Drug Detail:Slynd (Drospirenone)
Drug Class: Progestins
Highlights of Prescribing Information
SLYND (drospirenone) tablets, for oral use
Initial U.S. Approval: 2001
Indications and Usage for Slynd
SLYND is a progestin indicated for use by females of reproductive potential to prevent pregnancy. ( 1)
Slynd Dosage and Administration
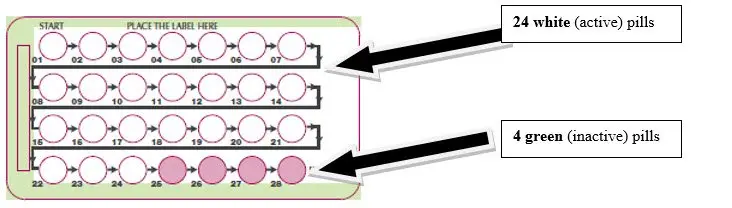
Take one tablet taken daily for 28 days; one white active tablet daily during the first 24 days and one green inactive tablet daily during the 4 following days. ( 2)
Dosage Forms and Strengths
SLYND consists of 24 white tablets each containing 4 mg of drospirenone and 4 green inert tablets. ( 3)
Contraindications
- Renal impairment ( 4)
- Adrenal insufficiency ( 4)
- Presence or history of progestin sensitive cancers ( 4)
- Liver tumors, benign or malignant, or hepatic impairment ( 4)
- Undiagnosed abnormal uterine bleeding ( 4)
Warnings and Precautions
- Hyperkalemia: Check serum potassium levels during the first treatment cycle in females receiving daily, long-term treatment for chronic conditions of diseases with medications that may increase serum potassium concentrations. ( 5.1)
- Thromboembolic disorders: Discontinue SLYND if a thromboembolic event occurs. ( 5.2)
- Bone loss: It is unknown if SLYND may cause a clinically relevant loss of bone mineral density. ( 5.3)
- Liver Disease: Discontinue use if jaundice or acute or chronic disturbances of liver function develops. ( 5.5)
- Ectopic pregnancy: Be alert to the possibility of ectopic pregnancy in females who become pregnant or complain of lower abdominal pain while on SLYND. ( 5.6)
- Risk of Hyperglycemia in Patients with Diabetes: Patients with diabetes may be at greater risk of hyperglycemia and may require additional medication adjustments or monitoring. ( 5.7)
- Bleeding Irregularities and Amenorrhea: May cause irregular bleeding or amenorrhea. Evaluate for other causes, such as pregnancy, if irregular bleeding or amenorrhea persists. ( 5.9)
Adverse Reactions/Side Effects
Most common adverse reactions (>1%) are: acne, metrorrhagia, headache, breast pain, weight increased, dysmenorrhea, nausea, vaginal hemorrhage, libido decreased, breast tenderness, menstruation irregular ( 6)
To report SUSPECTED ADVERSE REACTIONS, contact Exeltis USA, Inc. at 1-877-324-9349 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
Drugs or herbal products that induce certain enzymes (for example, CYP3A4) may decrease the effectiveness of SLYND or increase breakthrough bleeding. Counsel patients to use a back-up or alternative method of contraception when enzyme inducers are used with SLYND. ( 7.1)
Use In Specific Populations
- Pregnancy: Discontinue SLYND if pregnancy occurs ( 8.1)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 5/2019
Full Prescribing Information
1. Indications and Usage for Slynd
SLYND is a progestin indicated for use by females of reproductive potential to prevent pregnancy.
