Drug Detail:Simbrinza (Brimonidine and brinzolamide ophthalmic [ bri-mon-i-deen-and-brin-zoe-la-mide-off-thal-mik ])
Drug Class: Ophthalmic glaucoma agents
Highlights of Prescribing Information
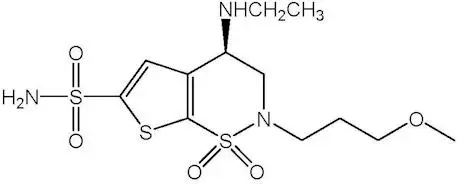
SIMBRINZA® (brinzolamide and brimonidine tartrate ophthalmic suspension)
Initial U.S. Approval: 2013
Indications and Usage for Simbrinza
SIMBRINZA is a fixed combination of a carbonic anhydrase inhibitor and an alpha-2 adrenergic receptor agonist indicated for the reduction of elevated intraocular pressure (IOP) in patients with open-angle glaucoma or ocular hypertension. (1)
Simbrinza Dosage and Administration
Shake well before use. Instill one drop in the affected eye(s) three times daily. If more than one topical ophthalmic drug is being used, the drugs should be administered at least five minutes apart. (2)
Dosage Forms and Strengths
Suspension containing 10 mg/mL brinzolamide and 2 mg/mL brimonidine tartrate. (3)
Contraindications
- Hypersensitivity to any component of this product. (4.1)
- Neonates and infants (Under the Age of two Years). (4.2)
Warnings and Precautions
- Potential for sulfonamide hypersensitivity reactions because of the brinzolamide component. (5.1)
- Potential for corneal endothelium cell loss. (5.2)
- Severe renal impairment may limit the metabolism of the brinzolamide component. (5.3)
Adverse Reactions/Side Effects
Most common adverse reactions occurring in approximately 3% to 5% of patients included blurred vision, eye irritation, dysgeusia (bad taste), dry mouth, and eye allergy. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Novartis Pharmaceuticals Corporation at 1-888-669-6682 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
- Oral Carbonic Anhydrase Inhibitors (CAIs) (7.1)
- High-dose Salicylate Therapy (7.2)
- Central Nervous System (CNS) Depressants (7.3)
- Antihypertensives/Cardiac Glycosides (7.4)
- Tricyclic Antidepressants (7.5)
- Monoamine Oxidase Inhibitors (MAOIs) (7.6)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 11/2019
Full Prescribing Information
1. Indications and Usage for Simbrinza
SIMBRINZA (brinzolamide and brimonidine tartrate ophthalmic suspension) 1% and 0.2% is a fixed combination of a carbonic anhydrase inhibitor and an alpha-2 adrenergic receptor agonist indicated for the reduction of elevated intraocular pressure (IOP) in patients with open-angle glaucoma or ocular hypertension.
2. Simbrinza Dosage and Administration
The recommended dose is one drop of SIMBRINZA in the affected eye(s) three times daily. Shake well before use. SIMBRINZA ophthalmic suspension may be used concomitantly with other topical ophthalmic drug products to lower IOP. If more than one topical ophthalmic drug is being used, the drugs should be administered at least five minutes apart.
3. Dosage Forms and Strengths
Suspension containing 10 mg/mL brinzolamide and 2 mg/mL brimonidine tartrate.
4. Contraindications
5. Warnings and Precautions
5.1 Sulfonamide Hypersensitivity Reactions
SIMBRINZA contains brinzolamide, a sulfonamide, and although administered topically is absorbed systemically. Therefore, the same types of adverse reactions that are attributable to sulfonamides may occur with topical administration of SIMBRINZA. Fatalities have occurred due to severe reactions to sulfonamides including Stevens-Johnson syndrome, toxic epidermal necrolysis, fulminant hepatic necrosis, agranulocytosis, aplastic anemia, and other blood dyscrasias. Sensitization may recur when a sulfonamide is re-administered irrespective of the route of administration. If signs of serious reactions or hypersensitivity occur, discontinue the use of this preparation [see Patient Counseling Information (17)].
5.2 Corneal Endothelium
Carbonic anhydrase activity has been observed in both the cytoplasm and around the plasma membranes of the corneal endothelium. There is an increased potential for developing corneal edema in patients with low endothelial cell counts. Caution should be used when prescribing SIMBRINZA to this group of patients.
6. Adverse Reactions/Side Effects
6.1 Clinical Trials Experience
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to the rates in the clinical trials of another drug and may not reflect the rates observed in practice.
