Drug Detail:Sylvant (Siltuximab [ sil-tux-i-mab ])
Drug Class: Interleukin inhibitors
Highlights of Prescribing Information
SYLVANT (siltuximab) for injection, for intravenous use
Initial U.S. Approval: 2014
Recent Major Changes
| Dosage and Administration (2.2) | 05/2018 |
| Contraindications (4) | 05/2018 |
| Warnings and Precautions, Infusion Related Reactions and Hypersensitivity (5.3) | 05/2018 |
Indications and Usage for Sylvant
SYLVANT is an interleukin-6 (IL-6) antagonist indicated for the treatment of patients with multicentric Castleman's disease (MCD) who are human immunodeficiency virus (HIV) negative and human herpesvirus-8 (HHV-8) negative. (1)
Limitations of Use
SYLVANT was not studied in patients with MCD who are HIV positive or HHV-8 positive because SYLVANT did not bind to virally produced IL-6 in a nonclinical study.
Sylvant Dosage and Administration
For intravenous infusion only.
Administer as an 11 mg/kg dose given over 1 hour by intravenous infusion every 3 weeks. (2)
Dosage Forms and Strengths
For injection
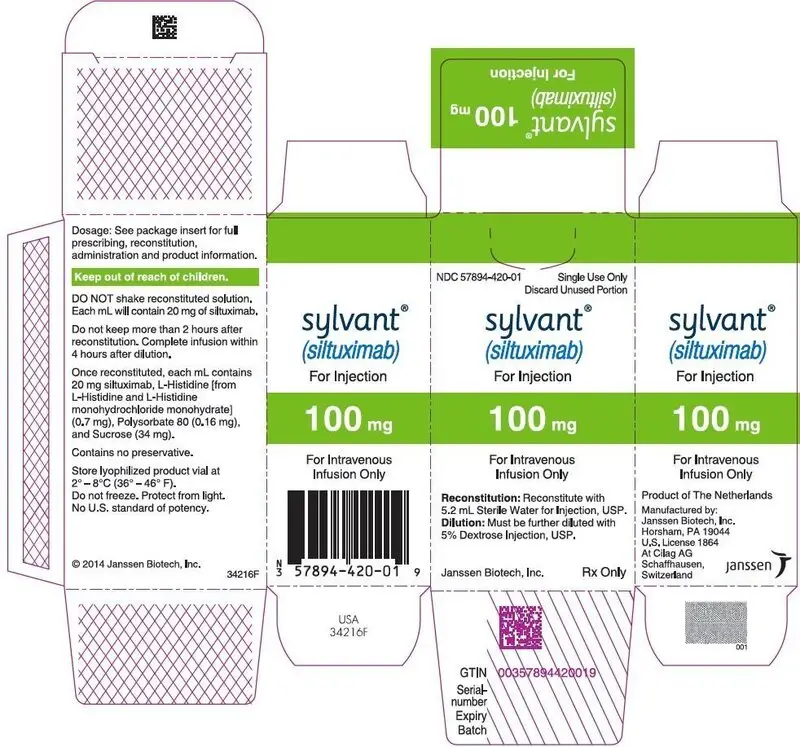
- 100 mg of lyophilized powder in a single-dose vial. (3)
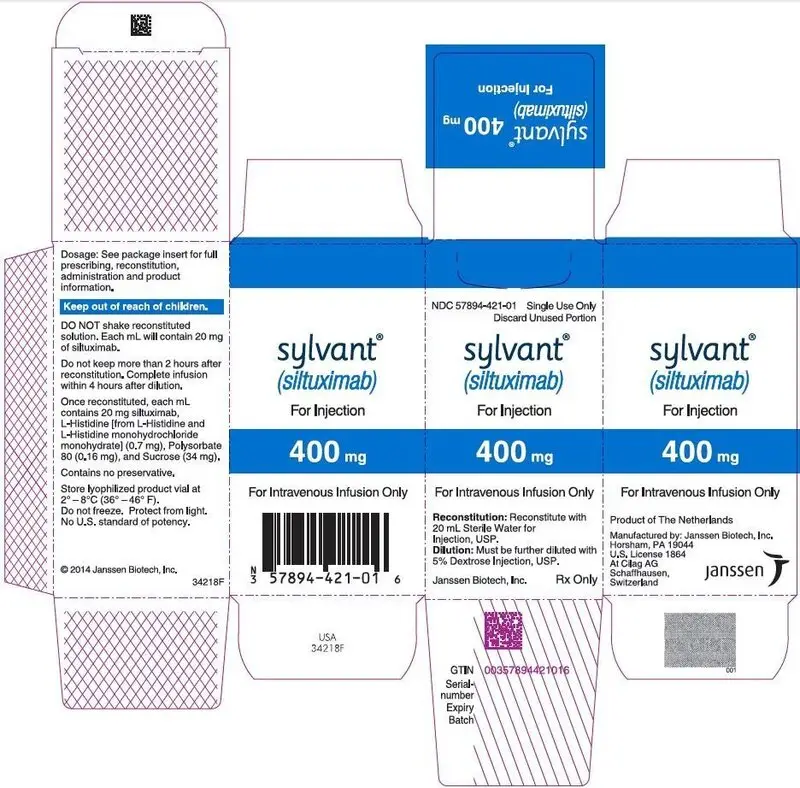
- 400 mg of lyophilized powder in a single-dose vial. (3)
Contraindications
Severe hypersensitivity reaction to siltuximab or any of the excipients in SYLVANT. (4)
Warnings and Precautions
- Concurrent Active Severe Infections: Do not administer SYLVANT to patients with severe infections, monitor for infections, institute prompt treatment, and interrupt SYLVANT until resolution of infection. (2, 5.1)
- Vaccinations: Do not administer live vaccines because IL-6 inhibition may interfere with the normal immune response to new antigens. (5.2)
- Infusion Related Reactions: Administer SYLVANT in a setting that provides resuscitation equipment, medication, and personnel trained to provide resuscitation. (5.3, 6.1)
