Drug Detail:Steglatro (Ertugliflozin [ er-too-gli-floe-zin ])
Drug Class: SGLT-2 inhibitors
Highlights of Prescribing Information
STEGLATRO® (ertugliflozin) tablets, for oral use
Initial U.S. Approval: 2017
Indications and Usage for Steglatro
STEGLATRO is a sodium glucose co-transporter 2 (SGLT2) inhibitor indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus. (1)
Limitations of Use:
Not recommended in patients with type 1 diabetes mellitus. It may increase the risk of diabetic ketoacidosis in these patients. (1)
Steglatro Dosage and Administration
- Assess renal function before initiating and as clinically indicated. (2.1)
- Correct volume depletion before initiating STEGLATRO (2.1)
- Recommended starting dose is 5 mg once daily, taken in the morning, with or without food. (2.2)
- Increase dose to 15 mg once daily in those tolerating STEGLATRO and needing additional glycemic control. (2.2)
- Use is not recommended in patients with an eGFR less than 45 mL/min/1.73 m2. (2.2)
Dosage Forms and Strengths
Tablets: 5 mg and 15 mg (3)
Contraindications
- Hypersensitivity to ertugliflozin or any of the excipients in STEGLATRO. (4)
- Patients on dialysis. (4, 5.3)
Warnings and Precautions
- Ketoacidosis: Assess patients who present with signs and symptoms of metabolic acidosis for ketoacidosis, regardless of blood glucose level. If suspected, discontinue, evaluate, and treat promptly. Before initiating, consider risk factors for ketoacidosis. Patients may require monitoring and temporary discontinuation of therapy in clinical situations known to predispose to ketoacidosis. (5.1)
- Lower Limb Amputation: Consider factors that may increase the risk of amputation before initiating STEGLATRO. Monitor patients for infections or ulcers of lower limbs, and discontinue if these occur. (5.2)
- Volume Depletion: May result in acute kidney injury. Before initiating, assess and correct volume status in patients with renal impairment or low systolic blood pressure, elderly patients, or patients on diuretics. Monitor for signs and symptoms during therapy. (5.3)
- Urosepsis and Pyelonephritis: Evaluate patients for signs and symptoms of urinary tract infections and treat promptly, if indicated. (5.4)
- Hypoglycemia: Consider a lower dose of insulin or insulin secretagogue to reduce risk of hypoglycemia when used in combination. (5.5)
- Necrotizing Fasciitis of the Perineum (Fournier's Gangrene): Serious, life-threatening cases have occurred in both females and males. Assess patients presenting with pain or tenderness, erythema, or swelling in the genital or perineal area, along with fever or malaise. If suspected, institute prompt treatment. (5.6)
- Genital Mycotic Infections: Monitor and treat if indicated. (5.7)
Adverse Reactions/Side Effects
Most common adverse reactions (incidence ≥ 5%) were female genital mycotic infections. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Merck Sharp & Dohme LLC at 1-877-888-4231 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch .
Drug Interactions
See full prescribing information for information on drug interactions and interference of STEGLATRO with laboratory tests. (7)
Use In Specific Populations
- Pregnancy: Advise females of the potential risk to a fetus especially during the second and third trimesters. (8.1)
- Lactation: Breastfeeding not recommended. (8.2)
- Geriatrics: Higher incidence of adverse reactions related to reduced intravascular volume. (5.3, 8.5)
- Renal Impairment: Higher incidence of adverse reactions related to reduced intravascular volume and renal function. (5.3, 8.6)
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 10/2022
Full Prescribing Information
1. Indications and Usage for Steglatro
STEGLATRO® is indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus.
2. Steglatro Dosage and Administration
2.1 Prior to Initiation of STEGLATRO
- Assess renal function prior to initiation of STEGLATRO and as clinically indicated [see Warnings and Precautions (5.3)].
- In patients with volume depletion, correct this condition before initiating STEGLATRO [see Warnings and Precautions (5.3), Use in Specific Populations (8.5, 8.6)].
2.2 Recommended Dosage
- The recommended starting dose of STEGLATRO is 5 mg once daily, taken in the morning, with or without food.
- For additional glycemic control, the dose may be increased to 15 mg once daily in patients tolerating STEGLATRO.
- Use of STEGLATRO is not recommended in patients with an eGFR less than 45 mL/min/1.73 m2.
- STEGLATRO is contraindicated in patients on dialysis.
3. Dosage Forms and Strengths
- Tablets: 5 mg, pink, triangular-shaped debossed with "701" on one side and plain on the other side.
- Tablets: 15 mg, red, triangular-shaped debossed with "702" on one side and plain on the other side.
4. Contraindications
- Hypersensitivity to ertugliflozin or any excipient in STEGLATRO, reactions such as angioedema have occurred [see Adverse Reactions (6.2)].
- Patients on dialysis [see Use in Specific Populations (8.6)].
5. Warnings and Precautions
5.1 Ketoacidosis
Reports of ketoacidosis, a serious life-threatening condition requiring urgent hospitalization, have been identified in clinical trials and postmarketing surveillance in patients with type 1 and type 2 diabetes mellitus receiving sodium glucose co-transporter-2 (SGLT2) inhibitors including STEGLATRO [see Adverse Reactions (6.1)]. Fatal cases of ketoacidosis have been reported in patients taking SGLT2 inhibitors. In placebo-controlled trials of patients with type 1 diabetes, the risk of ketoacidosis was increased in patients who received SGLT2 inhibitors compared to patients who received placebo. The risk of ketoacidosis may be greater with higher doses. STEGLATRO is not indicated for the treatment of patients with type 1 diabetes mellitus [see Indications and Usage (1)].
Patients treated with STEGLATRO who present with signs and symptoms consistent with severe metabolic acidosis should be assessed for ketoacidosis regardless of presenting blood glucose levels, as ketoacidosis associated with STEGLATRO may be present even if blood glucose levels are less than 250 mg/dL. If ketoacidosis is suspected, STEGLATRO should be discontinued, patient should be evaluated, and prompt treatment should be instituted. Treatment of ketoacidosis may require insulin, fluid and carbohydrate replacement.
In many of the reported cases, and particularly in patients with type 1 diabetes, the presence of ketoacidosis was not immediately recognized and institution of treatment was delayed because presenting blood glucose levels were below those typically expected for diabetic ketoacidosis (often less than 250 mg/dL). Signs and symptoms at presentation were consistent with dehydration and severe metabolic acidosis and included nausea, vomiting, abdominal pain, generalized malaise, and shortness of breath. In some but not all cases, factors predisposing to ketoacidosis such as insulin dose reduction, acute febrile illness, reduced caloric intake, surgery, pancreatic disorders suggesting insulin deficiency (e.g., type 1 diabetes, history of pancreatitis or pancreatic surgery), and alcohol abuse were identified.