2. Slynd Dosage and Administration
2.1 How to Use SLYND
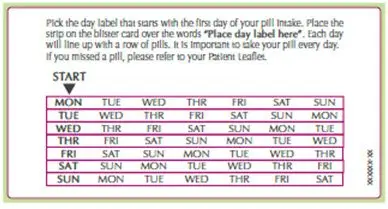
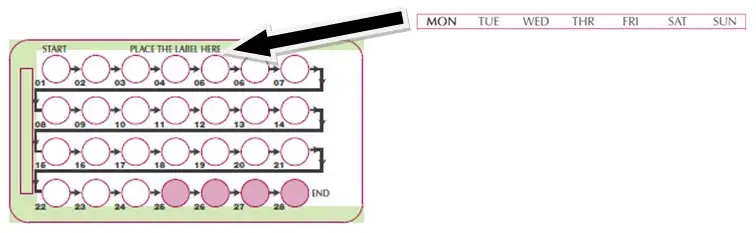
SLYND is dispensed in a blister card. SLYND should be started using a Day 1 start.
| Starting SLYND in females with no current use of hormonal contraception (Day 1 Start) Important: Consider the possibility of ovulation and conception prior to initiation of this product.
Tablet Color: • SLYND active tablets are white (Day 1 to Day 24). • SLYND inert tablets are green (Day 25 to Day 28). | Day 1 Start:
• Take first white active tablet on the first day of menses. • Take subsequent white active tablets once daily at the same time each day for a total of 24 days. • Take one green inert tablet daily for 4 days and at the same time of day that active tablets were taken. • Begin each subsequent pack on the same day of the week as the first cycle pack (i.e., on the day after taking the last inactive tablet). |
| Switching from another contraceptive method to SLYND | Start SLYND:
|
|
|
|
|
|
|
|
|
|
|
|
|
| Refer to the Patient Information and Instructions for Use for additional instructions for counseling patient concerning proper use | |
2.2 How to Take SLYND
SLYND (white active and green inert tablets) is swallowed whole once a day. Take one tablet daily for 28 consecutive days; one white active tablet daily during the first 24 days and one green inert tablet daily during the 4 following days. Tablets must be taken every day at about the same time of the day so that the interval between two tablets is always 24 hours.
2.3 Missed Doses
| Take the missed tablet as soon as possible. Continue taking one tablet a day until the pack is finished. |
| Take the last missed tablet as soon as possible. Continue one tablet a day until the pack is finished (one or more missed tablet(s) will remain in the blister pack). Additional non-hormonal contraception (such as condoms or spermicide) should be used as back-up if the patient has sex within 7 days after missing tablets. |
| Skip the missed pill days and continue taking one tablet a day until the pack is finished. |
2.4 Advice in Case of Gastrointestinal Disturbances
If vomiting or diarrhea occurs within 3-4 hours after tablet taking, the new tablet (scheduled for the next day) should be taken as soon as possible. The new tablet should be taken within 12 hours of the usual time of tablet-taking if possible. If more than two tablets are missed, the advice concerning missed tablets, including using backup non-hormonal contraception, given above is applicable.
3. Dosage Forms and Strengths
SLYND is supplied in blister cards, each containing 24 round, film-coated, unscored, white tablets and 4 round, film-coated, unscored green tablets.
- Each white tablet contains 4 mg of drospirenone. White tablets are debossed with an "E" on one side and a "D" on the other side
- Each green tablet is inert and does not contain drospirenone. Green tablets are debossed with an "E" on one side and a "4" on the other side.
4. Contraindications
SLYND is contraindicated in females with the following conditions:
- Renal impairment [see Warnings and Precautions (5.1) and Use in Specific Populations (8.7)]
- Adrenal insufficiency [see Warnings and Precautions (5.1)]
- Presence or history of cervical cancer or progestin sensitive cancers [see Warnings and Precautions (5.4)]
- Liver tumors, benign or malignant, or hepatic impairment [see Warnings and Precautions (5.5) and Use in Specific Populations (8.6)]
- Undiagnosed abnormal uterine bleeding [see Warnings and Precautions (5.8)]
5. Warnings and Precautions
5.1 Hyperkalemia
SLYND contains drospirenone, a progestin, which has anti-mineralocorticoid activity, including the potential for hyperkalemia in high-risk females, comparable to a 25 mg dose of spironolactone. SLYND is contraindicated in females with conditions that predispose to hyperkalemia (e.g. renal impairment, hepatic impairment, and adrenal insufficiency). Females receiving daily, long-term treatment for chronic conditions or diseases with medications that may increase serum potassium concentration should have their serum potassium concentration checked prior to starting treatment and during the first treatment cycle. Consider monitoring serum potassium concentration in females at increased risk for hyperkalemia i.e., those females who take a strong CYP3A4 inhibitor long-term and concomitantly with SLYND. Strong CYP3A4 inhibitors include azole antifungals (e.g. ketoconazole, itraconazole, voriconazole), HIV/HCV protease inhibitors (e.g., indinavir, boceprevir), and clarithromycin [ see Drug Interactions (7)]. Monitor females taking SLYND who later develop medical conditions and/or begin medication that put them at an increased risk for hyperkalemia.
