Drug Class: Antiviral combinations
Highlights of Prescribing Information
STRIBILD® (elvitegravir, cobicistat, emtricitabine, tenofovir disoproxil fumarate) tablets, for oral use
Initial U.S. Approval: 2012
WARNING: POSTTREATMENT ACUTE EXACERBATION OF HEPATITIS B
See full prescribing information for complete boxed warning.
Severe acute exacerbations of hepatitis B have been reported in patients coinfected with HIV-1 and HBV who have discontinued EMTRIVA or VIREAD, two of the components of STRIBILD. Hepatic function should be monitored closely in these patients. If appropriate, initiation of anti-hepatitis B therapy may be warranted. (5.1)
Indications and Usage for Stribild
STRIBILD is a four-drug combination of elvitegravir, an HIV integrase strand transfer inhibitor (HIV-1 INSTI), cobicistat, a CYP3A inhibitor, and emtricitabine and tenofovir disoproxil fumarate (TDF), both HIV nucleoside analog reverse transcriptase inhibitors (HIV NRTI) and is indicated as a complete regimen for the treatment of HIV-1 infection in adults and pediatric patients 12 years of age and older weighing at least 35 kg who have no antiretroviral treatment history or to replace the current antiretroviral regimen in those who are virologically suppressed (HIV-1 RNA less than 50 copies/mL) on a stable antiretroviral regimen for at least 6 months with no history of treatment failure and no known substitutions associated with resistance to the individual components of STRIBILD. (1, 14)
Stribild Dosage and Administration
- Testing: Prior to initiation of STRIBILD, test patients for hepatitis B virus infection. Prior to initiation and during use of STRIBILD, on a clinically appropriate schedule, assess serum creatinine, serum phosphorous, estimated serum creatinine clearance, urine glucose, and urine protein in all patients. In patients with chronic kidney disease, also assess serum phosphorus. (2.1)
- Recommended dosage: One tablet taken once daily with food. (2.2)
- Dosage in renal impairment: Initiation of STRIBILD in patients with estimated creatinine clearance below 70 mL per minute is not recommended. Discontinue in patients with estimated creatinine clearance below 50 mL per minute. (2.3)
Dosage Forms and Strengths
Tablets: 150 mg of elvitegravir, 150 mg of cobicistat, 200 mg of emtricitabine, and 300 mg of tenofovir disoproxil fumarate. (3)
Contraindications
Coadministration of STRIBILD is contraindicated with drugs that:
- Are highly dependent on CYP3A for clearance and for which elevated plasma concentrations are associated with serious adverse events. (4)
- Strongly induce CYP3A, which may lead to lower exposure of one or more components and loss of efficacy of STRIBILD and possible resistance. (4)
Warnings and Precautions
- New onset or worsening renal impairment: Can include acute renal failure and Fanconi syndrome. Avoid administering STRIBILD with concurrent or recent use of nephrotoxic drugs. (5.2)
- Lactic acidosis/severe hepatomegaly with steatosis: Discontinue treatment in patients who develop symptoms or laboratory findings suggestive of lactic acidosis or pronounced hepatotoxicity. (5.3)
- Risk of adverse reactions or loss of virologic response due to drug interactions: The concomitant use of STRIBILD and other drugs may result in known or potentially significant drug interactions, some of which may lead to loss of therapeutic effect of STRIBILD and possible development of resistance; clinically significant adverse reactions from greater exposures of concomitant drugs; or loss of therapeutic effect of concomitant drugs. (5.4)
- Decreases in bone mineral density (BMD): Consider monitoring BMD in patients with a history of pathologic fracture or other risk factors of osteoporosis or bone loss. (5.5)
- Immune reconstitution syndrome: May necessitate further evaluation and treatment. (5.6)
Adverse Reactions/Side Effects
Most common adverse drug reactions to STRIBILD (incidence greater than or equal to 10%, all grades) are nausea and diarrhea. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Gilead Sciences, Inc. at 1-800-GILEAD-5 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
- STRIBILD is a complete regimen for the treatment of HIV-1 infection; therefore, STRIBILD should not be administered with other antiretroviral medications for treatment of HIV-1 infection. (7.1)
- STRIBILD can alter the concentration of drugs metabolized by CYP3A or CYP2D6. Drugs that induce CYP3A can alter the concentrations of one or more components of STRIBILD. Consult the full prescribing information prior to and during treatment for potential drug-drug interactions. (4, 7.2, 7.3, 12.3)
Use In Specific Populations
- Pregnancy: Not recommended for use during pregnancy because of substantially lower exposures of cobicistat and elvitegravir during pregnancy. STRIBILD should not be initiated in pregnant individuals. (2.5, 8.1)
- Lactation: Breastfeeding is not recommended due to the potential for HIV transmission. (8.2)
- Pediatrics: Not recommended for patients less than 12 years of age or weighing less than 35 kg. (8.4)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 9/2021
Full Prescribing Information
WARNING: POSTTREATMENT ACUTE EXACERBATION OF HEPATITIS B
Severe acute exacerbations of hepatitis B have been reported in patients who are coinfected with HIV-1 and HBV and have discontinued EMTRIVA or VIREAD, which are components of STRIBILD. Hepatic function should be monitored closely, with both clinical and laboratory follow-up for at least several months in patients who are coinfected with HIV-1 and HBV and discontinue STRIBILD. If appropriate, initiation of anti-hepatitis B therapy may be warranted [see Warnings and Precautions (5.1)].
1. Indications and Usage for Stribild
STRIBILD® is indicated as a complete regimen for the treatment of HIV-1 infection in adults and pediatric patients 12 years of age and older weighing at least 35 kg who have no antiretroviral treatment history or to replace the current antiretroviral regimen in those who are virologically suppressed (HIV-1 RNA less than 50 copies/mL) on a stable antiretroviral regimen for at least 6 months with no history of treatment failure and no known substitutions associated with resistance to the individual components of STRIBILD [see Clinical Studies (14)].
2. Stribild Dosage and Administration
2.1 Testing Prior to Initiation and During Treatment with STRIBILD
Prior to initiation of STRIBILD, test patients for hepatitis B virus infection [see Warnings and Precautions (5.1)].
Prior to initiation and during use of STRIBILD, on a clinically appropriate schedule, assess serum creatinine, estimated creatinine clearance, urine glucose, and urine protein in all patients. In patients with chronic kidney disease, also assess serum phosphorus [see Warnings and Precautions (5.2)].
2.2 Recommended Dosage
STRIBILD is a four-drug fixed dose combination product containing 150 mg of elvitegravir, 150 mg of cobicistat, 200 mg of emtricitabine, and 300 mg of TDF. The recommended dosage of STRIBILD is one tablet taken orally once daily with food in adults and pediatric patients 12 years of age and older with a body weight at least 35 kg and creatinine clearance greater than or equal to 70 mL per minute [see Clinical Pharmacology (12.3)].