Before initiating STEGLATRO, consider factors in the patient history that may predispose to ketoacidosis, including pancreatic insulin deficiency from any cause, caloric restriction, and alcohol abuse.
For patients who undergo scheduled surgery, consider temporarily discontinuing STEGLATRO for at least 4 days prior to surgery [see Clinical Pharmacology (12.2, 12.3)].
Consider monitoring for ketoacidosis and temporarily discontinuing STEGLATRO in other clinical situations known to predispose to ketoacidosis (e.g., prolonged fasting due to acute illness or post-surgery). Ensure risk factors for ketoacidosis are resolved prior to restarting STEGLATRO.
Educate patients on the signs and symptoms of ketoacidosis and instruct patients to discontinue STEGLATRO and seek medical attention immediately if signs and symptoms occur.
5.2 Lower Limb Amputation
In a long-term cardiovascular outcomes study [see Clinical Studies 14.2], in patients with type 2 diabetes and established cardiovascular disease, the occurrence of non-traumatic lower limb amputations was reported with event rates of 4.7, 5.7, and 6.0 events per 1000 patient-years in the placebo, ertugliflozin 5 mg, and ertugliflozin 15 mg treatment arms, respectively.
Amputation of the toe and foot were most frequent (81 out of 109 patients with lower limb amputations). Some patients had multiple amputations, some involving both lower limbs.
Lower limb infections, gangrene, and diabetic foot ulcers were the most common precipitating medical events leading to the need for an amputation. Patients with amputations were more likely to be male, have higher A1C (%) at baseline, have a history of peripheral arterial disease, amputation or peripheral revascularization procedure, diabetic foot, and to have been taking diuretics or insulin.
Across seven STEGLATRO clinical trials, non-traumatic lower limb amputations were reported in 1 (0.1%) patient in the comparator group, 3 (0.2%) patients in the STEGLATRO 5 mg group, and 8 (0.5%) patients in the STEGLATRO 15 mg group.
Before initiating STEGLATRO, consider factors in the patient history that may predispose them to the need for amputations, such as a history of prior amputation, peripheral vascular disease, neuropathy and diabetic foot ulcers. Counsel patients about the importance of routine preventative foot care. Monitor patients receiving STEGLATRO for signs and symptoms of infection (including osteomyelitis), new pain or tenderness, sores or ulcers involving the lower limbs, and discontinue STEGLATRO if these complications occur.
5.3 Volume Depletion
STEGLATRO can cause intravascular volume contraction which may sometimes manifest as symptomatic hypotension or acute transient changes in creatinine [see Adverse Reactions (6.1)]. There have been postmarketing reports of acute kidney injury, some requiring hospitalization and dialysis, in patients with type 2 diabetes mellitus receiving SGLT2 inhibitors, including STEGLATRO. Patients with impaired renal function (eGFR less than 60 mL/min/1.73 m2) [see Use in Specific Populations (8.6)], elderly patients, patients with low systolic blood pressure, or patients on loop diuretics may be at increased risk for volume depletion or hypotension. Before initiating STEGLATRO in patients with one or more of these characteristics, assess volume status and renal function. In patients with volume depletion, correct this condition before initiating STEGLATRO. Monitor for signs and symptoms of volume depletion, and renal function after initiating therapy.
5.4 Urosepsis and Pyelonephritis
There have been postmarketing reports of serious urinary tract infections, including urosepsis and pyelonephritis, requiring hospitalization in patients receiving SGLT2 inhibitors. Treatment with SGLT2 inhibitors increases the risk for urinary tract infections. Evaluate patients for signs and symptoms of urinary tract infections and treat promptly, if indicated [see Adverse Reactions (6.1)].
5.5 Hypoglycemia with Concomitant Use with Insulin and Insulin Secretagogues
Insulin and insulin secretagogues (e.g., sulfonylurea) are known to cause hypoglycemia. STEGLATRO may increase the risk of hypoglycemia when used in combination with insulin and/or an insulin secretagogue [see Adverse Reactions (6.1)]. Therefore, a lower dose of insulin or insulin secretagogue may be required to minimize the risk of hypoglycemia when used in combination with STEGLATRO.
5.6 Necrotizing Fasciitis of the Perineum (Fournier's Gangrene)
Reports of necrotizing fasciitis of the perineum (Fournier's Gangrene), a rare but serious and life-threatening necrotizing infection requiring urgent surgical intervention, have been identified in postmarketing surveillance in patients with diabetes mellitus receiving SGLT2 inhibitors, including STEGLATRO. Cases have been reported in females and males. Serious outcomes have included hospitalization, multiple surgeries, and death.
Patients treated with STEGLATRO presenting with pain or tenderness, erythema, or swelling in the genital or perineal area, along with fever or malaise, should be assessed for necrotizing fasciitis. If suspected, start treatment immediately with broad-spectrum antibiotics and, if necessary, surgical debridement. Discontinue STEGLATRO, closely monitor blood glucose levels, and provide appropriate alternative therapy for glycemic control.
6. Adverse Reactions/Side Effects
The following important adverse reactions are described elsewhere in the labeling:
- Ketoacidosis [see Warnings and Precautions (5.1)]
- Lower Limb Amputation [see Warnings and Precautions (5.2)]
- Volume Depletion [see Warnings and Precautions (5.3)]
- Urosepsis and Pyelonephritis [see Warnings and Precautions (5.4)]
- Hypoglycemia with Concomitant Use with Insulin and Insulin Secretagogues [see Warnings and Precautions (5.5)]
- Necrotizing Fasciitis of the Perineum (Fournier's Gangrene) [see Warnings and Precautions (5.6)]
- Genital Mycotic Infections [see Warnings and Precautions (5.7)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Pool of Placebo-Controlled Trials Evaluating STEGLATRO 5 and 15 mg
The data in Table 1 are derived from a pool of three 26-week, placebo-controlled trials. STEGLATRO was used as monotherapy in one trial and as add-on therapy in two trials [see Clinical Studies (14)]. These data reflect exposure of 1,029 patients to STEGLATRO with a mean exposure duration of approximately 25 weeks. Patients received STEGLATRO 5 mg (N=519), STEGLATRO 15 mg (N=510), or placebo (N=515) once daily. The mean age of the population was 57 years and 2% were older than 75 years of age. Fifty-three percent (53%) of the population was male and 73% were Caucasian, 15% were Asian, and 7% were Black or African American. At baseline the population had diabetes for an average of 7.5 years, had a mean HbA1c of 8.1%, and 19.4% had established microvascular complications of diabetes. Baseline renal function (mean eGFR 88.9 mL/min/1.73 m2) was normal or mildly impaired in 97% of patients and moderately impaired in 3% of patients.