SIMBRINZA
In two clinical trials of three months duration 435 patients were treated with SIMBRINZA, and 915 were treated with the two individual components. The most frequently reported adverse reactions in patients treated with SIMBRINZA occurring in approximately 3% to 5% of patients in descending order of incidence were blurred vision, eye irritation, dysgeusia (bad taste), dry mouth, and eye allergy. Rates of adverse reactions reported with the individual components were comparable. Treatment discontinuation, mainly due to adverse reactions, was reported in 11% of SIMBRINZA patients.
Other adverse reactions that have been reported with the individual components during clinical trials are listed below.
Brinzolamide 1%
In clinical trials of brinzolamide ophthalmic suspension 1%, the most frequently reported adverse reactions reported in 5% to 10% of patients were blurred vision and bitter, sour or unusual taste. Adverse reactions occurring in 1% to 5% of patients were blepharitis, dermatitis, dry eye, foreign body sensation, headache, hyperemia, ocular discharge, ocular discomfort, ocular keratitis, ocular pain, ocular pruritus, and rhinitis.
The following adverse reactions were reported at an incidence below 1%: allergic reactions, alopecia, chest pain, conjunctivitis, diarrhea, diplopia, dizziness, dry mouth, dyspnea, dyspepsia, eye fatigue, hypertonia, keratoconjunctivitis, keratopathy, kidney pain, lid margin crusting or sticky sensation, nausea, pharyngitis, tearing, and urticaria.
Brimonidine Tartrate 0.2%
In clinical trials of brimonidine tartrate 0.2%, adverse reactions occurring in approximately 10% to 30% of the subjects, in descending order of incidence, included oral dryness, ocular hyperemia, burning and stinging, headache, blurring, foreign body sensation, fatigue/drowsiness, conjunctival follicles, ocular allergic reactions, and ocular pruritus.
Reactions occurring in approximately 3% to 9% of the subjects, in descending order included corneal staining/erosion, photophobia, eyelid erythema, ocular ache/pain, ocular dryness, tearing, upper respiratory symptoms, eyelid edema, conjunctival edema, dizziness, blepharitis, ocular irritation, gastrointestinal symptoms, asthenia, conjunctival blanching, abnormal vision, and muscular pain.
The following adverse reactions were reported in less than 3% of the patients: lid crusting, conjunctival hemorrhage, abnormal taste, insomnia, conjunctival discharge, depression, hypertension, anxiety, palpitations/arrhythmias, nasal dryness, and syncope.
7. Drug Interactions
7.1 Oral Carbonic Anhydrase Inhibitors
There is a potential for an additive effect on the known systemic effects of carbonic anhydrase inhibition in patients receiving an oral carbonic anhydrase inhibitor and brinzolamide ophthalmic suspension 1%, a component of SIMBRINZA ophthalmic suspension. The concomitant administration of SIMBRINZA and oral carbonic anhydrase inhibitors is not recommended.
7.2 High-Dose Salicylate Therapy
Carbonic anhydrase inhibitors may produce acid-base and electrolyte alterations. These alterations were not reported in the clinical trials with brinzolamide ophthalmic suspension 1%. However, in patients treated with oral carbonic anhydrase inhibitors, rare instances of acid-base alterations have occurred with high-dose salicylate therapy. Therefore, the potential for such drug interactions should be considered in patients receiving SIMBRINZA.
7.3 Central Nervous System Depressants
Although specific drug interaction studies have not been conducted with SIMBRINZA, the possibility of an additive or potentiating effect with central nervous system (CNS) depressants (alcohol, opiates, barbiturates, sedatives, or anesthetics) should be considered.
7.4 Antihypertensives/Cardiac Glycosides
Because brimonidine tartrate, a component of SIMBRINZA, may reduce blood pressure, caution in using drugs such as antihypertensives and/or cardiac glycosides with SIMBRINZA is advised.
7.5 Tricyclic Antidepressants
Tricyclic antidepressants have been reported to blunt the hypotensive effect of systemic clonidine. It is not known whether the concurrent use of these agents with SIMBRINZA in humans can lead to resulting interference with the IOP lowering effect. Caution is advised in patients taking tricyclic antidepressants, which can affect the metabolism and uptake of circulating amines.
7.6 Monoamine Oxidase Inhibitors
Monoamine oxidase inhibitors (MAOIs) may theoretically interfere with the metabolism of brimonidine tartrate and potentially result in an increased systemic side-effect such as hypotension. Caution is advised in patients taking MAOIs, which can affect the metabolism and uptake of circulating amines.
| SIMBRINZA
brinzolamide/brimonidine tartrate suspension/ drops |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Novartis Pharmaceuticals Corporation (002147023) |