2.3 Dosage Adjustment in Patients with Renal Impairment
Initiation of STRIBILD in patients with estimated creatinine clearance below 70 mL per minute is not recommended. Because STRIBILD is a fixed-dose combination tablet, STRIBILD should be discontinued if estimated creatinine clearance declines below 50 mL per minute during treatment with STRIBILD, as the dose interval adjustment required for emtricitabine and tenofovir disoproxil fumarate (DF) cannot be achieved [see Warnings and Precautions (5.2), Adverse Reactions (6.1), Use in Specific Populations (8.6), Clinical Pharmacology (12.3), and Clinical Studies (14)].
No data are available to make dose recommendations for pediatric patients with renal impairment.
2.4 Not Recommended in Patients with Severe Hepatic Impairment
STRIBILD is not recommended for use in patients with severe hepatic impairment [see Use in Specific Populations (8.7) and Clinical Pharmacology (12.3)].
2.5 Not Recommended During Pregnancy
STRIBILD is not recommended for use during pregnancy because of substantially lower exposures of cobicistat and elvitegravir during the second and third trimesters [see Use in Specific Populations (8.1)].
STRIBILD should not be initiated in pregnant individuals. An alternative regimen is recommended for individuals who become pregnant during therapy with STRIBILD [see Use in Specific Populations (8.1)].
3. Dosage Forms and Strengths
Each STRIBILD tablet contains 150 mg of elvitegravir, 150 mg of cobicistat, 200 mg of emtricitabine, and 300 mg of TDF (equivalent to 245 mg of tenofovir disoproxil).
The tablets are green, capsule shaped, film coated, and debossed with "GSI" on one side and the number "1" surrounded by a square box (  ) on the other side.
) on the other side.
4. Contraindications
Coadministration of STRIBILD is contraindicated with drugs that are highly dependent on CYP3A for clearance and for which elevated plasma concentrations are associated with serious and/or life-threatening events. These drugs and other contraindicated drugs (which may lead to reduced efficacy of STRIBILD and possible resistance) are listed below [see Drug Interactions (7.5) and Clinical Pharmacology (12.3)].
- Alpha 1-adrenoreceptor antagonist: alfuzosin
- Anticonvulsants: carbamazepine, phenobarbital, phenytoin
- Antimycobacterial: rifampin
- Antipsychotics: lurasidone, pimozide
- Ergot Derivatives: dihydroergotamine, ergotamine, methylergonovine
- Herbal Products: St. John's wort (Hypericum perforatum)
- Lipid-modifying Agents: lomitapide, lovastatin, simvastatin
- Phosphodiesterase-5 (PDE-5) Inhibitor: sildenafil when administered as Revatio® for the treatment of pulmonary arterial hypertension
- Sedative/hypnotics: triazolam, orally administered midazolam
5. Warnings and Precautions
5.1 Severe Acute Exacerbation of Hepatitis B in Patients Coinfected with HIV-1 and HBV
All patients with HIV-1 should be tested for the presence of hepatitis B virus (HBV) before initiating antiretroviral therapy [see Dosage and Administration (2.1)].
Severe acute exacerbations of hepatitis B (e.g., liver decompensated and liver failure) have been reported in patients who are coinfected with HIV-1 and HBV and have discontinued emtricitabine or TDF, two of the components of STRIBILD. Patients who are coinfected with HIV-1 and HBV should be closely monitored with both clinical and laboratory follow-up for at least several months after stopping treatment with STRIBILD. If appropriate, initiation of anti-hepatitis B therapy may be warranted, especially in patients with advanced liver disease or cirrhosis, since posttreatment exacerbation of hepatitis may lead to hepatic decompensation and liver failure.
5.2 New Onset or Worsening Renal Impairment
Renal impairment, including cases of acute renal failure and Fanconi syndrome (renal tubular injury with severe hypophosphatemia), has been reported with the use of TDF, a component of STRIBILD, and with the use of STRIBILD [see Adverse Reactions (6.2)].
In the clinical trials of STRIBILD over 144 weeks, 13 (1.9%) subjects in the STRIBILD group (N=701), 8 (2.3%) subjects in the atazanavir (ATV) + ritonavir (RTV) + TRUVADA® (emtricitabine 200 mg/TDF 300 mg) group (N=355), and no subjects in the ATRIPLA® (efavirenz 600 mg/emtricitabine 200 mg/TDF 300 mg) group (N=352) discontinued study drug due to a renal adverse reaction. Of these discontinuations, 8 in the STRIBILD group and 1 in the ATV+RTV+TRUVADA group occurred during the first 48 weeks. Four (0.6%) subjects who received STRIBILD developed laboratory findings consistent with proximal renal tubular dysfunction, leading to discontinuation of STRIBILD during the first 48 weeks of treatment. Two of the four subjects had renal impairment (i.e., estimated creatinine clearance less than 70 mL per minute) at baseline. The laboratory findings in these 4 subjects improved but did not completely resolve in all subjects upon discontinuation of STRIBILD. Renal replacement therapy was not required for these subjects. One (0.3%) subject who received ATV+RTV+TRUVADA developed laboratory findings consistent with proximal renal tubular dysfunction, leading to discontinuation of ATV+RTV+TRUVADA after Week 96.
STRIBILD should be avoided with concurrent or recent use of a nephrotoxic agent (e.g., high-dose or multiple nonsteroidal anti-inflammatory drugs [NSAIDs]) [see Drug Interactions (7.4)]. Cases of acute renal failure after initiation of high-dose or multiple NSAIDs have been reported in HIV-infected patients with risk factors for renal dysfunction who appeared stable on TDF. Some patients required hospitalization and renal replacement therapy. Alternatives to NSAIDs should be considered, if needed, in patients at risk for renal dysfunction.
Persistent or worsening bone pain, pain in extremities, fractures, and/or muscular pain or weakness may be manifestations of proximal renal tubulopathy and should prompt an evaluation of renal function in at-risk patients.
Prior to initiation and during use of STRIBILD, on a clinically appropriate schedule, assess serum creatinine, estimated creatinine clearance, urine glucose, and urine protein in all patients. In patients with chronic kidney disease, also assess serum phosphorus. Discontinue STRIBILD in patients who develop clinically significant decreases in renal function or evidence of Fanconi syndrome. Initiation of STRIBILD in patients with estimated creatinine clearance below 70 mL per minute is not recommended [see Dosage and Administration (2.1)].
Although cobicistat (a component of STRIBILD) may cause modest increases in serum creatinine and modest declines in estimated creatinine clearance without affecting renal glomerular function [see Adverse Reactions (6.1)], patients who experience a confirmed increase in serum creatinine of greater than 0.4 mg per dL from baseline should be closely monitored for renal safety.