Table 1 shows common adverse reactions associated with the use of STEGLATRO. These adverse reactions were not present at baseline, occurred more commonly on STEGLATRO than on placebo, and occurred in at least 2% of patients treated with either STEGLATRO 5 mg or STEGLATRO 15 mg.
| Number (%) of Patients | |||
|---|---|---|---|
| Placebo N = 515 | STEGLATRO 5 mg N = 519 | STEGLATRO 15 mg N = 510 |
|
|
|||
| Female genital mycotic infections† | 3.0% | 9.1% | 12.2% |
| Male genital mycotic infections‡ | 0.4% | 3.7% | 4.2% |
| Urinary tract infections§ | 3.9% | 4.0% | 4.1% |
| Headache | 2.3% | 3.5% | 2.9% |
| Vaginal pruritus¶ | 0.4% | 2.8% | 2.4% |
| Increased urination# | 1.0% | 2.7% | 2.4% |
| Nasopharyngitis | 2.3% | 2.5% | 2.0% |
| Back pain | 2.3% | 1.7% | 2.5% |
| Weight decreased | 1.0% | 1.2% | 2.4% |
| ThirstÞ | 0.6% | 2.7% | 1.4% |
Hypoglycemia
The incidence of hypoglycemia by study is shown in Table 2.
|
|||
| Monotherapy (26 weeks) | Placebo (N = 153) | STEGLATRO 5 mg (N =156) | STEGLATRO 15 mg (N = 152) |
| Overall [N (%)] | 1 (0.7) | 4 (2.6) | 4 (2.6) |
| Severe [N (%)] | 0 (0.0) | 0 (0.0) | 2 (1.3) |
| Add-on Combination Therapy with Metformin (26 weeks) | Placebo (N = 209) | STEGLATRO 5 mg (N = 207) | STEGLATRO 15 mg (N = 205) |
| Overall [N (%)] | 9 (4.3) | 15 (7.2) | 16 (7.8) |
| Severe [N (%)] | 1 (0.5) | 1 (0.5) | 0 (0.0) |
| Add-on Combination Therapy with Metformin and Sitagliptin (26 weeks) | Placebo (N = 153) | STEGLATRO 5 mg (N = 156) | STEGLATRO 15 mg (N = 153) |
| Overall [N (%)] | 5 (3.3) | 7 (4.5) | 3 (2.0) |
| Severe [N (%)] | 1 (0.7) | 1 (0.6) | 0 (0.0) |
| In Combination with Insulin and/or an Insulin Secretagogue in Patients with Moderate Renal Impairment (26 weeks) | Placebo (N = 133) | STEGLATRO 5 mg (N = 148) | STEGLATRO 15 mg (N = 143) |
| Overall [N (%)] | 48 (36.1) | 53 (35.8) | 39 (27.3) |
| Severe [N (%)] | 3 (2.3) | 5 (3.4) | 3 (2.1) |
| Add-on Combination with Insulin with or without Metformin (18 weeks) | Placebo (N = 347) | STEGLATRO 5 mg (N = 348) | STEGLATRO 15 mg (N = 370) |
| Overall [N (%)] | 130 (37.5) | 137 (39.4) | 144 (38.9) |
| Severe [N (%)] | 12 (3.5) | 13 (3.7) | 19 (5.1) |
| Add-on Combination with a Sulfonylurea (18 weeks) | Placebo (N =48) | STEGLATRO 5 mg (N =55) | STEGLATRO 15 mg (N =54) |
| Overall [N (%)] | 2 (4.2) | 4 (7.3) | 5 (9.3) |
| Severe [N (%)] | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Add-on Combination with Metformin and a Sulfonylurea (18 weeks) | Placebo (N = 117) | STEGLATRO 5 mg (N = 100) | STEGLATRO 15 mg (N = 113) |
| Overall [N (%)] | 17 (14.5) | 20 (20.0) | 30 (26.5) |
| Severe [N (%)] | 1 (0.9) | 2 (2.0) | 2 (1.8) |
6.2 Postmarketing Experience
Additional adverse reactions have been identified during postapproval use. Because these reactions are reported voluntarily from a population of uncertain size, it is generally not possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Necrotizing fasciitis of the perineum (Fournier's Gangrene)
- Angioedema
7. Drug Interactions
| Insulin and Insulin Secretagogues | |
|---|---|
| Clinical Impact: | The risk of hypoglycemia when STEGLATRO is used in combination with insulin and/or an insulin secretagogue. |
| Intervention: | A lower dose of insulin or insulin secretagogue may be required to minimize the risk of hypoglycemia when used in combination with STEGLATRO. |
| Lithium | |
| Clinical Impact: | Concomitant use of an SGLT2 inhibitor with lithium may decrease serum lithium concentrations. |
| Intervention: | Monitor serum lithium concentration more frequently during STEGLATRO initiation and dosage changes. |
| Positive Urine Glucose Test | |
| Clinical Impact: | SGLT2 inhibitors increase urinary glucose excretion and will lead to positive urine glucose tests. |
| Intervention: | Monitoring glycemic control with urine glucose tests is not recommended in patients taking SGLT2 inhibitors. Use alternative methods to monitor glycemic control. |
| Interference with 1,5-anhydroglucitol (1,5-AG) Assay | |
| Clinical Impact: | Measurements of 1,5-AG are unreliable in assessing glycemic control in patients taking SGLT2 inhibitors. |
| Intervention: | Monitoring glycemic control with 1,5-AG assay is not recommended. Use alternative methods to monitor glycemic control. |
8. Use In Specific Populations
8.4 Pediatric Use
Safety and effectiveness of STEGLATRO in pediatric patients under 18 years of age have not been established.
8.5 Geriatric Use
No dosage adjustment of STEGLATRO is recommended based on age. In STEGLATRO clinical trials, a total of 876 (25.7%) patients treated with STEGLATRO were 65 years and older, and 152 (4.5%) patients treated with STEGLATRO were 75 years and older. Patients 65 years and older had a higher incidence of adverse reactions related to volume depletion compared to younger patients; events were reported in 1.1%, 2.2%, and 2.6% of patients treated with comparator, STEGLATRO 5 mg, and STEGLATRO 15 mg, respectively [see Warnings and Precautions (5.3) and Adverse Reactions (6.1)].
In VERTIS CV, a total of 2780 (50.5%) patients treated with STEGLATRO were 65 years and older, and 595 (10.8%) patients treated with STEGLATRO were 75 years and older. Safety and efficacy were generally similar for patients age 65 years and older compared to patients younger than 65.
8.6 Renal Impairment
A 26-week placebo-controlled study of 313 patients with Stage 3 Chronic Kidney Disease (eGFR ≥30 to less than 60 mL/min/1.73 m2) treated with STEGLATRO did not demonstrate improvement in glycemic control.