Most females with hyperkalemia in the clinical development studies of SLYND had mild potassium elevations and/or isolated increases that returned to normal while still on study medication. No concurrent adverse reactions were attributed to hyperkalemia. In the pivotal trial, two females (0.2%) with persistent potassium elevations discontinued SLYND.
5.2 Thromboembolic Disorders
Epidemiological studies have not indicated an association between progestin-only preparations and an increased risk of myocardial infarction, cerebral thromboembolism, or venous thromboembolism.
Combined oral contraceptives containing drospirenone and ethinyl estradiol may be associated with a higher risk of venous thromboembolism (VTE) than those containing some other progestins in combination with ethinyl estradiol. It is unknown whether the risk of VTE is increased with drospirenone alone; however, if there is a risk, it is expected to be lower than that of drospirenone in combination with ethinyl estradiol.
When prescribing SLYND, consider the increased risk of thromboembolism inherent in the postpartum period and in females with a history of thromboembolism
Discontinue SLYND if arterial or venous thromboembolic events occur. Consider discontinuing SLYND, if feasible, in case of SLYND prolonged immobilization due to surgery or illness.
5.3 Bone Loss
Treatment with SLYND leads to decreased estradiol serum levels. It is unknown if this may cause a clinically relevant loss of bone mineral density.
5.4 Cervical Cancer
Some studies suggest that use of combination hormonal contraceptives containing progestin and estradiol has been associated with an increase in the risk of cervical cancer or intraepithelial neoplasia. However, there continues to be controversy about the extent to which such findings may be due to differences in sexual behavior and other factors.
5.5 Liver Disease
Discontinue SLYND if jaundice or acute or chronic disturbances of liver function develop. Do not resume use until markers of liver function return to normal and SLYND causation has been excluded.
SLYND is contraindicated in females with liver tumors, benign or malignant, or hepatic impairment [see Use in Specific Populations (8.6)].
5.6 Ectopic Pregnancy
Be alert to the possibility of ectopic pregnancy in females who become pregnant or complain of lower abdominal pain while on SLYND.
5.7 Risk of Hyperglycemia in Patients with Diabetes
Some patients receiving progestins, including SLYND, may exhibit a decrease in insulin sensitivity. Therefore, patients with diabetes may be at greater risk of hyperglycemia and may require additional medication adjustments or monitoring.
5.8 Bleeding Irregularities and Amenorrhea
Females using SLYND may experience unscheduled (breakthrough or intracyclic) bleeding and spotting, especially during the first three months of use. Bleeding irregularities may resolve over time or by changing to a different contraceptive product. If bleeding persists or occurs after previously regular cycles, evaluate for causes such as pregnancy or malignancy.
Based on subject diaries from four clinical trials of SLYND, 64.4% of females experienced unscheduled bleeding at Cycle 1. This percentage decreased to 40.3% by cycle 13.
A total of 91 out of 2593 subjects (0.4%) discontinued SLYND due to menstrual bleeding disorders including metrorrhagia, menstrual irregular, vaginal hemorrhage, menorrhagia, uterine hemorrhage, and amenorrhea.
If scheduled bleeding does not occur, consider the possibility of pregnancy. If the patient has not adhered to the prescribed dosing schedule (missed one or two active tablets or started taking them on a day later than she should have, consider the possibility of pregnancy at the time of the first missed period and perform appropriate diagnostic measures. If the patient has adhered to the prescribed dosing schedule and misses two consecutive periods, rule out pregnancy.