The emtricitabine and TDF components of STRIBILD are primarily excreted by the kidney. STRIBILD should be discontinued if estimated creatinine clearance declines below 50 mL per minute as dose interval adjustment required for emtricitabine and TDF cannot be achieved with the fixed-dose combination tablet [see Use in Specific Populations (8.6)].
5.3 Lactic Acidosis/Severe Hepatomegaly with Steatosis
Lactic acidosis and severe hepatomegaly with steatosis, including fatal cases, have been reported with the use of nucleoside analogs, including TDF and emtricitabine, components of STRIBILD, alone or in combination with other antiretrovirals. Treatment with STRIBILD should be suspended in any patient who develops clinical or laboratory findings suggestive of lactic acidosis or pronounced hepatotoxicity (which may include hepatomegaly and steatosis even in the absence of marked transaminase elevations).
5.4 Risk of Adverse Reactions or Loss of Virologic Response Due to Drug Interactions
The concomitant use of STRIBILD and other drugs may result in known or potentially significant drug interactions, some of which may lead to [see Contraindications (4) and Drug Interactions (7)]:
- Loss of therapeutic effect of STRIBILD and possible development of resistance.
- Clinically significant adverse reactions, potentially leading to severe, life-threatening, or fatal events, from greater exposures of concomitant drugs metabolized by CYP3A.
- Loss of therapeutic effect of concomitant drugs that utilize CYP3A to form active metabolites.
See Table 5 for steps to prevent or manage these possible and known significant drug interactions, including dosing recommendations [see Drug Interactions (7)]. Consider the potential for drug interactions prior to and during STRIBILD therapy; review concomitant medications during STRIBILD therapy; and monitor for the adverse reactions associated with the concomitant drugs.
5.6 Immune Reconstitution Syndrome
Immune reconstitution syndrome has been reported in patients treated with combination antiretroviral therapy, including STRIBILD. During the initial phase of combination antiretroviral treatment, patients whose immune system responds may develop an inflammatory response to indolent or residual opportunistic infections (such as Mycobacterium avium infection, cytomegalovirus, Pneumocystis jirovecii pneumonia (PCP), or tuberculosis), which may necessitate further evaluation and treatment.
Autoimmune disorders (such as Graves' disease, polymyositis, Guillain-Barré syndrome, and autoimmune hepatitis) have also been reported to occur in the setting of immune reconstitution; however, the time to onset is more variable and can occur many months after initiation of treatment.
6. Adverse Reactions/Side Effects
The following adverse reactions are discussed in other sections of the labeling:
- Severe Acute Exacerbations of Hepatitis B in Patients Coinfected with HIV-1 and HBV [see Warnings and Precautions (5.1)].
- New Onset or Worsening Renal Impairment [see Warnings and Precautions (5.2)].
- Lactic Acidosis/Severe Hepatomegaly with Steatosis [see Warnings and Precautions (5.3)].
- Bone Loss and Mineralization Defects [see Warnings and Precautions (5.5)].
- Immune Reconstitution Syndrome [see Warnings and Precautions (5.6)].
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of TDF. Because postmarketing reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. No additional postmarketing adverse reactions specific for emtricitabine have been identified.
Immune System Disorders
allergic reaction, including angioedema
Metabolism and Nutrition Disorders
lactic acidosis, hypokalemia, hypophosphatemia
Respiratory, Thoracic, and Mediastinal Disorders
dyspnea
Gastrointestinal Disorders
pancreatitis, increased amylase, abdominal pain
Hepatobiliary Disorders
hepatic steatosis, hepatitis, increased liver enzymes (most commonly AST, ALT, gamma GT)
Skin and Subcutaneous Tissue Disorders
rash
Musculoskeletal and Connective Tissue Disorders
rhabdomyolysis, osteomalacia (manifested as bone pain and which may contribute to fractures), muscular weakness, myopathy
Renal and Urinary Disorders
acute renal failure, renal failure, acute tubular necrosis, Fanconi syndrome, proximal renal tubulopathy, interstitial nephritis (including acute cases), nephrogenic diabetes insipidus, renal insufficiency, increased creatinine, proteinuria, polyuria
General Disorders and Administration Site Conditions
asthenia
The following adverse reactions, listed under the body system headings above, may occur as a consequence of proximal renal tubulopathy: rhabdomyolysis, osteomalacia, hypokalemia, muscular weakness, myopathy, hypophosphatemia.
7. Drug Interactions
7.1 Not Recommended with Other Antiretroviral Medications
STRIBILD is a complete regimen for the treatment of HIV-1 infection; therefore, STRIBILD should not be administered with other antiretroviral medications for treatment of HIV-1 infection. Complete information regarding potential drug-drug interactions with other antiretroviral medications is not provided [see Contraindications (4), Warnings and Precautions (5.4) and Clinical Pharmacology (12.3)].
7.2 Potential for STRIBILD to Affect Other Drugs
Cobicistat, a component of STRIBILD, is an inhibitor of CYP3A and CYP2D6 and an inhibitor of the following transporters: P-glycoprotein (P-gp), BCRP, OATP1B1, and OATP1B3. Thus, coadministration of STRIBILD with drugs that are primarily metabolized by CYP3A or CYP2D6, or are substrates of P-gp, BCRP, OATP1B1, or OATP1B3, may result in increased plasma concentrations of such drugs. Coadministration of STRIBILD with drugs that have active metabolite(s) formed by CYP3A may result in reduced plasma concentration of these active metabolite(s) (Table 5).
Elvitegravir is a modest inducer of CYP2C9 and may decrease the plasma concentrations of CYP2C9 substrates.
7.3 Potential for Other Drugs to Affect One or More Components of STRIBILD
Elvitegravir and cobicistat, components of STRIBILD, are metabolized by CYP3A. Cobicistat is also metabolized, to a minor extent, by CYP2D6.
Drugs that induce CYP3A activity are expected to increase the clearance of elvitegravir and cobicistat, resulting in decreased plasma concentration of cobicistat and elvitegravir, which may lead to loss of therapeutic effect of STRIBILD and development of resistance (Table 5).
Coadministration of STRIBILD with other drugs that inhibit CYP3A may decrease the clearance and increase the plasma concentration of cobicistat (Table 5).
7.4 Drugs Affecting Renal Function
Because emtricitabine and tenofovir, components of STRIBILD, are primarily excreted by the kidneys by a combination of glomerular filtration and active tubular secretion, coadministration of STRIBILD with drugs that reduce renal function or compete for active tubular secretion may increase concentrations of emtricitabine, tenofovir, and other renally eliminated drugs and this may increase the risk of adverse reactions. Some examples of drugs that are eliminated by active tubular secretion include, but are not limited to, acyclovir, cidofovir, ganciclovir, valacyclovir, valganciclovir, aminoglycosides (e.g., gentamicin), and high-dose or multiple NSAIDs [see Warnings and Precautions (5.2)].