In the VERTIS CV study, there were 1370 patients (25%) with an eGFR ≥90 mL/min/1.73 m2, 2929 patients (53%) with an eGFR of ≥60 to less than 90 mL/min/1.73 m2, 879 patients (16%) with an eGFR of ≥45 to less than 60 mL/min/1.73 m2, and 299 patients (5%) with eGFR of 30 to <45 mL/min/1.73 m2 treated with STEGLATRO. Similar effects on glycemic control at Week 18 were observed in patients treated with STEGLATRO in each eGFR subgroup and also in the overall patient population.
STEGLATRO is contraindicated in patients on dialysis [see Contraindications (4)].
No dosage adjustment is needed in patients with eGFR ≥45 mL/min/1.73 m2.
10. Overdosage
In the event of an overdose with STEGLATRO, contact the Poison Control Center. Employ the usual supportive measures as dictated by the patient's clinical status. Removal of ertugliflozin by hemodialysis has not been studied.
11. Steglatro Description
STEGLATRO (ertugliflozin) tablets for oral use contain ertugliflozin L-pyroglutamic acid, a SGLT2 inhibitor.
The chemical name of ertugliflozin L-pyroglutamic acid is (1S,2S,3S,4R,5S)-5-(4-chloro-3-(4-ethoxybenzyl)phenyl)-1-(hydroxymethyl)-6,8-dioxabicyclo[3.2.1]octane-2,3,4-triol, compound with (2S)-5-oxopyrrolidine-2-carboxylic acid. The molecular formula is C27H32ClNO10 and the molecular weight is 566.00.
The chemical structure is:
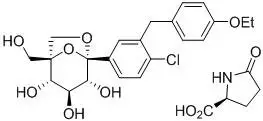
Ertugliflozin L-pyroglutamic acid is a white to off-white powder that is soluble in ethyl alcohol and acetone, slightly soluble in ethyl acetate and acetonitrile and very slightly soluble in water.
STEGLATRO is supplied as film-coated tablets, containing 6.48 or 19.43 mg of ertugliflozin L-pyroglutamic acid, which is equivalent to 5 and 15 mg of ertugliflozin.
Inactive ingredients are microcrystalline cellulose, lactose monohydrate, sodium starch glycolate, and magnesium stearate.
The film coating contains: hypromellose, lactose monohydrate, macrogol, triacetin, titanium dioxide and iron oxide red.
12. Steglatro - Clinical Pharmacology
12.1 Mechanism of Action
SGLT2 is the predominant transporter responsible for reabsorption of glucose from the glomerular filtrate back into the circulation. Ertugliflozin is an inhibitor of SGLT2. By inhibiting SGLT2, ertugliflozin reduces renal reabsorption of filtered glucose and lowers the renal threshold for glucose, and thereby increases urinary glucose excretion.
12.3 Pharmacokinetics
The pharmacokinetics of ertugliflozin are similar in healthy subjects and patients with type 2 diabetes mellitus. The steady state mean plasma AUC and Cmax were 398 ng∙hr/mL and 81.3 ng/mL, respectively, with 5 mg ertugliflozin once-daily treatment, and 1,193 ng∙hr/mL and 268 ng/mL, respectively, with 15 mg ertugliflozin once-daily treatment. Steady-state is reached after 4 to 6 days of once-daily dosing with ertugliflozin. Ertugliflozin does not exhibit time-dependent pharmacokinetics and accumulates in plasma up to 10-40% following multiple dosing.
Drug Interaction Studies
In Vivo Assessment of Drug Interactions
No dose adjustment of STEGLATRO is recommended when coadministered with commonly prescribed medicinal products. Ertugliflozin pharmacokinetics were similar with and without coadministration of metformin, glimepiride, sitagliptin, and simvastatin in healthy subjects (see Figure 1). Coadministration of ertugliflozin with multiple doses of 600 mg once-daily rifampin (an inducer of UGT and CYP enzymes) resulted in approximately 39% and 15% mean reductions in ertugliflozin AUC and Cmax, respectively, relative to ertugliflozin administered alone. These changes in exposure are not considered clinically relevant. Ertugliflozin had no clinically relevant effect on the pharmacokinetics of metformin, glimepiride, sitagliptin, and simvastatin when coadministered in healthy subjects (see Figure 2). Physiologically-based PK (PBPK) modeling suggests that coadministration of mefenamic acid (UGT inhibitor) may increase the AUC and Cmax of ertugliflozin by 1.51- and 1.19-fold, respectively. These predicted changes in exposure are not considered clinically relevant.
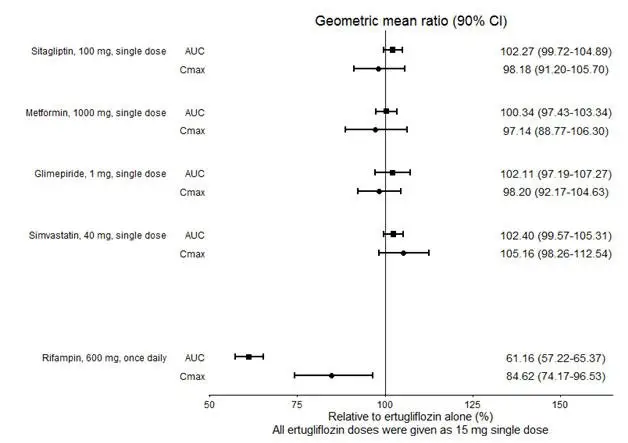 |
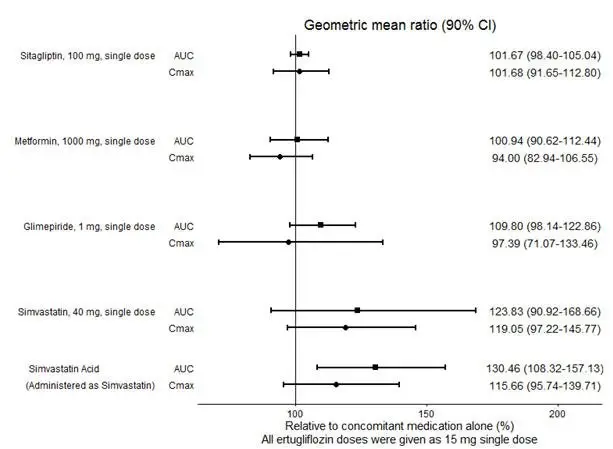 |
14. Clinical Studies
14.1 Glycemic Control Trials in Patients with Type 2 Diabetes Mellitus
STEGLATRO has been studied as monotherapy and in combination with metformin, sitagliptin, a sulfonylurea, insulin (with or without metformin), metformin plus sitagliptin, metformin plus a sulfonylurea and compared to a sulfonylurea (glimepiride). STEGLATRO has also been studied in patients with type 2 diabetes mellitus and moderate renal impairment.
