Drug Detail:Sitavig (Acyclovir (oral) [ a-sye-klo-veer ])
Drug Class: Purine nucleosides
Highlights of Prescribing Information
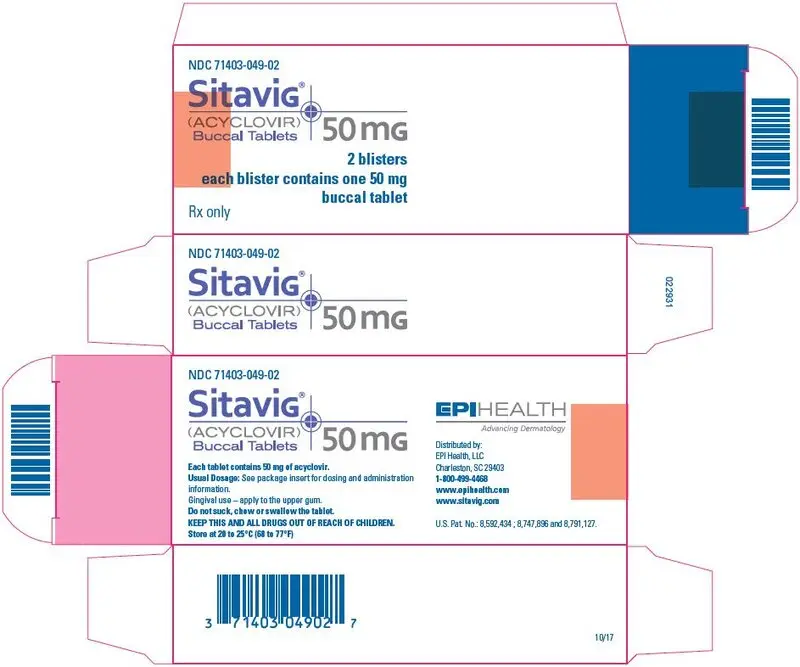
SITAVIG (acyclovir) buccal tablets
Initial U.S. Approval: 1982
Indications and Usage for Sitavig
SITAVIG is a deoxynucleoside analogue of DNA polymerase inhibitor, indicated for the treatment of recurrent herpes labialis (cold sores) in immunocompetent adults. (1).
Sitavig Dosage and Administration
- Application of one SITAVIG 50 mg buccal tablet as a single dose to the upper gum (canine fossa) region (2.1).
- SITAVIG should be applied within one hour after the onset of prodromal symptoms and before the appearance of any signs of herpes labialis.
- Do not crush, chew, suck or swallow tablets (2.2).
Dosage Forms and Strengths
50 mg buccal tablets (3).
Contraindications
Known hypersensitivity to acyclovir, milk protein concentrate, or any other component of the product (4).
Adverse Reactions/Side Effects
Most common adverse reactions (≥1%) are: headache and application site pain (6.1).
To report SUSPECTED ADVERSE REACTIONS, contact EPI Health, LLC at 1-800-499-4468 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
Due to the low dose and minimal systemic absorption of SITAVIG, drug interactions are unlikely (7)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 12/2019
Related/similar drugs
acyclovir, valacyclovir, Valtrex, Zovirax, tetracaine topicalFull Prescribing Information
1. Indications and Usage for Sitavig
SITAVIG is indicated for the treatment of recurrent herpes labialis (cold sores) in immunocompetent adults.
2. Sitavig Dosage and Administration
2.1 Basic Dosing Information
One SITAVIG 50 mg buccal tablet should be applied as a single dose to the upper gum region (canine fossa).
2.2 Administration Instructions
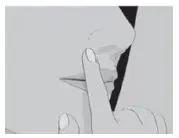
SITAVIG should be applied within one hour after the onset of prodromal symptoms and before the appearance of any signs of herpes labialis lesions. The tablet should be applied with a dry finger immediately after taking it out of the blister. The tablet should be placed to the upper gum just above the incisor tooth (canine fossa) and held in place with a slight pressure over the upper lip for 30 seconds to ensure adhesion. For comfort the rounded side should be placed to the upper gum, but either side of the tablet can be applied. Tablet should be applied on the same side of the mouth as the herpes labialis symptoms.
Once applied, SITAVIG stays in position and gradually dissolves during the day. [See Clinical Pharmacology (12.3)]. In addition,
- SITAVIG should not be crushed, chewed, sucked or swallowed.
- Food and drink can be taken normally when SITAVIG is in place. Avoid any situations which may interfere with adhesion of the tablet such as chewing gum, touching or pressing the tablet after placement, wearing upper dentures, and brushing teeth. If the teeth need to be cleaned while the tablet is in place, rinse the mouth gently. Drink plenty of liquids in the case of dry mouth.