7.5 Established and Other Potentially Significant Interactions
Table 5 provides a listing of established or potentially clinically significant drug interactions. The drug interactions described are based on studies conducted with either STRIBILD or the components of STRIBILD (elvitegravir, cobicistat, emtricitabine, and TDF) as individual agents and/or in combination, or are predicted drug interactions that may occur with STRIBILD [for magnitude of interaction see Clinical Pharmacology (12.3)]. The table includes potentially significant interactions but is not all inclusive [see Contraindications (4) and Clinical Pharmacology (12.3)].
| Concomitant Drug Class: Drug Name | Effect on Concentration† | Clinical Comment |
|---|---|---|
|
||
| Alpha 1-adrenoreceptor antagonist:
alfuzosin | ↑ alfuzosin | Coadministration with alfuzosin is contraindicated due to potential for serious and/or life-threatening reactions such as hypotension. |
| Antiarrhythmics:
e.g., amiodarone bepridil digoxin‡ disopyramide flecainide systemic lidocaine mexiletine propafenone quinidine | ↑ antiarrhythmics ↑ digoxin | Therapeutic concentration monitoring, if available, is recommended for antiarrhythmics when coadministered with STRIBILD. |
| Antibacterials:
clarithromycin | ↑ clarithromycin ↑ cobicistat | Patients with CLcr greater than or equal to 60 mL/minute:
No dose adjustment of clarithromycin is required. Patients with CLcr between 50 mL/minute and 60 mL/minute: The dose of clarithromycin should be reduced by 50%. |
| Anticoagulants:
Direct Oral Anticoagulants (DOACs) apixaban rivaroxaban betrixaban dabigatran edoxaban | ↑ apixaban | Due to potentially increased bleeding risk, dosing recommendations for coadministration with STRIBILD depends on the apixaban dose. Refer to apixaban dosing instructions for coadministration with strong CYP3A and P-gp inhibitors in apixaban prescribing information. |
| ↑ rivaroxaban | Coadministration of rivaroxaban with STRIBILD is not recommended because it may lead to an increased bleeding risk. | |
| ↑ betrixaban ↑ dabigatran ↑ edoxaban | Due to potentially increased bleeding risk, dosing recommendations for coadministration of betrixaban, dabigatran, or edoxaban with a P-gp inhibitor such as STRIBILD depends on DOAC indication and renal function. Refer to DOAC dosing instructions for coadministration with P-gp inhibitors in DOAC prescribing information. | |
| warfarin | Effect on warfarin unknown | Monitor international normalized ratio (INR) upon coadministration of warfarin with STRIBILD. |
| Anticonvulsants:
carbamazepine phenobarbital phenytoin | ↓ elvitegravir ↓ cobicistat | Coadministration with carbamazepine, phenobarbital, or phenytoin is contraindicated due to potential for loss of elvitegravir therapeutic effect and development of resistance. |
| oxcarbazepine | Alternative anticonvulsants should be considered when STRIBILD is coadministered with oxcarbazepine. | |
| clonazepam ethosuximide | ↑ clonazepam ↑ ethosuximide | Clinical monitoring is recommended upon coadministration of clonazepam or ethosuximide with STRIBILD. |
| Antidepressants:
Selective Serotonin Reuptake Inhibitors (SSRIs) e.g., paroxetine | ↑ SSRIs (except sertraline) ↑ TCAs ↑ trazodone | Careful dose titration of the antidepressant and monitoring for antidepressant response are recommended when coadministered with STRIBILD. |
| Tricyclic Antidepressants (TCAs) e.g., amitriptyline desipramine imipramine nortriptyline bupropion | ||
| trazodone | ||
| Antifungals:
itraconazole ketoconazole‡ voriconazole | ↑ elvitegravir ↑ cobicistat ↑ itraconazole ↑ ketoconazole ↑ voriconazole | When coadministered with STRIBILD, the maximum daily dose of ketoconazole or itraconazole should not exceed 200 mg per day. An assessment of benefit/risk ratio is recommended to justify use of voriconazole with STRIBILD. |
| Anti-gout:
colchicine | ↑ colchicine | STRIBILD is not recommended to be coadministered with colchicine to patients with renal or hepatic impairment. Treatment of gout-flares – coadministration of colchicine in patients receiving STRIBILD: 0.6 mg (1 tablet) × 1 dose, followed by 0.3 mg (half tablet) 1 hour later. Treatment course to be repeated no earlier than 3 days. Prophylaxis of gout-flares – coadministration of colchicine in patients receiving STRIBILD: If the original regimen was 0.6 mg twice a day, the regimen should be adjusted to 0.3 mg once a day. If the original regimen was 0.6 mg once a day, the regimen should be adjusted to 0.3 mg once every other day. Treatment of familial Mediterranean fever – coadministration of colchicine in patients receiving STRIBILD: Maximum daily dose of 0.6 mg (may be given as 0.3 mg twice a day). |
| Antimycobacterial:
rifampin | ↓ elvitegravir ↓ cobicistat | Coadministration with rifampin is contraindicated due to potential for loss of elvitegravir therapeutic effect and development of resistance. |
| rifabutin‡
rifapentine | Coadministration of STRIBILD with rifabutin or rifapentine is not recommended. | |
| Antiplatelets: | ||
| ticagrelor clopidogrel | ↑ ticagrelor ↓ clopidogrel active metabolite | Coadministration with ticagrelor is not recommended. Coadministration with clopidogrel is not recommended due to potential reduction of the antiplatelet activity of clopidogrel. |
| Antipsychotics: | ||
| lurasidone | ↑ lurasidone | Coadministration with lurasidone is contraindicated due to potential for serious and/or life-threatening reactions. |
| pimozide | ↑ pimozide | Coadministration with pimozide is contraindicated due to potential for serious and/or life-threatening reactions such as cardiac arrhythmias. |
| quetiapine | ↑ quetiapine | Initiation of STRIBILD in patients taking quetiapine:
Consider alternative antiretroviral therapy to avoid increases in quetiapine exposure. If coadministration is necessary, reduce the quetiapine dose to 1/6 of the current dose and monitor for quetiapine-associated adverse reactions. Refer to the quetiapine prescribing information for recommendations on adverse reaction monitoring. Initiation of quetiapine in patients taking STRIBILD: Refer to the quetiapine prescribing information for initial dosing and titration of quetiapine. |
| Other antipsychotics e.g., perphenazine risperidone thioridazine | ↑ antipsychotic | A decrease in the dose of antipsychotics that are metabolized by CYP3A4 or CYP2D6 may be needed when coadministered with STRIBILD. |
| Beta-Blockers:
e.g., metoprolol timolol | ↑ beta-blockers | Clinical monitoring is recommended and a dose decrease of the beta-blocker may be necessary when these agents are coadministered with STRIBILD. |
| Calcium Channel Blockers:
e.g., amlodipine diltiazem felodipine nicardipine nifedipine verapamil | ↑ calcium channel blockers | Clinical monitoring is recommended upon coadministration of calcium channel blockers with STRIBILD. |
| Corticosteroids:
e.g., betamethasone budesonide ciclesonide dexamethasone fluticasone methylprednisolone mometasone triamcinolone | ↓ elvitegravir ↓ cobicistat ↑ corticosteroids | Coadministration with oral dexamethasone or other systemic corticosteroids that induce CYP3A may result in loss of therapeutic effect and development of resistance to elvitegravir. Consider alternative corticosteroids. Coadministration with corticosteroids (all routes of administration) whose exposures are significantly increased by strong CYP3A inhibitors can increase the risk for Cushing's syndrome and adrenal suppression. Alternative corticosteroids including beclomethasone, prednisone, and prednisolone (whose PK and/or PD are less affected by strong CYP3A inhibitors relative to other studied steroids) should be considered, particularly for long-term use. |
| Endothelin Receptor Antagonists:
bosentan | ↑ bosentan | Coadministration of bosentan in patients on STRIBILD:
In patients who have been receiving STRIBILD for at least 10 days, start bosentan at 62.5 mg once daily or every other day based upon individual tolerability. Coadministration of STRIBILD in patients on bosentan: Discontinue use of bosentan at least 36 hours prior to initiation of STRIBILD. After at least 10 days following the initiation of STRIBILD, resume bosentan at 62.5 mg once daily or every other day based upon individual tolerability. |
| Ergot Derivatives:
dihydroergotamine, ergotamine, methylergonovine | ↑ ergot derivatives | Coadministration is contraindicated due to potential for serious and/or life-threatening reactions such as acute ergot toxicity characterized by peripheral vasospasm and ischemia of the extremities and other tissues. |
| Hepatitis C Antiviral Agents:
ledipasvir/sofosbuvir sofosbuvir/velpatasvir‡ sofosbuvir/velpatasvir/voxilaprevir | ↑ tenofovir | The safety of increased tenofovir concentrations in the setting of HARVONI® (ledipasvir/sofosbuvir) and STRIBILD has not been established. Coadministration is not recommended. Patients receiving STRIBILD concomitantly with EPCLUSA® (sofosbuvir/velpatasvir) or VOSEVI® (sofosbuvir/velpatasvir/voxilaprevir) should be monitored for adverse reactions associated with tenofovir disoproxil fumarate. |
| Herbal Products:
St. John's wort (Hypericum perforatum) | ↓ elvitegravir ↓ cobicistat | Coadministration is contraindicated due to potential for loss of elvitegravir therapeutic effect and development of resistance. |
| Hormonal Contraceptives:
drospirenone/ethinyl estradiol levonorgestrel norgestimate/ethinyl estradiol‡ | ↑ drospirenone ↑ levonorgestrel ↑ norgestimate ↓ ethinyl estradiol | Additional or alternative non-hormonal forms of contraception should be considered when estrogen based contraceptives are coadministered with STRIBILD. Plasma concentrations of drospirenone may be increased when coadministered with cobicistat- containing products. Clinical monitoring is recommended due to the potential for hyperkalemia. The effects of increases in the concentration of the progestational component norgestimate are not fully known and can include increased risk of insulin resistance, dyslipidemia, acne, and venous thrombosis. The potential risks and benefits associated with coadministration of norgestimate/ethinyl estradiol with STRIBILD should be considered, particularly in women who have risk factors for these events. Coadministration of STRIBILD with other hormonal contraceptives (e.g., contraceptive patch, contraceptive vaginal ring, or injectable contraceptives) or oral contraceptives containing progestogens other than drospirenone, levonorgestrel, or norgestimate has not been studied; therefore, alternative (non-hormonal) methods of contraception can be considered. |
| Immuno-suppressants:
e.g., cyclosporine sirolimus tacrolimus | ↑ immuno-suppressants | Therapeutic monitoring of the immunosuppressive agents is recommended upon coadministration with STRIBILD. |
| Lipid-modifying Agents:
HMG-CoA Reductase Inhibitors: | ||
| lovastatin simvastatin | ↑ lovastatin ↑ simvastatin | Coadministration with lovastatin or simvastatin is contraindicated due to potential for serious reactions such as myopathy including rhabdomyolysis. |
| atorvastatin | ↑ atorvastatin | Initiate atorvastatin with the lowest starting dose of atorvastatin and titrate carefully while monitoring for safety (e.g., myopathy). Do not exceed a dosage of atorvastatin 20 mg daily. |
| Other Lipid-modifying Agents: lomitapide | ↑ lomitapide | Coadministration with lomitapide is contraindicated due to potential for markedly increased transaminases. |
| Narcotic Analgesics:
buprenorphine/naloxone‡ | ↑ buprenorphine ↑ norbuprenorphine ↓ naloxone | Patients should be closely monitored for sedation and cognitive effects. |
| fentanyl | ↑ fentanyl | Careful monitoring of therapeutic and adverse effects of fentanyl (including potentially fatal respiratory depression) is recommended with coadministration. |
| tramadol | ↑ tramadol | A dose decrease may be needed for tramadol with concomitant use. |
| Inhaled Beta Agonist:
salmeterol | ↑ salmeterol | Coadministration of salmeterol and STRIBILD is not recommended because it may result in increased risk of cardiovascular adverse events associated with salmeterol, including QT prolongation, palpitations, and sinus tachycardia. |
| Medications or Oral Supplements Containing Polyvalent Cations (e.g., Mg, Al, Ca, Fe, Zn):
calcium or iron supplements, including multivitamins cation-containing antacids‡ or laxatives sucralfate buffered medications | ↓ elvitegravir | Separate STRIBILD and administration of medications, antacids, or oral supplements containing polyvalent cations by at least 2 hours. |
| Phosphodiesterase-5 (PDE-5) Inhibitors:
sildenafil tadalafil vardenafil | ↑ PDE-5 inhibitors | Coadministration of sildenafil with STRIBILD is contraindicated when used for treatment of pulmonary arterial hypertension (PAH), due to potential for PDE-5 inhibitor associated adverse reactions, including hypotension, syncope, visual disturbances, and priapism. Use of tadalafil for PAH:
The below PDE-5 inhibitors can be used along with increased monitoring for PDE-5-inhibitor associated adverse events:
|
| Sedative/hypnotics: | ||
| midazolam (oral), triazolam | ↑ midazolam ↑ triazolam | Coadministration with triazolam or orally administered midazolam is contraindicated due to potential for serious and/or life-threatening reactions such as prolonged or increased sedation or respiratory depression. Triazolam and orally administered midazolam are extensively metabolized by CYP3A. Coadministration of triazolam or orally administered midazolam with STRIBILD may cause large increases in the concentrations of these benzodiazepines. |
| Other benzodiazepines: e.g., parenterally administered midazolam clorazepate diazepam estazolam flurazepam buspirone zolpidem | ↑ sedatives/hypnotics | Coadministration of parenteral midazolam with STRIBILD should be done in a setting that ensures close clinical monitoring and appropriate medical management in case of respiratory depression and/or prolonged sedation. Dosage reduction for midazolam should be considered, especially if more than a single dose of midazolam is administered. With other sedative/hypnotics, dose reduction may be necessary and clinical monitoring is recommended. |
7.6 Drugs without Clinically Significant Interactions with STRIBILD
Based on drug interaction studies conducted with the components of STRIBILD, no clinically significant drug interactions have been observed or are expected when STRIBILD is combined with the following drugs: famciclovir, famotidine, methadone, omeprazole, prasugrel (active metabolite), and sertraline.