In patients with type 2 diabetes mellitus treatment with STEGLATRO reduced hemoglobin A1c (HbA1c) compared to placebo. Reduction in HbA1c was generally similar across subgroups defined by age, sex, race, geographic region, baseline body mass index (BMI), and duration of type 2 diabetes mellitus.
Monotherapy
A total of 461 patients with type 2 diabetes mellitus inadequately controlled (HbA1c between 7% and 10.5%) on diet and exercise participated in a randomized, double-blind, multi-center, 26-week, placebo-controlled study (NCT01958671) to evaluate the efficacy and safety of STEGLATRO monotherapy. These patients, who were either treatment naïve or not receiving any background antihyperglycemic treatment ≥8 weeks, entered a 2-week, single-blind, placebo run-in period and were randomized to placebo, STEGLATRO 5 mg, or STEGLATRO 15 mg, administered once daily.
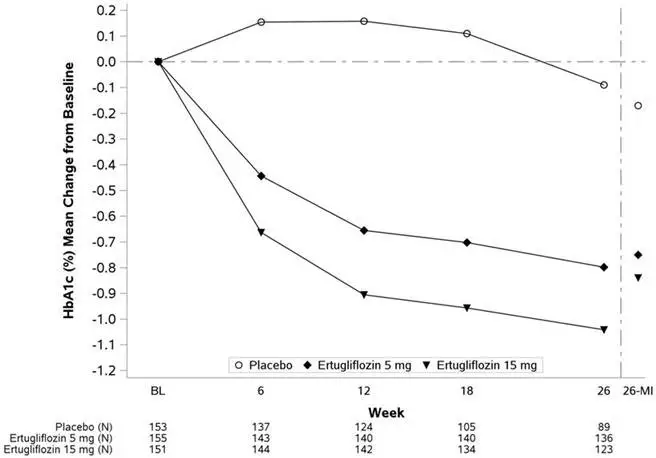
At Week 26, treatment with STEGLATRO at 5 mg or 15 mg daily provided statistically significant reductions in HbA1c compared to placebo. STEGLATRO also resulted in a greater proportion of patients achieving an HbA1c <7% compared with placebo (see Table 4 and Figure 3).
| Placebo | STEGLATRO 5 mg | STEGLATRO 15 mg | |
|---|---|---|---|
|
|||
| HbA1c (%) | N = 153 | N = 155 | N = 151 |
| Baseline (mean) | 8.1 | 8.2 | 8.4 |
| Change from baseline (LS mean†) | -0.2 | -0.7 | -0.8 |
| Difference from placebo (LS mean†, 95% CI) | -0.6‡ (-0.8, -0.4) | -0.7‡ (-0.9, -0.4) | |
| Patients [N (%)] with HbA1c <7% | 26 (16.9) | 47 (30.1) | 59 (38.8) |
| FPG (mg/dL) | N = 150 | N = 151 | N = 149 |
| Baseline (mean) | 180.2 | 180.9 | 179.1 |
| Change from baseline (LS mean†) | -11.6 | -31.0 | -36.4 |
| Difference from placebo (LS mean†, 95% CI) | -19.4‡ (-27.6, -11.2) | -24.8‡ (-33.2, -16.4) | |
The mean baseline body weight was 94.2 kg, 94.0 kg, and 90.6 kg in the placebo, STEGLATRO 5 mg, and STEGLATRO 15 mg groups, respectively. The mean changes from baseline to Week 26 were -1.0 kg, -3.0 kg, and -3.1 kg in the placebo, STEGLATRO 5 mg, and STEGLATRO 15 mg groups, respectively. The difference from placebo (95% CI) for STEGLATRO 5 mg was -2.0 kg (-2.8, -1.2) and for STEGLATRO 15 mg was -2.1 kg (-2.9, -1.3).
| Figure 3: HbA1c (%) Change Over Time in a 26-Week Placebo-Controlled Monotherapy Study of STEGLATRO in Patients with Type 2 Diabetes Mellitus* |
|---|
|
|
|
Combination Therapy
Add-on Combination Therapy with Metformin
A total of 621 patients with type 2 diabetes mellitus inadequately controlled (HbA1c between 7% and 10.5%) on metformin monotherapy (≥1,500 mg/day for ≥8 weeks) participated in a randomized, double-blind, multi-center, 26-week, placebo-controlled study (NCT02033889) to evaluate the efficacy and safety of STEGLATRO in combination with metformin. Patients entered a 2-week, single-blind, placebo run-in, and were randomized to placebo, STEGLATRO 5 mg, or STEGLATRO 15 mg administered once daily in addition to continuation of background metformin therapy.
At Week 26, treatment with STEGLATRO at 5 mg or 15 mg daily provided statistically significant reductions in HbA1c compared to placebo. STEGLATRO also resulted in a greater proportion of patients achieving an HbA1c <7% compared to placebo (see Table 5).
| Placebo | STEGLATRO 5 mg | STEGLATRO 15 mg | |
|---|---|---|---|
|
|||
| HbA1c (%) | N = 207 | N = 205 | N = 201 |
| Baseline (mean) | 8.2 | 8.1 | 8.1 |
| Change from baseline (LS mean†) | -0.2 | -0.7 | -0.9 |
| Difference from placebo (LS mean†, 95% CI) | -0.5‡ (-0.7, -0.4) | -0.7‡ (-0.9, -0.5) | |
| Patients [N (%)] with HbA1c <7% | 38 (18.4) | 74 (36.3) | 87 (43.3) |
| FPG (mg/dL) | N = 202 | N = 199 | N = 201 |
| Baseline (mean) | 169.1 | 168.1 | 167.9 |
| Change from baseline (LS mean†) | -8.7 | -30.3 | -40.9 |
| Difference from placebo (LS mean†, 95% CI) | -21.6‡ (-27.8, -15.5) | -32.3‡ (-38.5, -26.0) | |
The mean baseline body weight was 84.5 kg, 84.9 kg, and 85.3 kg in the placebo, STEGLATRO 5 mg, and STEGLATRO 15 mg groups, respectively. The mean changes from baseline to Week 26 were -1.4 kg, -3.2 kg, and -3.0 kg in the placebo, STEGLATRO 5 mg, and STEGLATRO 15 mg groups, respectively. The difference from placebo (95% CI) for STEGLATRO 5 mg was -1.8 kg (-2.4, -1.2) and for STEGLATRO 15 mg was -1.7 kg (-2.2, -1.1).
