Abuse and Dependence
9.2 Abuse
SEGLENTIS contains tramadol, a substance with a high potential for abuse similar to other opioids and can be abused and is subject to misuse, addiction, and criminal diversion [see Warnings and Precautions (5.1)].
All patients treated with opioids require careful monitoring for signs of abuse and addiction, since use of opioid analgesic products carries the risk of addiction even under appropriate medical use.
Prescription drug abuse is the intentional non-therapeutic use of a prescription drug, even once, for its rewarding psychological or physiological effects.
Drug addiction is a cluster of behavioral, cognitive, and physiological phenomena that develop after repeated substance use and includes: a strong desire to take the drug, difficulties in controlling its use, persisting in its use despite harmful, or potentially harmful, consequences, a higher priority given to drug use than to other activities and obligations, increased tolerance, and sometimes a physical withdrawal.
"Drug seeking" behavior is very common in persons with substance use disorders. Drug seeking tactics include emergency calls or visits near the end of office hours, refusal to undergo appropriate examination, testing or referral, repeated "loss" of prescriptions, tampering with prescriptions, and reluctance to provide prior medical records or contact information for other treating physician(s). "Doctor shopping" (visiting multiple prescribers) to obtain additional prescriptions is common among drug abusers and people suffering from untreated addiction. Preoccupation with achieving adequate pain relief can be appropriate behavior in a patient with poor pain control.
Abuse and addiction are separate and distinct from physical dependence and tolerance. Health care providers should be aware that addiction may not be accompanied by concurrent tolerance and symptoms of physical dependence in all addicts. In addition, abuse of opioids can occur in the absence of true addiction.
SEGLENTIS, like other opioids, can be diverted for non-medical use into illicit channels of distribution. Careful record-keeping of prescribing information, including quantity, frequency, and renewal requests, as required by state and federal law, is strongly advised.
Proper assessment of the patient, proper prescribing practices, periodic re-evaluation of therapy, and proper dispensing and storage are appropriate measures that help to limit abuse of opioid drugs.
9.3 Dependence
Both tolerance and physical dependence can develop during opioid therapy. Tolerance is the need for increasing doses of opioids to maintain a defined effect such as analgesia (in the absence of disease progression or other external factors). Tolerance may occur to both the desired and undesired effects of drugs, and may develop at different rates for different effects.
Physical dependence is a physiological state in which the body adapts to the drug after a period of regular exposure, resulting in withdrawal symptoms after abrupt discontinuation or a significant dosage reduction of a drug. Withdrawal also may be precipitated through the administration of drugs with opioid antagonist activity (e.g., naloxone, nalmefene), mixed agonist/antagonist analgesics (e.g., pentazocine, butorphanol, nalbuphine), or partial agonists (e.g., buprenorphine). Physical dependence may not occur to a clinically significant degree until after several days to weeks of continued opioid usage.
Do not abruptly discontinue SEGLENTIS in a patient physically dependent on opioids. Rapid tapering of SEGLENTIS in a patient physically dependent on opioids may lead to serious withdrawal symptoms, uncontrolled pain, and suicide. Rapid discontinuation has also been associated with attempts to find other sources of opioid analgesics, which may be confused with drug-seeking for abuse.
When discontinuing SEGLENTIS, gradually taper the dosage using a patient-specific plan that considers the following: the dose of SEGLENTIS the patient has been taking, the duration of treatment, and the physical and psychological attributes of the patient. To improve the likelihood of a successful taper and minimize withdrawal symptoms, it is important that the opioid tapering schedule is agreed upon by the patient. In patients taking opioids for a long duration at high doses, ensure that a multimodal approach to pain management, including mental health support (if needed), is in place prior to initiating an opioid analgesic taper [see Dosage and Administration (2.4), Warnings and Precautions (5.28)]. If SEGLENTIS is abruptly discontinued in a physically-dependent patient, a withdrawal syndrome may occur. Some or all of the following can characterize this syndrome: restlessness, lacrimation, rhinorrhea, yawning, perspiration, chills, myalgia, and mydriasis. Other signs and symptoms also may develop, including irritability, anxiety, backache, joint pain, weakness, abdominal cramps, insomnia, nausea, anorexia, vomiting, diarrhea, or increased blood pressure, respiratory rate, or heart rate.
Infants born to mothers physically dependent on opioids will also be physically dependent and may exhibit respiratory difficulties and withdrawal signs [see Use in Specific Populations (8.1)].
10. Overdosage
11. Seglentis Description
SEGLENTIS (celecoxib and tramadol hydrochloride) tablets contains a co-crystal with molecular weight of 681.2, composed of tramadol hydrochloride, an analgesic and opioid agonist, and celecoxib, a nonsteroidal anti-inflammatory drug, in a 1:1 molecular ratio.
The chemical name for tramadol hydrochloride is (1RS,2RS)-2-[(dimethylamino)methyl]-1-(3-methoxyphenyl)cyclohexanol hydrochloride (C16H26ClNO2). The structural formula is:
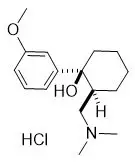
The molecular weight of tramadol hydrochloride is 299.84 (the molecular weight of tramadol is 263.38).