- If SITAVIG does not adhere or falls off within the first 6 hours, the same tablet should be repositioned immediately. If the tablet cannot be repositioned, a new tablet should be placed.
- If SITAVIG is swallowed within the first 6 hours, the patient should drink a glass of water and a new tablet should be applied. [see Patient Counseling Information (17)].
- SITAVIG does not need to be reapplied if the tablet falls out or is swallowed after the first 6 hours.
3. Dosage Forms and Strengths
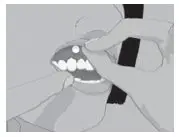
SITAVIG is a buccal tablet containing 50 mg of acyclovir. SITAVIG tablets are round, off-white tablets, with a rounded side and a flat side. The tablets are marked with an "AL21" on the flat side.
4. Contraindications
SITAVIG is contraindicated in patients with known hypersensitivity (e.g., anaphylaxis) to acyclovir, milk protein concentrate, or any other component of the product.
6. Adverse Reactions/Side Effects
6.1 Clinical Trial Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
The overall safety of SITAVIG was assessed in 378 adult subjects having at least 4 herpes labialis episodes the previous year.
One randomized, double-blind, placebo controlled trial was conducted in patients with recurrent herpes labialis (cold sores). In this trial, 378 HSV infected subjects used SITAVIG as a single dose, and 397 subjects used placebo.
Selected treatment emergent adverse events without regard to causality and reported in at least 1% of patients can be seen in Table 1.
| Event | SITAVIG N = 378 | Placebo N = 397 |
|---|---|---|
| Nervous System Disorders | ||
| Headache | 3% | 3% |
| Dizziness | 1% | 1% |
| Lethargy | 1% | 0 |
| Gastrointestinal system Disorders | ||
| Gingival Pain | 1% | 0.3% |
| Aphthous Stomatitis | 1% | 0 |
| Administration Site Conditions | ||
| Application Site Pain | 1% | 1% |
| Application Site Irritation | 1% | 0 |
| Skin and Subcutaneous Disorders | ||
| Erythema | 1% | 0.3% |
| Rash | 1% | 0.3% |
The treatment emergent adverse events considered related to treatment that occurred in greater than or equal to 1% of patients included headache (1% SITAVIG vs. 2% placebo) and application site pain (1% both arms). There was no discontinuation of SITAVIG due to adverse drug reactions. Most treatment related adverse events were mild or moderate in severity. One report of headache from both treatment arms was classified as severe.
7. Drug Interactions
No interaction studies have been performed with SITAVIG. Acyclovir is primarily eliminated unchanged in the urine via active tubular secretion. Drugs administered concomitantly that compete with tubular secretion may increase acyclovir plasma concentrations. However, due to the low dose and minimal systemic absorption of SITAVIG, systemic drug interactions are unlikely.
8. Use In Specific Populations
8.1 Pregnancy
There are no available data on SITAVIG use in pregnant women. However, published observational studies over decades of use of acyclovir have not identified a drug-associated risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes. Systemic exposure of acyclovir following buccal administration of SITAVIG is minimal [see Clinical Pharmacology (12.3)]. Animal reproduction studies have not been conducted with SITAVIG. Animal reproduction studies with systemic exposure of acyclovir have been conducted. Refer to oral and parental acyclovir prescribing information for additional details.
8.2 Lactation
There are no data on the presence of acyclovir in human milk following buccal administration. There are no data on the effects of acyclovir on the breastfed infant or milk production. Systemic exposure following buccal administration of acyclovir is minimal [see Clinical Pharmacology (12.3)]. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for SITAVIG and any potential adverse effects on the breastfed child from SITAVIG or from the underlying maternal condition.
8.4 Pediatric Use
Safety and effectiveness of SITAVIG in pediatric patients have not been established. The ability of pediatric patients to comply with the application instructions has not been evaluated. Use in younger children is not recommended due to potential risk of choking.
10. Overdosage
Acyclovir absorption and systemic exposure following application of SITAVIG are minimal. Overdose is therefore unlikely [see Clinical Pharmacology (12.3)].
Symptomatic and supportive care is the basis for management.
11. Sitavig Description
SITAVIG (acyclovir) buccal tablet is applied topically to the gum and releases acyclovir as the buccal tablet gradually dissolves [see Clinical Pharmacology (12.3)].
Acyclovir is a synthetic purine nucleoside analogue active against herpes viruses. The chemical name of acyclovir is 2-amino-1,9-dihydro-9-[(2-hydroxyethoxy)methyl]-6H-purin-6-one; it has a molecular formula of C8H11N5O3 and a molecular weight of 225. The structural formula is shown in Figure 1.