The mean baseline systolic blood pressure was 129.3 mmHg, 130.5 mmHg, and 130.2 mmHg in the placebo, STEGLATRO 5 mg, and STEGLATRO 15 mg groups, respectively. The mean changes from baseline to Week 26 were -1.8 mmHg, -5.1 mmHg, and -5.7 mmHg in the placebo, STEGLATRO 5 mg, and STEGLATRO 15 mg groups, respectively. The difference from placebo (95% CI) for STEGLATRO 5 mg was -3.3 mmHg (-5.6, -1.1) and for STEGLATRO 15 mg was -3.8 mmHg (-6.1, -1.5).
Active Controlled Study versus Glimepiride as Add-on Combination Therapy with Metformin
A total of 1,326 patients with type 2 diabetes mellitus inadequately controlled (HbA1c between 7% and 9%) on metformin monotherapy participated in a randomized, double-blind, multi-center, 52-week, active comparator controlled study (NCT01999218) to evaluate the efficacy and safety of STEGLATRO in combination with metformin. These patients, who were receiving metformin monotherapy (≥1,500 mg/day for ≥8 weeks), entered a 2-week, single-blind, placebo run-in period and were randomized to glimepiride, STEGLATRO 5 mg, or STEGLATRO 15 mg administered once daily in addition to continuation of background metformin therapy. Glimepiride was initiated at 1 mg/day and titrated up to a maximum dose of 6 or 8 mg/day (depending on maximum approved dose in each country) or a maximum tolerated dose or down-titrated to avoid or manage hypoglycemia. The mean daily dose of glimepiride was 3.0 mg.
STEGLATRO 15 mg was non-inferior to glimepiride after 52 weeks of treatment. (See Table 6.)
| Glimepiride | STEGLATRO 5 mg | STEGLATRO 15 mg | |
|---|---|---|---|
|
|||
| HbA1c (%) | N = 437 | N = 447 | N = 440 |
| Baseline (mean) | 7.8 | 7.8 | 7.8 |
| Change from baseline (LS mean†) | -0.6 | -0.5 | -0.5 |
| Difference from glimepiride (LS mean†, 95% CI) | 0.2‡ (0.0, 0.3) | 0.1‡ (-0.0, 0.2) | |
| Patients [N (%)] with HbA1c <7% | 208 (47.7) | 177 (39.5) | 186 (42.2) |
The mean baseline body weight was 86.8 kg, 87.9 kg, and 85.6 kg in the glimepiride, STEGLATRO 5 mg, and STEGLATRO 15 mg groups, respectively. The mean changes from baseline to Week 52 were 0.6 kg, -2.6 kg, and -3.0 kg in the glimepiride, STEGLATRO 5 mg, and STEGLATRO 15 mg groups, respectively. The difference from glimepiride (95% CI) for STEGLATRO 5 mg was -3.2 kg (-3.7, -2.7) and for STEGLATRO 15 mg was -3.6 kg (-4.1, -3.1).
Add-on Combination Therapy with Metformin and Sitagliptin
A total of 463 patients with type 2 diabetes mellitus inadequately controlled (HbA1c between 7% and 10.5%) on metformin (≥1,500 mg/day for ≥8 weeks) and sitagliptin 100 mg once daily participated in a randomized, double-blind, multi-center, 26-week, placebo-controlled study (NCT02036515) to evaluate the efficacy and safety of STEGLATRO. Patients entered a 2-week, single-blind, placebo run-in period and were randomized to placebo, STEGLATRO 5 mg, or STEGLATRO 15 mg.
At Week 26, treatment with STEGLATRO at 5 mg or 15 mg daily provided statistically significant reductions in HbA1c. STEGLATRO also resulted in a higher proportion of patients achieving an HbA1c <7% compared to placebo (see Table 7).
| Placebo | STEGLATRO 5 mg | STEGLATRO 15 mg | |
|---|---|---|---|
|
|||
| HbA1c (%) | N = 152 | N = 155 | N = 152 |
| Baseline (mean) | 8.0 | 8.1 | 8.0 |
| Change from baseline (LS mean†) | -0.2 | -0.7 | -0.8 |
| Difference from placebo (LS mean†, 95% CI) | -0.5‡ (-0.7, -0.3) | -0.6‡ (-0.8, -0.4) | |
| Patients [N (%)] with HbA1c <7% | 31 (20.2) | 54 (34.6) | 64 (42.3) |
| FPG (mg/dL) | N = 152 | N = 156 | N = 152 |
| Baseline (mean) | 169.6 | 167.7 | 171.7 |
| Change from baseline (LS mean†) | -6.5 | -25.7 | -32.1 |
| Difference from placebo (LS mean†, 95% CI) | -19.2‡ (-26.8, -11.6) | -25.6‡ (-33.2, -18.0) | |
The mean baseline body weight was 86.5 kg, 87.6 kg, and 86.6 kg in the placebo, STEGLATRO 5 mg, and STEGLATRO 15 mg groups, respectively. The mean changes from baseline to Week 26 were -1.0 kg, -3.0 kg, and -2.8 kg in the placebo, STEGLATRO 5 mg, and STEGLATRO 15 mg groups, respectively. The difference from placebo (95% CI) for STEGLATRO 5 mg was -1.9 kg (-2.6, -1.3) and for STEGLATRO 15 mg was -1.8 kg (-2.4, -1.2).
The mean baseline systolic blood pressure was 130.2 mmHg, 132.1 mmHg, and 131.6 mmHg in the placebo, STEGLATRO 5 mg, and STEGLATRO 15 mg groups, respectively. The mean changes from baseline to Week 26 were -0.2 mmHg, -3.8 mmHg, and -4.5 mmHg in the placebo, STEGLATRO 5 mg, and STEGLATRO 15 mg groups, respectively. The difference from placebo (95% CI) for STEGLATRO 5 mg was -3.7 mmHg (-6.1, -1.2) and for STEGLATRO 15 mg was -4.3 mmHg (-6.7, -1.9).
Add-on Combination Therapy with Insulin (with or without Metformin)
In an 18-week randomized, double-blind, multi-center, placebo-controlled, glycemic sub-study of VERTIS CV (eValuation of ERTugliflozin efficacy and Safety CardioVascular, NCT01986881, study details see 14.2), a total of 1065 patients with type 2 diabetes mellitus and established atherosclerotic cardiovascular disease with inadequate glycemic control (HbA1c between 7% and 10.5%) on background therapy of insulin ≥20 units/day (59% also on metformin ≥1,500 mg/day) were randomized to placebo, STEGLATRO 5 mg or STEGLATRO 15 mg once daily treatment.