6. Adverse Reactions/Side Effects
The following clinically significant adverse reactions are described elsewhere in other sections of the labeling:
- Hyperkalemia [see Warnings and Precautions (5.1)]
- Bleeding Irregularities and Amenorrhea [see Warnings and Precautions (5.8)]
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The data described below reflect the exposure of SLYND in females of reproductive potential desiring to prevent pregnancy based on four clinical studies including Study CF111/303 [ see Clinical Studies (14)] . The mean time of SLYND exposure ranged from 197 to 328 days. The demographic profile for the pooled study data was: mean age 28 years; mean BMI 25 kg/m 2; racial distribution was 83% White; 14% Black; 1% Asian and 2% Other.
| Adverse Reaction | Total
N = 2598 n (%) |
|---|---|
| Any adverse reaction | 627 (24.1) |
| Acne | 98 (3.8) |
| Metrorrhagia | 72 (2.8) |
| Headache | 71 (2.7) |
| Breast pain | 57 (2.2) |
| Weight increased | 50 (1.9) |
| Dysmenorrhea | 49 (1.9) |
| Nausea | 47 (1.8) |
| Vaginal hemorrhage | 45 (1.7) |
| Libido decreased | 33 (1.3) |
| Breast tenderness | 31 (1.2) |
| Menstruation irregular | 30 (1.2) |
7. Drug Interactions
Consult the labeling of all concurrently-used drugs to obtain further information about interactions with hormonal contraceptives or the potential for enzyme alterations.
8. Use In Specific Populations
8.2 Lactation
8.4 Pediatric Use
Safety and efficacy of SLYND have been established in females of reproductive age. Safety and efficacy are expected to be the same for postpubertal adolescents under the age of 16 and users 16 years and older.
Study CF111/304 evaluated the bleeding associated with SLYND in females ≥12 years of age. Bleeding data were generally consistent with those from Study CF111/303 in adult females [ see Clinical Studies (14)].
Use of this product before menarche is not indicated.
8.5 Geriatric Use
SLYND has not been studied in postmenopausal females and is not indicated in this population.
8.6 Hepatic Impairment
SLYND is contraindicated in females with hepatic impairment [ see Contraindications (4), Warnings and Precautions (5.5)] . The mean exposure to drospirenone (DRSP) in females with moderate liver impairment is approximately three times higher than the exposure in females with normal liver function. SLYND has not been studied in females with severe hepatic impairment [ see Clinical Pharmacology (12.3)].
8.7 Renal Impairment
SLYND is contraindicated in females with renal impairment [ see Contraindications (4), Warnings and Precautions (5.1)].
In subjects with creatinine clearance (CLcr) of 50–79 mL/min, serum DRSP levels were comparable to those in a control group with CLcr ≥ 80 mL/min. In subjects with CLcr of 30–49 mL/min, serum DRSP concentrations were on average 37% higher than those in the control group. In addition, there is a potential to develop hyperkalemia in subjects with renal impairment whose serum potassium is in the upper reference range, and who are concomitantly using potassium sparing drugs [see Drug Interactions (7.2) and Clinical Pharmacology (12.3)] .
10. Overdosage
There have been no reports of serious deleterious effects from overdosage of SLYND. Symptoms that may occur include are nausea, vomiting, and vaginal bleeding. There are no antidotes and treatment should be to provide symptomatic support.
Drospirenone is a spironolactone analogue which has antimineralocorticoid properties. Therefore, serum potassium and sodium, and evidence of metabolic acidosis, should be monitored in cases of overdose.
11. Slynd Description
SLYND (drospirenone) is for use as an oral contraceptive. It is supplied as clear to a slightly opaque PVC-PVDC/Aluminum blister cards, each holding of 24 white tablets each containing 4 mg of drospirenone, a synthetic progestational compound and 4 green inert tablets.