Figure 1: Structural Formula of Acyclovir
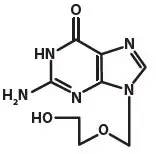
Acyclovir drug substance is a white or almost white crystalline powder.
SITAVIG contains 50 mg of acyclovir, USP and the following inactive ingredients: hypromellose, USP; milk protein concentrate; sodium lauryl sulfate, NF; magnesium stearate, NF; microcrystalline cellulose, NF; povidone, USP; colloidal silicon dioxide, NF.
12. Sitavig - Clinical Pharmacology
12.1 Mechanism of Action
Acyclovir is an antiviral drug active against α-herpesviruses [see Microbiology (12.4)].
12.3 Pharmacokinetics
Absorption and Distribution
Salivary
The pharmacokinetic parameters of acyclovir after a single dose of SITAVIG in the saliva and plasma of healthy volunteers are provided in Table 2.
| PK Parameters (N = 12) | Salivary Mean ±SD (Min - Max) | Plasma*
Mean ±SD (Min - Max) |
|---|---|---|
|
||
| AUC0-24h (mcg∙h/mL) | 2900 ± 2400 (849 - 9450) | 0.225 + 0.132 (0.027-0.422) |
| Cmax (mcg/mL) | 440 ± 241 (149 – 959) | 0.028 + 0.010 (0.017-0.055) |
| Tmax (hour)† | 7.04 (3.07 – 18.05) | 12 (5-16) |
In the Phase 3 study, the levels of acyclovir in saliva were measured within 24 hours of SITAVIG application in 56 patients with recurrent herpes labialis (mean value 88.1 micrograms per mL) and were within the range of those observed in the PK study in healthy volunteers.
In healthy volunteers, the median duration of buccal adhesion was 14 hours following application of a single SITAVIG 50 mg tablet.
12.4 Microbiology
Resistance
In Cell Culture
Acyclovir-resistant HSV-1 and HSV-2 strains were isolated in cell culture. Acyclovirresistant HSV resulted from mutations in the viral thymidine kinase (TK; pUL23) and DNA polymerase (POL; pUL30) genes. Frameshifts were commonly isolated and result in premature truncation of the HSV TK product with consequent decreased susceptibility to acyclovir. Mutations in the viral TK gene may lead to complete loss of TK activity (TK negative), reduced levels of TK activity (TK partial), or alteration in the ability of viral TK to phosphorylate the drug without an equivalent loss in the ability to phosphorylate thymidine (TK altered). In cell culture the following resistance-associated substitutions in TK of HSV-1 and HSV-2 were observed (Table 3).
| HSV-1 | TK | P5A, H7Q, L50V, G56V, G59A, G61A, K62N, T63A, E83K, P84S, D116N, P131S, R163H, A167V, P173L, Q185R, R216S, R220H, T245M, R281stop, T287M, M322K |
| HSV-2 | TK | L69P, C172R, T288M |
| HSV-1 | POL | D368A, Y557S, E597D, V621S, L702H, N815S, V817M,G841C |
| HSV-2 | POL | - |
In HSV-Infected Patients
Clinical HSV-1 and HSV-2 isolates obtained from patients who failed treatment for their α-herpesvirus infections were evaluated for genotypic changes in the TK and POL genes and for phenotypic resistance to acyclovir (Table 4). HSV isolates with frameshift mutations and resistance-associated substitutions in TK and POL were identified. The listing of substitutions in the HSV TK and POL leading to decreased susceptibility to acyclovir is not all inclusive and additional changes will likely be identified in HSV variants isolated from patients who fail acyclovir-containing regimens. The possibility of viral resistance to acyclovir should be considered in patients who fail to respond or experience recurrent viral shedding during therapy.