- Gastrointestinal (GI) perforation: Promptly evaluate patients presenting with symptoms that may be associated or suggestive of GI perforation. (5.4)
Adverse Reactions/Side Effects
The most common adverse reactions (>10% of patients) were rash, pruritus, upper respiratory tract infection, increased weight, and hyperuricemia. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Janssen Biotech, Inc. at 1-800-526-7736 (1-800-JANSSEN) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. (6)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 5/2018
Related/similar drugs
siltuximabFull Prescribing Information
1. Indications and Usage for Sylvant
SYLVANT is indicated for the treatment of patients with multicentric Castleman's disease (MCD) who are human immunodeficiency virus (HIV) negative and human herpesvirus-8 (HHV-8) negative.
2. Sylvant Dosage and Administration
2.1 Dosage
Administer SYLVANT 11 mg/kg over 1 hour as an intravenous infusion every 3 weeks until treatment failure.
Perform hematology laboratory tests prior to each dose of SYLVANT therapy for the first 12 months and every 3 dosing cycles thereafter. If treatment criteria outlined in Table 1 are not met, consider delaying treatment with SYLVANT. Do not reduce dose.
| Laboratory parameter | Requirements before first SYLVANT administration | Retreatment criteria |
|---|---|---|
|
||
| Absolute Neutrophil Count | ≥1.0 × 109/L | ≥1.0 × 109/L |
| Platelet count | ≥75 × 109/L | ≥50 × 109/L |
| Hemoglobin* | <17 g/dL | <17 g/dL |
Do not administer SYLVANT to patients with severe infections until the infection resolves.
Discontinue SYLVANT in patients with severe infusion related reactions, anaphylaxis, severe allergic reactions, or cytokine release syndromes. Do not reinstitute treatment.
