Drug Detail:Symdeko (Ivacaftor and tezacaftor [ eye-va-kaf-tor-and-tez-a-kaf-tor ])
Drug Class: CFTR combinations
Highlights of Prescribing Information
SYMDEKO® (tezacaftor/ivacaftor) tablets; (ivacaftor) tablets, for oral use
Initial U.S. Approval: 2018
Recent Major Changes
| Warnings and Precautions, Hypersensitivity Reactions, Including Anaphylaxis (5.2) | 08/2023 |
Indications and Usage for Symdeko
SYMDEKO is a combination of tezacaftor and ivacaftor, indicated for the treatment of cystic fibrosis (CF) in patients age 6 years and older who are homozygous for the F508del mutation or who have at least one mutation in the cystic fibrosis transmembrane conductance regulator (CFTR) gene that is responsive to tezacaftor/ivacaftor based on in vitro data and/or clinical evidence. (12.1, 14)
If the patient's genotype is unknown, an FDA-cleared CF mutation test should be used to detect the presence of a CFTR mutation followed by verification with bi-directional sequencing when recommended by the mutation test instructions for use.
Symdeko Dosage and Administration
- Pediatric patients age 6 to less than 12 years weighing less than 30 kg: one tablet (containing tezacaftor 50 mg/ivacaftor 75 mg) in the morning and one tablet (containing ivacaftor 75 mg) in the evening, approximately 12 hours apart. SYMDEKO should be taken with fat-containing food. (2.1, 2.2, 12.3)
- Adults and pediatric patients age 12 years and older or pediatric patients age 6 to less than 12 years weighing 30 kg or more: one tablet (containing tezacaftor 100 mg/ivacaftor 150 mg) in the morning and one tablet (containing ivacaftor 150 mg) in the evening, approximately 12 hours apart. SYMDEKO should be taken with fat-containing food. (2.1, 2.2, 12.3)
- Reduce dose in patients with moderate and severe hepatic impairment. (2.3, 8.6, 12.3)
- See full prescribing information for dosage modifications due to drug interactions with SYMDEKO. (2.4, 7.2, 12.3)
Dosage Forms and Strengths
- Tablets: tezacaftor 50 mg/ivacaftor 75 mg fixed-dose combination tablets co-packaged with ivacaftor 75 mg tablets. (3)
- Tablets: tezacaftor 100 mg/ivacaftor 150 mg fixed-dose combination tablets co-packaged with ivacaftor 150 mg tablets. (3)
Contraindications
- None. (4)
Warnings and Precautions
- Elevated transaminases (ALT or AST): Transaminases (ALT and AST) should be assessed prior to initiating SYMDEKO, every 3 months during the first year of treatment, and annually thereafter. In patients with a history of transaminase elevations, more frequent monitoring should be considered. Dosing should be interrupted in patients with significant elevations of transaminases, e.g., patients with ALT or AST >5 × upper limit of normal (ULN), or ALT or AST >3 × ULN with bilirubin >2 × ULN. Following resolution of transaminase elevations, consider the benefits and risks of resuming treatment. (5.1, 6)
- Hypersensitivity reactions: Anaphylaxis has been reported with SYMDEKO in the postmarketing setting. Initiate appropriate therapy in the event of a hypersensitivity reaction. (5.2)
- Use with CYP3A inducers: Concomitant use with strong CYP3A inducers (e.g., rifampin, St. John's wort) substantially decrease exposure of ivacaftor and may decrease the exposure of tezacaftor, which may reduce therapeutic effectiveness. Therefore, co-administration is not recommended. (5.3, 7.1, 12.3)
- Cataracts: Non-congenital lens opacities/cataracts have been reported in pediatric patients treated with SYMDEKO. Baseline and follow-up examinations are recommended in pediatric patients initiating SYMDEKO treatment. (5.4, 8.4)
Adverse Reactions/Side Effects
The most common adverse drug reactions to SYMDEKO (occurring in ≥3% of patients) were headache, nausea, sinus congestion, and dizziness. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Vertex Pharmaceuticals Incorporated at 1-877-634-8789 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
CYP3A inhibitors: Reduce SYMDEKO dose when co-administered with strong (e.g., ketoconazole) or moderate (e.g., fluconazole) CYP3A inhibitors. Avoid food containing grapefruit. (2.4, 7.2, 12.3)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 8/2023
Full Prescribing Information
1. Indications and Usage for Symdeko
SYMDEKO is indicated for the treatment of cystic fibrosis (CF) in patients age 6 years and older who are homozygous for the F508del mutation or who have at least one mutation in the cystic fibrosis transmembrane conductance regulator (CFTR) gene that is responsive to tezacaftor/ivacaftor based on in vitro data and/or clinical evidence [see Clinical Pharmacology (12.1) and Clinical Studies (14)].
If the patient's genotype is unknown, an FDA-cleared CF mutation test should be used to detect the presence of a CFTR mutation followed by verification with bi-directional sequencing when recommended by the mutation test instructions for use.
2. Symdeko Dosage and Administration
2.1 General Dosing Information
Swallow the tablets whole.