| Note: Many additional pathways to acyclovir resistance likely exist. | ||
| HSV-1 | TK | G6C, R32H, R41H, R51W, Y53C/D/H, Y53stop, D55N, G56D/S, P57H, H58N/R/Y, G59R, G61A, K62N, T63I, Q67stop, S74stop, Y80N, E83K, P84L, Y87H, W88R, R89Q/W, E95stop, T103P, Q104H, Q104stop, H105P, D116N, M121L/R, S123R, Q125H, M128L, G129D, I143V, A156V, D162A/H/N, R163G/H, L170P, Y172C, P173L, A174P, A175V, R176Q/W, R176stop, L178R, S181N, V187M, A189V, V192A, G200C/D/S, T201P, V204G, A207P, L208F/H, R216C/H, R220C/H, R221H, R222C/H, L227F, T245M/P, L249P, Q250Stop, C251G, R256W, E257K, Q261R, T287M, L288Stop, L291P/R, L297S, L315S, L327R, C336Y, Q342Stop, T354P, L364P, A365T |
| HSV-2 | TK | R34C, G39E, R51W, Y53N, G59P, G61W, S66P, A72S, D78N, P85S, A94V, N100H, I101S, Q105P, T131P, D137stop, F140L, L158P, S169P, R177W, S182N, M183I, V192M, G201D, R217H, R221C/H, Q222stop, R223H, Y239stop, R271V, P272S, D273R, T287M, C337Y |
| HSV-1 | POL | K532T, Q570R, L583V, A605V, A657T, D672N, V715G, A719T/V, S724N, F733C, E771Q, S775N, L778M, E798K, V813M, N815S, G841S, I890M, G901V, V958L, H1228D |
| HSV-2 | POL | E250Q, D307N, K533E, A606V, C625R, R628C, E678G, A724V, S725G, S729N, I731F, Q732R, M789K/T, V818A, N820S, Y823C, Q829R, T843A, M910T, D912N/V, A915V, F923L, T934A, R964H |
Cross-resistance
Cross-resistance has been observed among HSV isolates carrying frameshift mutations and resistance-associated substitutions, which confer reduced susceptibility to penciclovir (PCV), famciclovir (FCV), and foscarnet (FOS) [Table 5].
| Cross-resistant to PCV/FCV | HSV-1 TK | G6C, R32H, R51W, Y53C/H, H58N, G61A, S74Stop, E83K, P84L, T103P, Q104Stop, D116N, M121R, I143V, R163H, L170P, Y172C, A174P, R176Q/W, Q185R, A189V, G200D, L208H, R216C, R220H, R222C/H, T245M, Q250Stop, R256W, R281Stop, T287M, L315S, M322K, C336Y |
| Cross-resistant to PCV/FCV | HSV-1 POL | A657T, D672N, V715G, A719V, S724N, E798K, N815S, G841S |
| Cross-resistant to PCV/FCV | HSV-2 TK | G39E, R51W, Y53N, R177W, R221H, T288M |
| Cross-resistant to PCV/FCV | HSV-2 POL | K533E, A606V, C625R, R628C, S729N, Q732R, M789K/T, V818A, N820S, F923L, T934A |
| Cross-resistant to FOS | HSV-1 POL | D368A, A605V, D672N, L702H, V715G, A719T/V, S724N, L778M, E798K, V813M, N815S, V817M, G841C/S, I890M |
| Cross-resistant to FOS | HSV-2 POL | K533E, A606V, C625R, R628C, A724V, S725G, S729N, I731F, Q732R, M789K/T, V818A, Y823C D912V, F923L, T934A, R964H |
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Systemic exposure following buccal administration of acyclovir is minimal. Results from previous studies of carcinogenesis, mutagenesis and fertility for acyclovir are not included in the full prescribing information for SITAVIG due to the minimal exposure that results from buccal administration. Information on these studies following systemic exposure is available in the full prescribing information for acyclovir products approved for oral and parenteral administration.
16. How is Sitavig supplied
SITAVIG buccal tablets are supplied as off-white tablets containing 50 mg of acyclovir. SITAVIG tablets have a rounded side and a flat side and are imprinted with AL21 on one side. SITAVIG tablets are packaged in blisters of two tablets (NDC 71403-049-02).
17. Patient Counseling Information
See FDA-approved patient labeling (Patient Information and Instructions for Use).
17.1 Instructions for Use
Read the Instructions for Use that comes with SITAVIG before you start using it. Talk to your doctor or pharmacist if you have any questions.
Important:
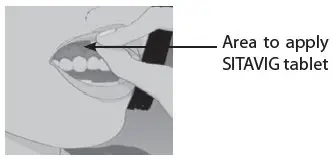
SITAVIG should be applied to the area of the upper gum above the incisor tooth.
SITAVIG tablets should not be crushed, sucked, chewed or swallowed.
If it comes out before 6 hours have gone by, reapply it. If this does not work then a new tablet should be applied.
It should not be applied to the inside of the lip or cheek.
- Tablet should be applied on the same side of the mouth as the herpes labialis symptoms.
- Do not remove SITAVIG if it sticks to your upper gum. If SITAVIG does not stick or falls off of your upper gum within the first 6 hours that you apply it, place it back onto your upper gum. If it still does not stick, replace it with a new SITAVIG tablet.