2.2 Instructions for Preparation and Administration
Use aseptic technique for reconstitution and preparation of dosing solution.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
1. Calculate the dose (mg), total volume (mL) of reconstituted SYLVANT solution required and the number of vials needed. A 21-gauge 1½ inch needle is recommended for preparation. Infusion bags (250 mL) must contain Dextrose 5% in Water and must be made of polyvinyl chloride (PVC), or polyolefin (PO), or polypropylene (PP), or polyethylene (PE). Alternatively PE bottles may be used.
2. Allow the vial(s) of SYLVANT to come to room temperature over approximately 30 minutes. SYLVANT should remain at room temperature for the duration of the preparation.
3. Aseptically reconstitute each SYLVANT vial as instructed in Table 2.
| Strength | Amount of Sterile Water for Injection, USP required for reconstitution | Post-reconstitution concentration |
|---|---|---|
| 100 mg vial | 5.2 mL | 20 mg/mL |
| 400 mg vial | 20 mL | 20 mg/mL |
Gently swirl the reconstituted vials to aid the dissolution of the lyophilized powder. DO NOT SHAKE or SWIRL VIGOROUSLY. Do not remove the contents until all of the solids have been completely dissolved. The lyophilized powder should dissolve in less than 60 minutes.
Once reconstituted, and prior to further dilution, inspect the vials for particulates and discoloration. Do not use if particles or solution discoloration are present or if visibly opaque. The reconstituted product should be kept for no more than two hours prior to addition into the infusion bag.
4. Dilute the reconstituted SYLVANT solution dose to 250 mL with sterile Dextrose 5% in Water by withdrawing a volume equal to the total calculated volume of reconstituted SYLVANT from the Dextrose 5% in Water, 250 mL bag. Slowly add the total calculated volume (mL) of reconstituted SYLVANT solution to the Dextrose 5% in Water infusion bag. Gently invert the bag to mix the solution.
5. Administer the diluted SYLVANT solution in 5% Dextrose in Water 250 mL by intravenous infusion over a period of 1 hour using administration sets lined with PVC, or polyurethane (PU), or PE, containing a 0.2-micron inline polyethersulfone (PES) filter. The infusion should be completed within 4 hours of the dilution of the reconstituted solution to the infusion bag.
6. Do not infuse SYLVANT concomitantly in the same intravenous line with other agents.
7. SYLVANT does not contain preservatives. Do not store any unused portion of the reconstituted product or of the infusion solution. Waste material should be disposed of in accordance with local requirements.
3. Dosage Forms and Strengths
SYLVANT (siltuximab) for injection is available as:
100 mg of lyophilized powder in a single-dose vial for intravenous infusion.
400 mg of lyophilized powder in a single-dose vial for intravenous infusion.
4. Contraindications
Severe hypersensitivity reaction to siltuximab or any of the excipients in SYLVANT [see Warnings and Precautions (5.3)]. Hypersensitivity reactions, including anaphylactic reaction, hypersensitivity, and drug hypersensitivity have been reported in patients treated with siltuximab.
5. Warnings and Precautions
5.1 Concurrent Active Severe Infections
Do not administer SYLVANT to patients with severe infections until the infection resolves. SYLVANT may mask signs and symptoms of acute inflammation including suppression of fever and of acute Phase reactants such as C-reactive protein (CRP). Monitor patients receiving SYLVANT closely for infections. Institute prompt anti-infective therapy and do not administer further SYLVANT until the infection resolves.
5.2 Vaccinations
Do not administer live vaccines to patients receiving SYLVANT because IL-6 inhibition may interfere with the normal immune response to new antigens.