Drospirenone is chemically described as (6R,7R,8R,9S,10R,13S,14S,15S,16S,17S)-1,3',4',6,6a,7,8,9,10,11, 12,13,14,15,15a,16-hexadecahydro10,13-dimethylspiro-[17H-dicyclopropa- [6,7:15,16]cyclopenta[a]phenanthrene-17,2'(5H)-furan]-3,5'(2H)-dione). It has a molecular weight of 366.5, a molecular formula of C 24H 30O 3, and the structural formula below:
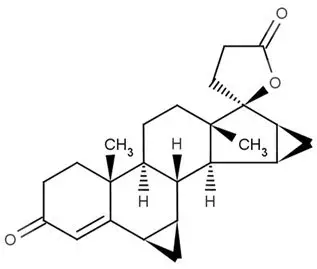
Drospirenone is a white to almost white or slightly yellow crystalline powder. It is a progestin and neutral molecule with slight solubility in water
The active tablet is a 5 mm, round, unscored, film-coated, white tablet that contains 4mg of drospirenone as the active ingredient, and microcrystalline cellulose NF, anhydrous lactose NF, colloidal silicon dioxide NF, magnesium stearate NF, polyvinyl alcohol partially hydrolyzed NF, talc NF, titanium dioxide NF, and polyethylene glycol NF as the inactive ingredients. Each tablet is debossed with the letter "E" on one side and the letter "D" on the other sides.
The inert tablet is a 5 mm, round, unscored, film-coated, green tablet that does not contain drospirenone. Each inert green tablet contains the following inactive ingredients: Lactose monohydrate NF, corn starch NF, povidone 30000 NF, colloidal silicon dioxide NF, magnesium stearate NF, hypromellose NF, talc NF, titanium dioxide USP, polysorbate 2910 NF, triacetin NF, FD&C blue 2 aluminum lake and yellow ferric oxide.
12. Slynd - Clinical Pharmacology
12.1 Mechanism of Action
SLYND progestin-only oral contraceptive lowers the risk of becoming pregnant primarily by suppressing ovulation.
12.2 Pharmacodynamics
Drospirenone is a spironolactone analogue with anti-mineralocorticoid activity.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
In a 24-month oral carcinogenicity study in mice with doses up to 10 mg/kg/day DRSP, equating to 2 times the maximum clinical exposure (based on AUC), there was an increase in carcinomas of the harderian gland in the high dose DRSP group. In a similar study in rats given doses up to 10 mg/kg/day DRSP, 10 times the maximum clinical exposure (based on AUC), there was an increased incidence of benign and total (benign and malignant) adrenal gland pheochromocytomas in the high dose DRSP group. Mutagenesis studies for DRSP were conducted in vivo and in vitro and no evidence of mutagenic activity was observed.
17. Patient Counseling Information
Advise the patient to read the FDA-approved Patient Labeling (Patient Information and Instructions for Use).
16. How is Slynd supplied
16.1 How Supplied
SLYND (drospirenone) tablets is packaged in clear to a slightly opaque PVC-PVDC/Aluminum blister cards. Each blister card holds 24 white round active film-coated tablets, each containing 4mg of drospirenone and 4 green round inert film-coated tablets that do not contain drospirenone. SLYND is supplied in cardboard cartons containing a blister card of 28 tablets: NDC 0642-7470-01
PATIENT INFORMATION SLYND (slind) (drospirenone) tablets, for oral use
Progestin pills help to lower the chance of becoming pregnant when taken as directed. They do not protect against HIV infection (AIDS) and other sexually transmitted diseases (STDs).
What is SLYND?
SLYND is a birth control pill (oral contraceptive) also called a POP (progestin only pill) that is used by females who can become pregnant to prevent pregnancy.
The progestin drospirenone may increase potassium levels in your blood. You should not take SLYND if you have kidney, liver or adrenal disease because this could cause serious heart problems as well as other health problems. Other medicines may also increase potassium levels in your blood. If you are currently on daily, long-term treatment for a chronic health condition with any of the medicines listed below, talk to your healthcare provider about whether SLYND is right for you. If you take any of the medicines listed below for a chronic health condition you should have a blood test to check the potassium level in your blood before you start taking SLYND and during the first month that you take SLYND.