- Do not re-apply SITAVIG if it falls out or you swallow it after it has been in place 6 hours or longer.
- If you swallow SITAVIG within the first 6 hours of applying it, drink a glass of water and place a new SITAVIG tablet onto your upper gum.
| This Patient Information has been approved by the U.S. Food and Drug Administration. | Revised: 12/2019 |
| Patient Information SITAVIG (SIT-a-vig) (acyclovir) buccal tablets |
|
What is SITAVIG?
|
|
| Who should not use SITAVIG? Do not use SITAVIG if you are allergic to:
|
|
Before using SITAVIG, tell your healthcare provider about all of your medical conditions, including if you:
|
|
| How should I use SITAVIG? See the detailed Instructions for Use for information about how to use SITAVIG.
|
|
| What should I avoid while using SITAVIG?
You should avoid activities that may prevent SITAVIG from sticking to your gum, including: touching or pressing SITAVIG after you apply it, wearing upper dentures, chewing gum, and brushing your teeth during the treatment day. |
|
| What are the possible side effects of SITAVIG? The most common side effects of SITAVIG include: headache and pain where SITAVIG is applied. These are not all the possible side effects of SITAVIG. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
|
How should I store SITAVIG?
|
|
| General information about the safe and effective use of SITAVIG
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use SITAVIG for a condition for which it was not prescribed. Do not give SITAVIG to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about SITAVIG that is written for health professionals. |
|
| What are the ingredients in SITAVIG?
Active ingredient: acyclovir Inactive ingredients: hypromellose, milk protein concentrate, sodium lauryl sulfate, magnesium stearate, microcrystalline cellulose, povidone, colloidal silicon dioxide. Manufactured By: Farméa, 10 rue Bouché Thomas, ZAC d'orgemont, 49 000 Angers - France For more information call 1-800-499-4468. |
|
Instructions for Use
SITAVIG (SIT-a-vig)
(acyclovir) buccal tablets
Read the Instructions for Use that comes with SITAVIG before you start using it. Talk to your healthcare provider or pharmacist if you have any questions.
Important:
- SITAVIG should be applied within 1 hour after you have the first symptom of a cold sore, such as itching, redness, burning, or tingling, and before a cold sore appears.
- SITAVIG should be applied on the same side of your mouth as the cold sore symptoms.
- SITAVIG should be applied to your upper gum, just above your incisor tooth.
- SITAVIG should not be applied to the inside of your lip or your cheek.
- If SITAVIG does not stick to your upper gum or falls off of your upper gum within the first 6 hours after you apply it, the same tablet should be placed back onto your upper gum right away. If this SITAVIG tablet does not stay in place, apply another SITAVIG tablet to your upper gum.
- Do not re-apply SITAVIG if it falls out or you swallow it after it has been in place 6 hours or longer.
- If you swallow SITAVIG within the first 6 hours of applying it, drink a glass of water and place a new SITAVIG tablet onto your upper gum.
How to apply SITAVIG:
| Step 1: | Before you apply SITAVIG, find the area on your upper gum, just above either the left or the right incisor. The incisor tooth is the tooth just to the right or left of your two front teeth (See Figure A). This is where you should apply SITAVIG. |
| Figure A | |
| Step 2: | Peel back the cover of the blister pack. Take 1 SITAVIG out of the blister pack. When removed from the blister pack, SITAVIG must be used right away. SITAVIG is round on one side and flat on the other side (See Figure B). |
| Figure B | |
| Step 3: | Place the flat side of SITAVIG on your dry fingertip. Apply the round side of SITAVIG to your upper gum (See Figure C). The flat side will be facing the inside of your lip. |
| Figure C | |
| Step 4: | Hold SITAVIG in place by applying a slight pressure with your finger on the outside of your upper lip, over the area where SITAVIG is placed, for 30 seconds. This will help SITAVIG stick to your gum (See Figure D). |
| Figure D | |
| Step 5: | Leave the SITAVIG tablet in place until it dissolves. |
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Manufactured By:
Farméa
10 rue Bouché Thomas
ZAC d'orgemont
49 000 Angers - France
Distributed By:
EPI Health, LLC
Charleston, SC 29403
1-800-499-4468
www.epihealth.com
www.sitavig.com
U.S. Pat. No.: 8,592,434 ; 8,747,896 and 8,791,127.
©EPI Health, LLC
Revision date: 12/2019
SIT-PI-0220
023220
| SITAVIG
acyclovir tablet, delayed release |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - EPI Health, Inc (080638894) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| EPI Health, Inc | 080638894 | manufacture(71403-049) , analysis(71403-049) , label(71403-049) , pack(71403-049) | |