5.3 Infusion Related Reactions and Hypersensitivity
SYLVANT may cause infusion related reactions and anaphylaxis. Approximately 945 patients have been treated with SYLVANT in clinical trials. Of these, one patient experienced an anaphylactic reaction. Data from 254 patients treated with SYLVANT monotherapy forms the basis of the safety evaluation of infusion related reactions. Infusion related reactions were reported in 5.1% of these patients. Two (0.8%) were Grade 3 or higher, and 1 (0.4%) was serious; none were fatal. Symptoms of infusion reactions consisted of back pain, chest pain or discomfort, nausea and vomiting, flushing, erythema, and palpitations.
In long-term treatment of MCD patients with siltuximab at the recommended dosage of 11 mg/kg every 3 weeks, infusion related reactions or hypersensitivity reactions occurred at a frequency of 6.3% (1.3% for severe reactions).
Stop the infusion of SYLVANT if the patient develops signs of anaphylaxis. Discontinue further therapy with SYLVANT.
Stop the infusion if the patient develops a mild to moderate infusion reaction. If the reaction resolves, the SYLVANT infusion may be restarted at a lower infusion rate. Consider medication with antihistamines, acetaminophen, and corticosteroids. Discontinue SYLVANT if the patient does not tolerate the infusion following these interventions [see Adverse Reactions (6)].
Administer SYLVANT in a setting that provides resuscitation equipment, medication, and personnel trained to provide resuscitation.
5.4 Gastrointestinal Perforation
Gastrointestinal (GI) perforation has been reported in clinical trials although not in MCD trials. Use with caution in patients who may be at increased risk for GI perforation. Promptly evaluate patients presenting with symptoms that may be associated or suggestive of GI perforation.
6. Adverse Reactions/Side Effects
The following adverse reactions are also discussed in other sections of the labeling:
- Concurrent active severe infections [see Warnings and Precautions (5.1)]
- Infusion-related reactions and hypersensitivity [see Warnings and Precautions (5.3)]
- Gastrointestinal perforation [see Warnings and Precautions (5.4)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
Study 1, in MCD, was an international, multicenter, randomized Phase 2 study of every 3 week infusions comparing SYLVANT and best supportive care (BSC) to placebo and BSC. There were 53 patients randomized to the SYLVANT arm at a dosage of 11 mg/kg and 26 patients randomized to the placebo arm. Of the 26 placebo-treated patients, 13 patients subsequently crossed-over to receive SYLVANT. The median age was 48 years (range 20 to 78), 66% male, 48% Asian, 39% White, 4% Black or African American, 7% other. The patients randomized to SYLVANT received a median of 19 infusions (range 1 to 50) compared to patients randomized to placebo who received a median of 8 infusions (range 2 to 32). To control for disparate exposure between arms, Table 3 reports the per patient incidence of adverse reactions that occurred during the first 8 infusions. Adverse reactions that occurred >3% in the SYLVANT arm are presented.
The most common adverse reactions (> 10% compared to placebo) during treatment with SYLVANT in the MCD clinical trial were rash, pruritus, upper respiratory tract infection, increased weight, and hyperuricemia.