- medicines to treat fungal infections, such as ketoconazole, itraconazole, or voriconazole
- medicines to treat Human Immunodeficiency Virus (HIV) infection or Hepatitis C infection, such as indinavir or boceprevir
- clarithromycin
How does SLYND work for contraception?
Your chance of getting pregnant depends on how well you follow the directions for taking your birth control pills. The better you follow the directions, the less chance you have of getting pregnant.
Based on the results of one clinical study of a 28-day regimen of SLYND about 4 out of 100 females may get pregnant within the first year they use SLYND.
The following chart shows the chance of getting pregnant for females who use different methods of birth control. Each box on the chart contains a list of birth control methods that are similar in effectiveness. The most effective methods are at the top of the chart. The box on the bottom of the chart shows the chance of getting pregnant for females who do not use birth control and are trying to get pregnant.
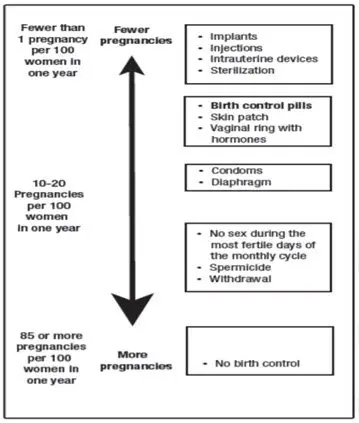
Do not take SLYND if you:
- have kidney disease or kidney failure.
- have reduced adrenal gland function (adrenal insufficiency).
- have or have had cervical cancer or any cancer that is sensitive to female hormones.
- have liver disease, including liver tumors.
- have unexplained vaginal bleeding.
Tell your healthcare provider if you have or have had any of these conditions. Your healthcare provider can suggest a different method of birth control.
If any of these conditions happen while you are taking SLYND, stop taking SLYND right away and talk to your healthcare provider. Use non-hormonal contraception when you stop taking SLYND.
Before you take SLYND, tell your healthcare provider about all of your medical conditions, including if you:
- are pregnant or think you may be pregnant.
- have ever had blood clots in your legs (deep vein thrombosis), lungs (pulmonary embolism) or a stroke or heart attack (myocardial infarction).
- have or have had depression
Tell your healthcare provider about all the medicine you take including prescription and over-the-counter medicines, vitamins and herbal supplements, such as St. John's Wort.
SLYND may affect the way other medicines work, and other medicines may affect how well SLYND works.
Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine.
How should I take SLYND?
Read the detailed Instructions for Use at the end of this Patient Information leaflet about the right way to take SLYND.
What are the possible serious side effects of SLYND?
SLYND may cause serious side effects, including:
-
High potassium levels in your blood (hyperkalemia). Certain medicines and conditions can also increase the potassium levels in your blood. Your healthcare provider may check the potassium levels in your blood before and during treatment with SLYND.
Call you healthcare provider or go to a hospital emergency room right away if you have signs or symptoms of high potassium levels in your blood including:
- weakness or numbness in an arm or leg
- palpitations (feel like your heart is racing or fluttering) or irregular heartbeat
- nausea
- vomiting
- severe pain in your chest
- shortness of breath.
-
Blood clot forming in blood vessels (thromboembolism problems). Tell your healthcare provider if you have had a blood clot. Tell your healthcare provider if you plan to have surgery or are not able to be active due to illness or injury.
Call your healthcare provider or go to a hospital emergency room right away if you have:
- leg pain that will not go away
- a sudden, severe headache unlike your usual headaches
- sudden, severe shortness of breath
- sudden change in vision or blindness
- chest pain
- weakness or numbness in your arm or leg
- trouble speaking
- Bone loss. It is not known if the decrease in a sex hormone that happens with SLYND can result in decreased bone density (bone loss).
- Cervical cancer. See " Do birth control pills cause cancer?"
- Liver problems, including rare liver tumors. Call your healthcare provider right away if you have yellowing of your skin or eyes.
- Ectopic pregnancy (pregnancy in your tubes). If you get pregnant while using SLYND, you might have an ectopic pregnancy. That means that the pregnancy is not in the uterus. Ectopic pregnancy is a medical emergency that often requires surgery. If you have severe abdominal (belly) pain, call your healthcare provider or go to a hospital emergency room right away.