At Week 18, treatment with STEGLATRO at 5 mg or 15 mg daily provided statistically significant reductions in HbA1c compared to placebo (see Table 8).
| Placebo | STEGLATRO 5 mg | STEGLATRO 15 mg | |
|---|---|---|---|
| SE: standard error. | |||
|
|||
| HbA1c (%) | N = 346 | N = 346 | N = 367 |
| Baseline (mean) | 8.4 | 8.4 | 8.4 |
| Change from baseline (LS mean†, SE) | -0.2 (0.05) | -0.7 (0.05) | -0.7 (0.05) |
| Difference from placebo (LS mean†, 95% CI) | -0.5‡ (-0.6, -0.4) | -0.5‡ (-0.7, -0.4) | |
| Patients [N (%)] with HbA1c <7%§ | 37 (10.7) | 79 (22.8) | 81 (22.1) |
| FPG (mg/dL) | N = 343 | N = 346 | N = 368 |
| Baseline (mean) | 167.4 | 173.8 | 175.4 |
| Change from baseline (LS mean†, SE) | -6.3 (2.91) | -25.6 (2.90) | -29.8 (2.86) |
| Difference from placebo (LS mean†, 95% CI) | -19.2‡ (-26.8, -11.6) | -23.4‡ (-30.9, -16.0) | |
The mean baseline body weights were 93.3 kg, 93.8 kg, and 92.1 kg in the placebo, STEGLATRO 5 mg, and STEGLATRO 15 mg groups, respectively. The mean changes from baseline to Week 18 were - 0.2 kg, -1.6 kg, and -1.9 kg in the placebo, STEGLATRO 5 mg, and STEGLATRO 15 mg groups, respectively. The differences from placebo (95% CI) for STEGLATRO 5 mg were - 1.4 kg (- 1.9, - 0.9) and for STEGLATRO 15 mg was -1.6 kg (-2.1, -1.1).
The mean baseline systolic blood pressures were 134.0 mmHg, 135.6 mmHg, and 133.7 mmHg in the placebo, STEGLATRO 5 mg, and STEGLATRO 15 mg groups, respectively. The mean changes from baseline to Week 18 were 0.7 mmHg, -2.2 mmHg, and -1.7 mmHg in the placebo, STEGLATRO 5 mg, and STEGLATRO 15 mg groups, respectively. The differences from placebo (95% CI) for STEGLATRO 5 mg was – 2.9 mmHg (-4.9, -1.0) and for STEGLATRO 15 mg were -2.5 mmHg (- 4.4, - 0.5).
Add-on Combination Therapy with Metformin and Sulfonylurea
In an 18-week randomized, double-blind, multi-center, placebo-controlled, glycemic sub-study of VERTIS CV (NCT01986881, study details see 14.2), a total of 330 patients with type 2 diabetes mellitus and established atherosclerotic cardiovascular disease with inadequate glycemic control (HbA1c between 7% and 10.5%) with background therapy of metformin ≥1,500 mg/day and a sulfonylurea (SU) were randomized to placebo, STEGLATRO 5 mg or STEGLATRO 15 mg once daily treatment.
At Week 18, treatment with STEGLATRO at 5 mg or 15 mg daily provided statistically significant reductions in HbA1c compared to placebo (see Table 9).
| Placebo | STEGLATRO 5 mg | STEGLATRO 15 mg | |
|---|---|---|---|
| SE: standard error | |||
|
|||
| HbA1c (%) | N = 116 | N = 99 | N = 113 |
| Baseline (mean) | 8.3 | 8.4 | 8.3 |
| Change from baseline (LS mean†, SE) | -0.3 (0.08) | -0.8 (0.09) | -0.9 (0.08) |
| Difference from placebo (LS mean†, 95% CI) | -0.6‡ (-0.8, -0.3) | -0.7‡ (-0.9, -0.4) | |
| Patients [N (%)] with HbA1c <7%§ | 17 (14.7) | 39 (39.4) | 38 (33.6) |
| FPG (mg/dL) | N = 117 | N = 99 | N = 113 |
| Baseline (mean) | 177.3 | 183.5 | 174.0 |
| Change from baseline (LS mean†, SE) | -3.5 (3.65) | -31.3 (3.87) | -33.0 (3.67) |
| Difference from placebo (LS mean†, 95% CI) | -27.9‡ (-37.8, -17.9) | -29.5‡ (-39.0, -19.9) | |
The mean baseline body weights were 90.5 kg, 92.1 kg, and 92.9 kg in the placebo, STEGLATRO 5 mg, and STEGLATRO 15 mg groups, respectively. The mean changes from baseline to Week 18 were - 0.6 kg, -2.0 kg, and - 2.2 kg in the placebo, STEGLATRO 5 mg, and STEGLATRO 15 mg groups, respectively. The differences from placebo (95% CI) for STEGLATRO 5 mg were - 1.4 kg (- 2.2, - 0.7) and for STEGLATRO 15 mg was - 1.6 kg (- 2.3, - 0.9).
Use in Patients with Type 2 Diabetes Mellitus and Moderate Renal Impairment
26-Week Placebo-Controlled Study
The efficacy of STEGLATRO was assessed in a multicenter, randomized, double-blind, placebo-controlled study (NCT01986855) of patients with type 2 diabetes mellitus and moderate renal impairment (468 patients with eGFR ≥30 to <60 mL/min/1.73 m2). In this study, 202 patients exposed to STEGLATRO (5 mg or 15 mg) had an eGFR between 45 and 60 mL/min/1.73 m2 and 111 patients exposed to STEGLATRO (5 mg or 15 mg) had an eGFR between 30 and 45 mL/min/1.73 m2. The mean duration of diabetes for the study population was approximately 14 years, and the majority of patients were receiving background insulin (55.9%) and/or sulfonylurea (40.3%) therapy. Approximately 50% had a history of cardiovascular disease or heart failure.
STEGLATRO did not show efficacy in this study. The HbA1c reductions from baseline to Week 26 were not significantly different between placebo and STEGLATRO 5 mg or 15 mg [see Use in Specific Populations (8.6)].
14.2 Cardiovascular Outcomes in Patients with Type 2 Diabetes Mellitus and Established Cardiovascular Disease
The effect of STEGLATRO on cardiovascular risk in adult patients with type 2 diabetes and established atherosclerotic cardiovascular disease was evaluated in the VERTIS CV study (NCT01986881), a multicenter, multi-national, randomized, double-blind, placebo-controlled, event-driven trial. The study compared the risk of experiencing a major adverse cardiovascular event (MACE) between STEGLATRO and placebo when these were added to and used concomitantly with standard of care treatments for diabetes and atherosclerotic cardiovascular disease.
A total of 8246 patients were randomized (placebo N=2747, STEGLATRO 5 mg N=2752, STEGLATRO 15 mg N=2747) and followed for a median of 3 years. Approximately 88% of the study population was Caucasian, 6% Asian, and 3% Black. The mean age was 64 years and approximately 70% were male.