| Body System/Adverse Reactions | SYLVANT+BSC*
n=53 | Placebo+BSC n=26 |
||
|---|---|---|---|---|
| All Grades | Grades 3–4 | All Grades | Grades 3–4 | |
|
||||
| Skin disorders | ||||
| Rash (rash, rash generalized, rash maculo-papular, rash popular and rash pruritic) | 15 (28%) | 1 (2%) | 3 (12%) | 0 |
| Pruritus | 15 (28%) | 0 | 2 (8%) | 0 |
| Skin hyperpigmentation | 2 (4%) | 0 | 0 | 0 |
| Eczema | 2 (4%) | 0 | 0 | 0 |
| Psoriasis | 2 (4%) | 0 | 0 | 0 |
| Dry skin | 2 (4%) | 0 | 0 | 0 |
| Infections | ||||
| Lower respiratory tract | 4 (8%) | 2 (4%) | 1 (4%) | 1 (4%) |
| Upper respiratory tract | 14 (26%) | 1 (2%) | 4 (15%) | 1 (4%) |
| Blood and lymphatic system disorders | ||||
| Thrombocytopenia | 5 (9%) | 2 (4%) | 1 (4%) | 1 (4%) |
| General disorders | ||||
| Edema (general and localized) | 14 (26%) | 4 (8%) | 7 (27%) | 0 |
| Gastrointestinal disorders | ||||
| Constipation | 4 (8%) | 0 | 1 (4%) | 0 |
| Metabolism | ||||
| Hypertriglyceridemia | 4 (8%) | 0 | 0 | 0 |
| Hypercholesterolemia | 2 (4%) | 0 | 0 | 0 |
| Hyperuricemia | 6 (11%) | 1 (2%) | 0 | 0 |
| Respiratory, thoracic and mediastinal disorders | ||||
| Oropharyngeal pain | 4 (8%) | 0 | 1 (4%) | 0 |
| Renal and urinary disorders | ||||
| Renal impairment | 4 (8%) | 0 | 0 | 0 |
| Nervous system disorders | ||||
| Headache | 4 (8%) | 0 | 1 (4%) | 0 |
| Investigations | ||||
| Weight increased | 10 (19%) | 1 (2%) | 0 | 0 |
| Vascular disorders | ||||
| Hypotension | 2 (4%) | 1 (2%)† | 0 | 0 |
Study CNTO328MCD2002 (referred to as Study 2) (NCT01400503) was an open label, long term extension study of patients with MCD treated on prior trials. The median duration of siltuximab treatment was 5.52 years (range: 0.8 to 10.8 years); more than 50% of patients received siltuximab treatment for ≥5 years. The rate of serious or Grade ≥3 adverse events did not increase over time as a function of cumulative exposure.
Other important adverse reactions reported in MCD clinical studies, all of which were very common, were:
Infections and infestations: nasopharyngitis, urinary tract infection
Blood and lymphatic system disorders: neutropenia
Nervous system disorders: dizziness
Vascular disorders: hypertension
Gastrointestinal disorders: nausea, abdominal pain, vomiting, diarrhea, gastroesophageal reflux disease, mouth ulceration
7. Drug Interactions
7.1 Cytochrome P450 Substrates
Cytochrome P450s in the liver are down regulated by infection and inflammation stimuli including cytokines such as IL-6. Inhibition of IL-6 signaling in patients treated with SYLVANT may restore CYP450 activities to higher levels leading to increased metabolism of drugs that are CYP450 substrates compared to metabolism prior to treatment with SYLVANT.
Upon initiation or discontinuation of SYLVANT, in patients being treated with CYP450 substrates with a narrow therapeutic index, perform therapeutic monitoring of effect (e.g., warfarin) or drug concentration (e.g., cyclosporine or theophylline) as needed and adjust dose. The effect of SYLVANT on CYP450 enzyme activity can persist for several weeks after stopping therapy. Exercise caution when SYLVANT is co-administered with CYP3A4 substrate drugs where a decrease in effectiveness would be undesirable (e.g., oral contraceptives, lovastatin, atorvastatin).
8. Use In Specific Populations
8.3 Nursing Mothers
It is not known whether siltuximab is excreted in human milk or absorbed systemically after ingestion. Because many drugs and immunoglobulins are excreted in human milk, and because of the potential for adverse reactions in nursing infants from SYLVANT, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
8.4 Pediatric Use
The safety and efficacy of SYLVANT have not been established in pediatric patients.
8.5 Geriatric Use
Of the patients treated with SYLVANT monotherapy in clinical studies 127 (35%) were 65 years and older. No overall differences in safety profile were observed between these patients and younger patients, and other reported clinical experience has not identified differences in the safety profile between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out. Clinical studies did not include sufficient numbers of patients aged 65 years and older to determine the effect of age on efficacy in MCD population.