- Risk of high blood sugar levels in people with diabetes. If you have diabetes, you may need to monitor your blood sugar level more often or adjust your diabetes medicine.
- Changes in menstrual bleeding. Irregular vaginal bleeding, especially between menstrual periods, and irregular periods or the absence of menstrual periods are common side effects of SLYND, but can sometimes be serious. Tell your healthcare provider if you have any of these changes in menstrual bleeding.
- Depression, especially if you have had depression in the past. Call your healthcare provider immediately if you have any thoughts of harming yourself.
What are the most common side effects of SLYND?
The most common side effects of SLYND include:
|
|
These are not all the possible side effects of SLYND.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
What else should I know about taking SLYND?
- If you are scheduled for any lab tests, tell your healthcare provider you are taking SLYND. Certain blood tests may be affected by SLYND.
How should I store SLYND?
- Store SLYND at room temperature between 68°F to 77°F (20°C to 25°C).
- Keep SLYND and all medicines out of the reach of children.
General information about the safe and effective use of SLYND.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use SLYND for a condition for which it was not prescribed. Do not give SLYND to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about SLYND that is written for health professionals.
Do birth control pills cause cancer?
Hormonal contraceptives do not appear to cause breast cancer. However if you have breast cancer now, have had it in the past, or you have (or have had) another cancer that may be sensitive to hormones, do not use hormonal contraceptives.
Women who use hormonal contraceptives may have a higher chance of getting cervical cancer. However, this may be due to other reasons such as having more sexual partners and exposure to the human papilloma virus (HPV).
What if I want to become pregnant?
You may stop taking SLYND whenever you wish. Consider a visit with your healthcare provider for a pre-pregnancy checkup before you stop taking SLYND.
What should I know about my period when taking SLYND?
Some females may miss a period. Irregular vaginal bleeding or spotting may happen while you are taking SLYND, especially during the first few months of use. If the irregular vaginal bleeding or spotting continues or happens again after you have had regular menstrual cycles call your healthcare provider. It is important to continue taking your pills on a regular schedule to prevent a pregnancy.
What if I miss my scheduled period when using SLYND?
Some females miss periods on hormonal birth control, even when they are not pregnant. However, if you go 2 or more months in a row without a period, or you miss your period after a month where you did not use all of your SLYND correctly, call you healthcare provider because you may be pregnant. Also call your healthcare provider if you have symptoms of pregnancy such as morning sickness or unusual breast tenderness. Stop taking SLYND if you are pregnant.
What are the ingredients in SLYND?
White tablets
Active ingredient: drospirenone
Inactive ingredients: microcrystalline cellulose, anhydrous lactose, colloidal silicon dioxide, magnesium stearate, polyvinyl alcohol partially hydrolyzed, talc, titanium dioxide, and polyethylene glycol.
Green tablets
Inactive ingredients: lactose monohydrate, corn starch, povidone 30000, colloidal silicon dioxide, magnesium stearate, hypromellose, talc, titanium dioxide, polysorbate 2910, triacetin, FD&C blue 2 aluminum lake and yellow ferric oxide.
PRINCIPAL DISPLAY PANEL - 4 mg Tablet Blister Card Carton
NDC 0642-7470-01
Rx only
Slynd
®
(drospirenone) tablets, 4 mg
1 blister card with 28 tablets / Oral Use Exeltis
THIS PRODUCT (LIKE ALL ORAL CONTRACEPTIVES) IS INTENDED TO PREVENT PREGNANCY. IT DOES
NOT PROTECT AGAINST HIV INFECTION (AIDS) AND OTHER SEXUALLY TRANSMITTED DISEASES.
| SLYND
drospirenone kit |
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| Labeler - Exeltis USA, Inc. (071170534) |
| Registrant - Exeltis USA, Inc. (071170534) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Laboratorios Leon Farma, S.A. | 467782459 | manufacture(0642-7470) , analysis(0642-7470) | |