8. Use In Specific Populations
8.1 Pregnancy
Data
8.2 Lactation
Risk Summary
The Centers for Disease Control and Prevention recommend that HIV-infected mothers not breastfeed their infants to avoid risking postnatal transmission of HIV.
Based on limited published data, emtricitabine and tenofovir have been shown to be present in human breast milk. It is not known whether elvitegravir or cobicistat are present in human breast milk, while elvitegravir and cobicistat have been shown to be present in rat milk (see Data).
It is not known if the components of STRIBILD affect milk production or have effects on the breastfed child. Because of the potential for: (1) HIV transmission (in HIV-negative infants); (2) developing viral resistance (in HIV-positive infants); and (3) adverse reactions in a breastfed infant similar to those seen in adults, instruct mothers not to breastfeed if they are receiving STRIBILD (see Data).
8.4 Pediatric Use
The pharmacokinetics, safety, and virologic and immunologic responses were evaluated in 50 treatment-naïve, HIV-1 infected subjects aged 12 to less than 18 years weighing at least 35 kg receiving STRIBILD through 48 weeks in an open-label trial (Study 112). The safety and efficacy of STRIBILD in these subjects was similar to that in antiretroviral treatment-naïve adults [see Dosage and Administration (2.2), Adverse Reactions (6.1), Clinical Pharmacology (12.3), and Clinical Studies (14.4)].
Safety and effectiveness of STRIBILD in pediatric patients less than 12 years of age or weighing less than 35 kg have not been established.
8.5 Geriatric Use
Clinical studies of STRIBILD did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. In general, caution should be exercised in the administration of STRIBILD in elderly patients, keeping in mind the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy [see Clinical Pharmacology (12.3)].
8.6 Renal Impairment
Initiation of STRIBILD in patients with estimated creatinine clearance below 70 mL per min is not recommended. Because STRIBILD is a fixed-dose combination tablet, STRIBILD should be discontinued if estimated creatinine clearance declines below 50 mL per minute during treatment with STRIBILD as dose interval adjustment required for emtricitabine and TDF cannot be achieved [see Warnings and Precautions (5.2), Adverse Reactions (6.1), Clinical Pharmacology (12.3), and Clinical Studies (14)].
No data are available to make dose recommendations for pediatric patients with renal impairment.
8.7 Hepatic Impairment
No dose adjustment of STRIBILD is required in patients with mild (Child-Pugh Class A) or moderate (Child-Pugh Class B) hepatic impairment. No pharmacokinetic or safety data are available regarding the use of STRIBILD in patients with severe hepatic impairment (Child-Pugh Class C). Therefore, STRIBILD is not recommended for use in patients with severe hepatic impairment [see Dosage and Administration (2.4) and Clinical Pharmacology (12.3)].
10. Overdosage
No data are available on overdose of STRIBILD in patients. If overdose occurs the patient must be monitored for evidence of toxicity. Treatment of overdose with STRIBILD consists of general supportive measures, including monitoring of vital signs as well as observation of the clinical status of the patient.
11. Stribild Description
STRIBILD is a fixed-dose combination tablet containing elvitegravir, cobicistat, emtricitabine, and TDF for oral administration.
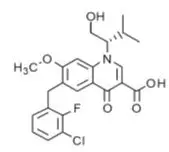
- Elvitegravir is an HIV-1 integrase strand transfer inhibitor.
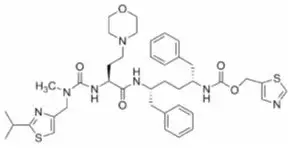
- Cobicistat is a mechanism-based inhibitor of cytochrome P450 (CYP) enzymes of the CYP3A family.
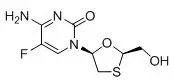
- Emtricitabine is a synthetic nucleoside analog of cytidine. EMTRIVA is the brand name for emtricitabine.
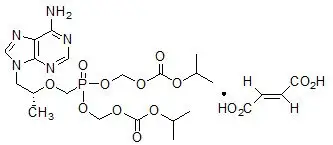
- Tenofovir DF is converted in vivo to tenofovir, an acyclic nucleoside phosphonate (nucleotide) analog of adenosine 5'-monophosphate. VIREAD is the brand name for TDF.
Each tablet contains 150 mg of elvitegravir, 150 mg of cobicistat, 200 mg of emtricitabine, and 300 mg of TDF (equivalent to 245 mg of tenofovir disoproxil). The tablets include the following inactive ingredients: lactose monohydrate, microcrystalline cellulose, silicon dioxide, croscarmellose sodium, hydroxypropyl cellulose, sodium lauryl sulfate, and magnesium stearate. The tablets are film coated with a coating material containing indigo carmine (FD&C Blue #2) aluminum lake, polyethylene glycol, polyvinyl alcohol, talc, titanium dioxide, and yellow iron oxide.
12. Stribild - Clinical Pharmacology
12.1 Mechanism of Action
STRIBILD is a fixed-dose combination of antiretroviral drugs elvitegravir (boosted by the CYP3A inhibitor cobicistat), emtricitabine, and TDF [see Microbiology (12.4)].