The chemical name for celecoxib is 4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl] benzenesulfonamide and is a diaryl-substituted pyrazole (C17H14F3N3O2S). The molecular weight is 381.38 and it has the following chemical structure:
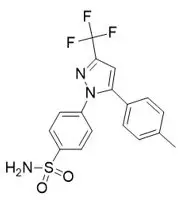
SEGLENTIS coated tablets contain 56 mg celecoxib and 44 mg of tramadol hydrochloride (equivalent to 39 mg tramadol) in a co-crystal structure. Tablets are white to off-white in color. Inactive ingredients in the tablet are sodium lauryl sulfate, crospovidone, mannitol, sodium stearyl fumarate, talc, cellulose microcrystalline, copovidone and color mixture (polyvinyl alcohol partially hydrolyzed, titanium dioxide, polyethylene glycol and talc).
12. Seglentis - Clinical Pharmacology
12.1 Mechanism of Action
SEGLENTIS is a co-crystal that contains tramadol, an opioid agonist and inhibitor of norepinephrine and serotonin re-uptake, and celecoxib, a nonsteroidal anti-inflammatory drug, in a 1:1 molecular ratio.
12.3 Pharmacokinetics
Absorption
Tramadol is presented in SEGLENTIS as a racemate. After Tramadol immediate-release (IR) administration both the [-] and [+] forms of both tramadol and M1 are detected in the circulation.
The rate and extent of absorption of tramadol and celecoxib in SEGLENTIS show differences in absorption compared to Tramadol IR Tablets or Celecoxib capsule when those drugs are administered individually and concomitantly in a single four way cross-over study.
The PK parameters of tramadol, tramadol-M1 metabolite and celecoxib after single dose oral administration of SEGLENTIS Tablets, Tramadol IR Tablets, Celecoxib Capsule or Tramadol IR Tablets and Celecoxib Capsule administered concomitantly is shown in Table 3.
| Analyte | PK Parameter * | 2 × SEGLENTIS Tablets (112 mg celecoxib + 88 mg tramadol) | 2 × 50 mg Tramadol IR Tablets | 1× 100 mg Celecoxib Capsule | 2 × 50 mg Tramadol IR Tablets + 100 mg Celecoxib Capsule |
|---|---|---|---|---|---|
| n=33 | n=32 | n=33 | n=32 | ||
|
|||||
| Tramadol | Cmax (ng/mL) | 214 (29) | 305 (23) | - | 312 (22) |
| Tmax (h) † | 3.0 (1.25, 8.0) | 2.0 (0.75, 3.0) | - | 1.9 (1.0, 6.0) | |
| AUC0-t (ng∙h/mL) | 2507 (36) | 2709 (35) | - | 2888 (34) | |
| AUC0-∞ (ng∙h/mL) | 2590 (35) ‡ | 2802(32) § | - | 2990 (32) § | |
| T½ (h) | 6.5 (15) | 6.1 (17) | - | 6.2 (16) | |
| Tramadol-M1 metabolite | Cmax (ng/mL) | 55 (29) | 78 (29) | - | 78 (29) |
| Tmax (h) † | 4.0 (2.5, 8.0) | 2.5 (1.25, 6.0) | - | 2.5 (1.25, 8.0) | |
| AUC0-t (ng∙h/mL) | 846 (27) | 965 (25) | - | 1010 (25) | |
| AUC0-∞ (ng∙h/mL) | 880 (24) ‡ | 1002 (21) § | - | 1049 (21) § | |
| T½ (h) | 7.2 (14) | 6.7 (14) | - | 7.0 (15) | |
| Celecoxib | Cmax (ng/mL) | 259 (34) | - | 318 (47) | 165 (46) |
| Tmax (h) † | 1.5 (0.75, 6.0) | - | 3.0 (1.25, 8.0) | 2.5 (1.0, 12.0) | |
| AUC0-t (ng∙h/mL) | 1930 (41) | - | 2348 (40) | 1929 (38) | |
| AUC0-∞ (ng∙h/mL) | 2128 (42) ¶ | - | 2553 (43) # | 2224 (39) Þ | |
| T½ (h) | 13 (27) | - | 11 (46) | 14 (29) | |
Elimination
Tramadol is eliminated primarily through metabolism by the liver and the metabolites are eliminated primarily by the kidneys.
The mean terminal plasma elimination half-life of tramadol were 6.5 hours and 9.0 hours after single-dose and multiple-dose administration of SEGLENTIS tablets, respectively. There was no change in the elimination half-life of celecoxib (13 hours) after single or multiple dose administration of SEGLENTIS tablets.
Specific Populations
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
There are no animal or laboratory studies with SEGLENTIS (product composed of tramadol and celecoxib) to evaluate carcinogenesis, mutagenesis, or impairment of fertility. Data on the individual components are described below.
16. How is Seglentis supplied
SEGLENTIS (celecoxib and tramadol hydrochloride) tablets are coated tablets containing celecoxib 56 mg and tramadol hydrochloride 44 mg. The tablets are white to off-white elongated coated tablets debossed with "100 on one side and "CTC" on the other side and are available as follows:
Bottles of 30 tablets: NDC 66869-564-30
Bottles of 35 tablets: NDC 66869-564-35
Bottles of 90 tablets: NDC 66869-564-90
| SEGLENTIS
celecoxib and tramadol hydrochloride tablet |
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
 |
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
| Labeler - Kowa Pharmaceuticals America, Inc. (119459589) |