8.6 Patients with Renal Impairment
Based on a population pharmacokinetic analysis using data from clinical trials in patients, no significant difference in siltuximab clearance was observed in patients with pre-existing renal impairment (creatinine clearance (CLCr) ≥ 15 mL/min) compared to patients with baseline normal renal function (CLCr ≥ 90 mL/min). No initial dosage adjustment is necessary for patients with CLCr ≥ 15 mL/min. The potential effect of end stage renal disease on siltuximab pharmacokinetics cannot be determined [see Clinical Pharmacology (12.3)].
8.7 Patients with Hepatic Impairment
Based on a population pharmacokinetic analysis using data from clinical trials in patients, no significant difference in siltuximab clearance was observed in patients with pre-existing mild to moderate hepatic impairment (Child-Pugh Class A and B, respectively) compared to patients with baseline normal hepatic function. No initial dosage adjustment is necessary for patients with mild to moderate hepatic impairment. Patients with baseline severe hepatic impairment (Child-Pugh Class C) were not included in clinical trials [see Clinical Pharmacology (12.3)].
11. Sylvant Description
Siltuximab is a human-mouse chimeric monoclonal antibody that binds human interleukin-6 (IL-6) and is produced by Chinese hamster ovary cells.
SYLVANT (siltuximab) for injection is supplied as a sterile, white, preservative free, lyophilized powder in single-dose vials.
Each SYLVANT 100 mg single-dose vial contains 100 mg siltuximab, 3.7 mg L-histidine (from L-histidine and L-histidine monohydrochloride monohydrate), 0.8 mg polysorbate 80, and 169 mg sucrose.
Each SYLVANT 400 mg single-dose vial contains 400 mg siltuximab, 14.9 mg L-histidine (from L-histidine and L-histidine monohydrochloride monohydrate), 3.2 mg polysorbate 80, and 677 mg sucrose.
Following reconstitution with Sterile Water for Injection, USP (per section 2.2), the resulting pH is approximately 5.2. The resulting solution contains 20 mg/mL siltuximab to be administered by intravenous infusion following dilution [see Dosage and Administration (2.2)].
12. Sylvant - Clinical Pharmacology
12.1 Mechanism of Action
Siltuximab binds human IL-6 and prevents the binding of IL-6 to both soluble and membrane-bound IL-6 receptors. IL-6 has been shown to be involved in diverse normal physiologic processes such as induction of immunoglobulin secretion. Overproduction of IL-6 has been linked to systemic manifestations in patients with MCD.
12.3 Pharmacokinetics
The pharmacokinetics of siltuximab were evaluated in patients with multicentric Castleman's disease and hematological and non-hematological malignancies. The serum siltuximab pharmacokinetics are adequately described by a linear two-compartment intravenous model with first-order elimination.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
No carcinogenicity or genotoxicity studies have been conducted with siltuximab.
Two fertility studies were conducted. In one study, drug-treated male mice were mated with untreated females and in the second study drug-treated female mice were mated with untreated males. A murine analog of siltuximab was administered subcutaneously at doses up to 100 mg/kg/week for a total of 7 doses in both studies. There was no effect on male or female fertility parameters. In addition, siltuximab did not produce any toxicity in the reproductive organs in cynomolgus monkeys in the 6-month repeat-dose toxicology study at doses up to 46 mg/kg (approximately 7 times) the systemic exposure in patients at the recommended dose.
16. How is Sylvant supplied
| SYLVANT
siltuximab injection, powder, lyophilized, for solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| SYLVANT
siltuximab injection, powder, lyophilized, for solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Janssen Biotech, Inc. (099091753) |
| Registrant - Janssen Pharmaceuticals, Inc. (063137772) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Janssen Biologics BV | 409612918 | API MANUFACTURE(57894-420, 57894-421) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Janssen Sciences Ireland UC | 986030167 | API MANUFACTURE(57894-420, 57894-421) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Cilag AG | 483237103 | MANUFACTURE(57894-420, 57894-421) | |