12.2 Pharmacodynamics
12.3 Pharmacokinetics
The pharmacokinetic properties of the components of STRIBILD are provided in Table 6. The multiple dose pharmacokinetic parameters of elvitegravir, cobicistat, emtricitabine, and tenofovir are provided in Table 7.
| Elvitegravir | Cobicistat | Emtricitabine | Tenofovir | |
|---|---|---|---|---|
| NC=Not Calculated | ||||
|
||||
| Absorption | ||||
| Tmax (h) | 4 | 3 | 3 | 2 |
| Effect of light meal (relative to fasting)* | ↑34% (↑19, ↑51) | ↑3% (↓10, ↑17) | ↓5% (↓9, 0) | ↑24% (↑18, ↑30) |
| Effect of high fat meal (relative to fasting)* | ↑87% (↑66, ↑110) | ↓17% (↓27, ↓5) | ↓4% (↓8, 0) | ↑23% (↑17, ↑29) |
| Distribution | ||||
| % Bound to human plasma proteins | ~99 | ~98 | <4 | <0.7 |
| Source of protein binding data | Ex vivo | In vitro | In vitro | In vitro |
| Blood-to-plasma ratio | 0.73 | 0.5 | 0.6 | NC |
| Metabolism | ||||
| Metabolism | CYP3A (major) UGT1A1/3 (minor) | CYP3A (major) CYP2D6 (minor) | Not significantly metabolized | |
| Elimination | ||||
| Major route of elimination | Metabolism | Glomerular filtration and active tubular secretion | ||
| T1/2 (h)† | 12.9 | 3.5 | 10 | 12–18 |
| % Of dose excreted in urine‡ | 6.7 | 8.2 | 70 | 70–80 |
| % Of dose excreted in feces‡ | 94.8 | 86.2 | 13.7 | NC |
| Parameter Mean ± SD [range, min:max] | Elvitegravir* | Cobicistat† | Emtricitabine† | Tenofovir† |
|---|---|---|---|---|
| SD=Standard Deviation | ||||
|
||||
| Cmax
(microgram per mL) | 1.7 ± 0.4 [0.4:3.7] | 1.1 ± 0.4 [0.1:2.1] | 1.9 ± 0.5 [0.6:3.6] | 0.45 ± 0.2 [0.2:1.2] |
| AUCtau
(microgram∙hour per mL) | 23.0 ± 7.5 [4.4:69.8] | 8.3 ± 3.8 [0.5:18.3] | 12.7 ± 4.5 [5.2:34.1] | 4.4 ± 2.2 [2.1:18.2] |
| Ctrough
(microgram per mL) | 0.45 ± 0.26 [0.05:2.34] | 0.05 ± 0.13 [0.01:0.92] | 0.14 ± 0.25 [0.04:1.94] | 0.10 ± 0.08 [0.04:0.58] |
12.4 Microbiology
14. Clinical Studies
14.1 Description of Clinical Trials
The efficacy and safety of STRIBILD were evaluated in the studies summarized in Table 13.
| Trial | Population | Study Arms (N)* | Timepoint (Week) |
|---|---|---|---|
|
|||
| Study 102 †,‡ | Adults with no antiretroviral treatment history | STRIBILD (348) ATRIPLA (352) | 144 |
| Study 103 †,‡ | STRIBILD (353) TRUVADA+atazanavir+ritonavir (355) |
||
| Study 115‡,§ | Virologically suppressed adults without a history of virologic failure¶ | STRIBILD (293) TRUVADA+PI+ritonavir (140) | 48 |
| Study 121‡,§ | STRIBILD (291) TRUVADA+NNRTI (143) |
||
| Study 112# | Treatment-naïve adolescents between the ages of 12 to less than 18 years | STRIBILD (50) | 48 |
14.2 Clinical Trial Results in HIV-1 Infected Adult Subjects with No Antiretroviral Treatment History
In Study 102, subjects were randomized in a 1:1 ratio to receive either STRIBILD (N=348) once daily or ATRIPLA (N=352) once daily. The mean age was 38 years (range 18–67), 89% were male, 63% were White, 28% were Black, and 2% were Asian. Twenty-four percent of subjects identified as Hispanic/Latino. The mean baseline plasma HIV-1 RNA was 4.8 log10 copies per mL (range 2.6–6.5). The mean baseline CD4+ cell count was 386 cells per mm3 (range 3–1348), and 13% had CD4+ cell counts less than 200 cells per mm3. Thirty-three percent of subjects had baseline viral loads greater than 100,000 copies per mL.
In Study 103, subjects were randomized in a 1:1 ratio to receive either STRIBILD (N=353) once daily or ATV 300 mg + RTV 100 mg + TRUVADA (N=355) once daily. The mean age was 38 years (range 19–72), 90% were male, 74% were White, 17% were Black, and 5% were Asian. Sixteen percent of subjects identified as Hispanic/Latino. The mean baseline plasma HIV-1 RNA was 4.8 log10 copies per mL (range 1.7–6.6). The mean baseline CD4+ cell count was 370 cells per mm3 (range 5–1132), and 13% had CD4+ cell count less than 200 cells per mm3. Forty-one percent of subjects had baseline viral loads greater than 100,000 copies per mL.
In both studies, subjects were stratified by baseline HIV-1 RNA (less than or equal to 100,000 copies per mL or greater than 100,000 copies per mL).
Treatment outcomes of Study 102 and Study 103 through 144 weeks are presented in Table 14.
| Study 102 | Study 103 | |||
|---|---|---|---|---|
| STRIBILD N=348 | ATRIPLA N=352 | STRIBILD N=353 | ATV+RTV+TRUVADA N=355 |
|
|
||||
| Virologic Success
HIV-1 RNA <50 copies/mL | 80% | 75% | 78% | 75% |
| Treatment Difference | 4.9% (95% CI = −1.3%, 11.1%) | 3.1% (95% CI = −3.2%, 9.4%) | ||
| Virologic Failure† | 7% | 10% | 8% | 7% |
| No Virologic Data in Week 144 Window | ||||
| Discontinued Study Drug Due to AE or Death‡ | 6% | 8% | 6% | 8% |
| Discontinued Study Drug Due to Other Reasons and Last Available HIV-1 RNA <50 copies/mL§ | 5% | 7% | 8% | 9% |
| Missing Data During Window but on Study Drug | 1% | 0% | 1% | 1% |
In Study 102, the mean increase from baseline in CD4+ cell count at Week 144 was 298 cells per mm3 in the STRIBILD-treated subjects and 272 cells per mm3 in the ATRIPLA -treated subjects. In Study 103, the mean increase from baseline in CD4+ cell count at Week 144 was 261 cells per mm3 in the STRIBILD-treated subjects and 269 cells per mm3 in the ATV+RTV+TRUVADA-treated subjects.
14.3 Clinical Trial Results in Virologically Suppressed HIV-1 Infected Adult Subjects with No History of Virologic Failure
In Study 115, subjects had to be on either their first or second antiretroviral regimen with no history of virologic failure, with no current or past history of resistance to the antiretroviral components of STRIBILD, and must have been suppressed (HIV-1 RNA <50 copies/mL) on a ritonavir-boosted PI in combination with TRUVADA for at least 6 months prior to screening. Subjects were randomized in a 2:1 ratio to either switch to STRIBILD (STRIBILD arm, N=293; randomized and dosed) or stay on their baseline antiretroviral regimen for 48 weeks (PI+RTV+TRUVADA arm, N=140; randomized and dosed). Subjects had a mean age of 41 years (range 21–76), 86% were male, 80% were White, and 15% were Black. The mean baseline CD4+ cell count was 610 cells per mm3 (range 74–1919). At screening subjects were receiving atazanavir (40%), darunavir (40%), lopinavir (17%), fosamprenavir (3%), or saquinavir (<1%) as the PI in their regimen.