All patients in the study had inadequately controlled type 2 diabetes mellitus at baseline (HbA1c greater than or equal to 7%). The mean duration of type 2 diabetes mellitus was 13 years, the mean HbA1c at baseline was 8.2% and the mean eGFR was 76 mL/min/1.73 m2. At baseline, patients were treated with one (32%) or more (67%) antidiabetic medications including biguanides (metformin) (76%), insulin (47%), sulfonylureas (41%) DPP-4 inhibitors (11%) and GLP-1 receptor agonists (3%).
Almost all patients (99%) had established atherosclerotic cardiovascular disease at baseline including: a documented history of coronary artery disease (76%), cerebrovascular disease (23%) or peripheral artery disease (19%). Approximately 24% patients had a history of heart failure (HF). At baseline, the mean systolic blood pressure was 133 mmHg, the mean diastolic blood pressure was 77 mmHg, the mean LDL was 89 mg/dL, and the mean HDL was 44 mg/dL. At baseline, approximately 81% of patients were treated with renin angiotensin system inhibitors, 69% with beta-blockers, 43% with diuretics, 82% with statins, 4% with ezetimibe, and 89% with antiplatelet agents.
The primary endpoint in VERTIS CV was the time to first occurrence of MACE. A major adverse cardiovascular event was defined as occurrence of either a cardiovascular death or a nonfatal myocardial infarction (MI) or a nonfatal stroke. The statistical analysis plan pre-specified that the 5 and 15 mg doses would be combined for the analysis. A Cox proportional hazards model was used to test for non-inferiority against the pre-specified risk margin of 1.3 for the hazard ratio of MACE. Type-1 error was controlled across multiple tests using a hierarchical testing strategy.
The incidence rate of MACE was similar between the STEGLATRO-treated and placebo-treated patients. The estimated hazard ratio of MACE associated with STEGLATRO relative to placebo was 0.97 with 95.6% confidence interval (0.85, 1.11). The upper bound of this confidence interval excluded a risk larger than 1.3 (Table 10). Results for the individual 5 mg and 15 mg doses were consistent with results for the combined dose group.
| Endpoint† | Placebo (N=2747) | STEGLATRO (N=5499) | Hazard Ratio vs Placebo (CI) ‡ |
||
|---|---|---|---|---|---|
| N (%) | Event Rate (per 100 person-years) | N (%) | Event Rate (per 100 person-years) | ||
| N=Number of patients, CI=Confidence interval, CV=Cardiovascular, MI=Myocardial infarction. | |||||
|
|||||
| MACE (CV death, non-fatal MI, or non-fatal stroke) Composite | 327 (11.9) | 4.0 | 653 (11.9) | 3.9 | 0.97 (0.85, 1.11) |
| Components of Composite Endpoint | |||||
| Non-fatal MI | 148 (5.4) | 1.6 | 310 (5.6) | 1.7 | 1.04 (0.86, 1.27) |
| Non-fatal Stroke | 78 (2.8) | 0.8 | 157 (2.9) | 0.8 | 1.00 (0.76, 1.32) |
| CV death | 184 (6.7) | 1.9 | 341 (6.2) | 1.8 | 0.92 (0.77, 1.11) |
16. How is Steglatro supplied
STEGLATRO (ertugliflozin) tablets are available as follows:
| Strength | Description | How Supplied | NDC |
|---|---|---|---|
| 5 mg tablets | pink, triangular-shaped, biconvex tablets, with “701” debossed on one side and plain on the other side | unit-of-use bottles of 30 | 0006-5363-03 |
| unit-of-use bottles of 90 | 0006-5363-06 | ||
| bulk bottles of 500 | 0006-5363-07 | ||
| 15 mg tablets | red, triangular-shaped, biconvex tablets, with “702” debossed on one side and plain on the other side | unit-of-use bottles of 30 | 0006-5364-03 |
| unit-of-use bottles of 90 | 0006-5364-06 | ||
| bulk bottles of 500 | 0006-5364-07 |
17. Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
| Medication Guide STEGLATRO® (steh-GLA-troh) (ertugliflozin) tablets, for oral use |
|||
|---|---|---|---|
| This Medication Guide has been approved by the U.S. Food and Drug Administration. | Revised: 10/2022 | ||
| Read this Medication Guide carefully before you start taking STEGLATRO and each time you get a refill. There may be new information. This information does not take the place of talking with your doctor about your medical condition or your treatment. | |||
What is the most important information I should know about STEGLATRO? STEGLATRO may cause serious side effects, including:
|
|||
|
|
||
If you get any of these symptoms during treatment with STEGLATRO, if possible check for ketones in your urine, even if your blood sugar is less than 250 mg/dL.
|
|||
|
|
||
| Talk to your doctor about what to do if you get symptoms of a yeast infection of the vagina or penis. Your doctor may suggest you use an over-the-counter antifungal medicine. Talk to your doctor right away if you use an over-the-counter antifungal medicine and your symptoms do not go away. | |||
What is STEGLATRO?
|
|||
Do not take STEGLATRO if you:
|
|||
Before you take STEGLATRO, tell your doctor about all of your medical conditions, including if you:
|
|||
How should I take STEGLATRO?
|
|||
| What are the possible side effects of STEGLATRO? STEGLATRO may cause serious side effects, including: See "What is the most important information I should know about STEGLATRO?"
|
|||
|
|
|
|
|
|||
|
|
|
|
The most common side effects of STEGLATRO include:
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
|||
How should I store STEGLATRO?
|
|||
| General information about the safe and effective use of STEGLATRO.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use STEGLATRO for a condition for which it was not prescribed. Do not give STEGLATRO to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or doctor for information about STEGLATRO that is written for health professionals. For more information about STEGLATRO, go to www.steglatro.com or call 1-800-622-4477. |
|||
| What are the ingredients in STEGLATRO? Active ingredient: ertugliflozin. Inactive ingredients: microcrystalline cellulose, lactose monohydrate, sodium starch glycolate, and magnesium stearate. The tablet film coating contains the following inactive ingredients: hypromellose, lactose monohydrate, macrogol, triacetin, titanium dioxide, and iron oxide red. |
|||
| Manufactured for: Merck Sharp & Dohme LLC Rahway, NJ 07065, USA For patent information, go to: www.msd.com/research/patent Copyright © 2017-2022 Merck & Co., Inc., Rahway, NJ, USA, and its affiliates. All rights reserved. usmg-mk8835-t-2210r005 |
|||
| STEGLATRO
ertugliflozin tablet, film coated |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| STEGLATRO
ertugliflozin tablet, film coated |
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
| Labeler - Merck Sharp & Dohme LLC (118446553) |