In Study 121, subjects had to be on either their first or second antiretroviral regimen with no history of virologic failure, with no current or past history of resistance to the antiretroviral components of STRIBILD, and must have been suppressed (HIV-1 RNA <50 copies/mL) on a NNRTI in combination with TRUVADA for at least 6 months prior to screening. Subjects were randomized in a 2:1 ratio to either switch to STRIBILD (STRIBILD arm, N=291; randomized and dosed) or stay on their baseline antiretroviral regimen for 48 weeks (NNRTI+TRUVADA arm, N=143; randomized and dosed). Subjects had a mean age of 41 years (range 20–72); 93% were male, 78% were White, and 17% were Black. The mean baseline CD4+ cell count was 588 cells per mm3 (range 100–1614). Randomization was stratified by use of efavirenz in the baseline regimen. At screening subjects were receiving efavirenz (78%) (predominantly as ATRIPLA [74%]), nevirapine (17%), rilpivirine (4%) (as COMPLERA® [4%]), or etravirine (1%) as the NNRTI in their regimen.
Virologic outcomes of Study 115 and Study 121 are presented in Table 15. Five treated subjects were excluded from the efficacy analysis: in Study 115, three STRIBILD subjects had protocol-prohibited documented resistance and one PI+RTV+TRUVADA subject was not on a protease inhibitor-based regimen at screening; in Study 121, one STRIBILD subject had protocol-prohibited documented resistance.
| Study GS-US-236-0115* | Study GS-US-236-0121* | |||
|---|---|---|---|---|
| STRIBILD N=290 | PI+RTV+TRUVADA N=139 | STRIBILD N=290 | NNRTI+TRUVADA N=143 |
|
|
||||
| Virologic Success HIV-1 RNA <50 copies/mL | 94% | 87% | 93% | 88% |
| Virologic Failure† | 1% | 1% | 1% | 1% |
| No Virologic Data in Week 48 Window | 6% | 12% | 6% | 11% |
| Discontinued Study Drug Due to AE or Death‡ | 2% | 1% | 2% | 1% |
| Discontinued Study Drug Due to Other Reasons and Last Available HIV-1 RNA <50 copies/mL§ | 4% | 10% | 4% | 9% |
| Missing Data During Window but on Study Drug | 0% | 0% | 0% | 1% |
14.4 Clinical Trial Results in HIV-1Treatment-Naïve Adolescent Subjects Aged 12 to Less than 18 Years
In Study 112, the efficacy, safety, and pharmacokinetics of STRIBILD were evaluated in a single group, open-label trial in HIV-1 infected treatment-naïve adolescents aged 12 to less than 18 years of age and weighing at least 35 kg (N=50). Mean age was 15 years (range 12–17); 70% were male, 68% black, and 28% Asian. At baseline, mean plasma HIV-1 RNA was 4.60 log10 copies per mL (range 3.18–5.73), mean CD4+ cell count was 399 cells per mm3 (range 133–734), and mean CD4+ percentage was 20.9% (range 4.5%–41.1%). Twenty percent had baseline plasma HIV-1 RNA >100,000 copies per mL.
At Week 48, 44 of 50 (88%) adolescent patients treated with STRIBILD achieved HIV-1 RNA <50 copies per mL and 4 had HIV-1 RNA ≥50 copies per mL; 1 patient discontinued study drug; 1 had no virologic data at Week 48. The mean decrease from baseline in HIV-1 RNA was −3.16 log10 copies per mL; mean increase from baseline in CD4+ cell count was 229 cells per mm3. No emergent resistance to STRIBILD was detected through Week 48.
16. How is Stribild supplied
STRIBILD tablets are green, capsule shaped, film coated, and debossed with "GSI" on one side and the number "1" surrounded by a square box (  ) on the other side. Each bottle contains 30 tablets (NDC 61958-1201-1) and a silica gel desiccant, and is closed with a child-resistant closure.
) on the other side. Each bottle contains 30 tablets (NDC 61958-1201-1) and a silica gel desiccant, and is closed with a child-resistant closure.
17. Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Patient Information).
| This Patient Information has been approved by the U.S. Food and Drug Administration. | Revised: 09/2021 | |
| PATIENT INFORMATION
STRIBILD® (STRY-bild) (elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate) tablets |
||
|
Important: Ask your healthcare provider or pharmacist about medicines that should not be taken with STRIBILD. For more information, see the section "What should I tell my healthcare provider before taking STRIBILD?" |
||
|
What is the most important information I should know about STRIBILD? STRIBILD can cause serious side effects, including:
See "What are the possible side effects of STRIBILD?" for more information about side effects. |
||
|
What is STRIBILD? STRIBILD is a prescription medicine that is used without other antiretroviral medicines to treat Human Immunodeficiency Virus-1 (HIV-1) in people 12 years of age and older:
HIV-1 is the virus that causes AIDS (Acquired Immune Deficiency Syndrome). STRIBILD contains the medicines elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate. It is not known if STRIBILD is safe and effective in children under 12 years of age or who weigh less than 77 lbs. |
||
|
Do not take STRIBILD if you also take a medicine that contains:
|
||
|
What should I tell my healthcare provider before taking STRIBILD? Before taking STRIBILD, tell your healthcare provider about all of your medical conditions, including if you:
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Some medicines may interact with STRIBILD. Keep a list of your medicines and show it to your healthcare provider and pharmacist when you get a new medicine.
|
||
|
How should I take STRIBILD?
|
||
|
What are the possible side effects of STRIBILD? STRIBILD may cause the following serious side effects, including:
The most common side effects of STRIBILD include: |
||
|
|
|
|
These are not all the possible side effects of STRIBILD. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
||
|
How should I store STRIBILD?
Keep STRIBILD and all medicines out of reach of children. |
||
|
General information about the safe and effective use of STRIBILD. Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use STRIBILD for a condition for which it was not prescribed. Do not give STRIBILD to other people, even if they have the same symptoms you have. It may harm them. You can ask your healthcare provider or pharmacist for information about STRIBILD that is written for health professionals. For more information, call 1-800-445-3235 or go to www.STRIBILD.com. |
||
|
What are the ingredients in STRIBILD? Active ingredients: elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate Inactive ingredients: lactose monohydrate, microcrystalline cellulose, silicon dioxide, croscarmellose sodium, hydroxypropyl cellulose, sodium lauryl sulfate, and magnesium stearate. The tablets are film coated with a coating material containing indigo carmine (FD&C blue #2) aluminum lake, polyethylene glycol, polyvinyl alcohol, talc, titanium dioxide, and yellow iron oxide. Manufactured and distributed by: Gilead Sciences, Inc. Foster City, CA 94404 |
||
| STRIBILD
elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate tablet, film coated |
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
| Labeler - Gilead Sciences, Inc. (185049848) |