SYMDEKO should be taken with fat-containing food, such as food recommended in standard nutritional guidelines. Examples of meals or snacks that contain fat are those prepared with butter or oils or those containing eggs, cheeses, nuts, whole milk, or meats, etc. [see Clinical Pharmacology (12.3)].
2.2 Recommended Dosage in Adults, Adolescents, and Children Age 6 Years and Older
Adults, adolescents, and children age 6 years and older should be dosed according to Table 1. The morning and the evening dose should be taken approximately 12 hours apart.
| Age | Morning (one tablet) | Evening (one tablet) |
|---|---|---|
| 6 to <12 years weighing <30 kg | tezacaftor 50 mg/ivacaftor 75 mg | ivacaftor 75 mg |
| 6 to <12 years weighing ≥30 kg | tezacaftor 100 mg/ivacaftor 150 mg | ivacaftor 150 mg |
| ≥12 years | tezacaftor 100 mg/ivacaftor 150 mg | ivacaftor 150 mg |
2.3 Recommended Dosage for Patients with Hepatic Impairment
For dose adjustment for patients with hepatic impairment, refer to Table 2.
Studies have not been conducted in patients with severe hepatic impairment (Child-Pugh Class C), but exposure of tezacaftor and ivacaftor is expected to be higher than in patients with moderate hepatic impairment. Therefore, SYMDEKO should be used with caution at an adjusted dose after weighing the risks and benefits of treatment in these patients [see Use in Specific Populations (8.6), Clinical Pharmacology (12.3), and Patient Counseling Information (17)].
| Hepatic Impairment | Morning | Evening | |
|---|---|---|---|
| Patients Age 6 to <12 Years Weighing <30kg | Patients Age 6 to <12 Years Weighing ≥30 kg and Patients Age ≥12 Years | All Patients | |
| Mild (Child-Pugh Class A) | No dose adjustment | No dose adjustment | No dose adjustment |
| Moderate (Child-Pugh Class B) | One tablet of tezacaftor 50 mg/ivacaftor 75 mg once daily | One tablet of tezacaftor 100 mg/ivacaftor 150 mg once daily | No ivacaftor dose |
| Severe (Child-Pugh Class C) | One tablet of tezacaftor 50 mg/ivacaftor 75 mg once daily (or less frequently) | One tablet of tezacaftor 100 mg/ivacaftor 150 mg once daily (or less frequently) |
|
2.4 Dosage Adjustment for Patients Taking Drugs that are CYP3A Inhibitors
The dosing regimen of SYMDEKO should be adjusted when co-administered with moderate and strong CYP3A inhibitors.
Moderate CYP3A inhibitors:
When co-administered with moderate CYP3A inhibitors (e.g., fluconazole, erythromycin), the dosing regimen should be adjusted as in Table 3 [see Drug Interactions (7.2), Clinical Pharmacology (12.3), and Patient Counseling Information (17)].
| Day 1 | Day 2 | Day 3 | Day 4* | |
|---|---|---|---|---|
|
||||
| Patients Age 6 to <12 Years Weighing <30 kg | ||||
| Morning | ||||
| Tezacaftor 50 mg/ivacaftor 75 mg tablet | ✓ | - | ✓ | - |
| Ivacaftor 75 mg tablet | - | ✓ | - | ✓ |
| Evening | ||||
| Ivacaftor 75 mg tablet | - | - | - | - |
| Patients Age 6 to <12 Years Weighing ≥30 kg and Patients Age ≥12 Years | ||||
| Morning | ||||
| Tezacaftor 100 mg/ivacaftor 150 mg tablet | ✓ | - | ✓ | - |
| Ivacaftor 150 mg tablet | - | ✓ | - | ✓ |
| Evening | ||||
| Ivacaftor 150 mg tablet | - | - | - | - |
Strong CYP3A inhibitors:
When co-administered with strong CYP3A inhibitors (e.g., ketoconazole, itraconazole, posaconazole, voriconazole, telithromycin, and clarithromycin), the dosing regimen should be adjusted as in Table 4 [see Drug Interactions (7.2), Clinical Pharmacology (12.3), and Patient Counseling Information (17)].
| Day 1 | Day 2 and Day 3 | Day 4* | |
|---|---|---|---|
|
|||
| Patients Age 6 to <12 Years Weighing <30 kg | |||
| Morning | |||
| Tezacaftor 50 mg/ivacaftor 75 mg tablet | ✓ | - | ✓ |
| Evening† | |||
| Ivacaftor 75 mg tablet | - | - | - |
| Patients Age 6 to <12 Years Weighing ≥30 kg and Patients Age ≥12 Years | |||
| Morning | |||
| Tezacaftor 100 mg/ivacaftor 150 mg tablet | ✓ | - | ✓ |
| Evening† | |||
| Ivacaftor 150 mg tablet | - | - | - |
Food or drink containing grapefruit should be avoided during treatment with SYMDEKO [see Drug Interactions (7.2) and Patient Counseling Information (17)].
3. Dosage Forms and Strengths
Tablets: Tezacaftor 50 mg/ivacaftor 75 mg fixed-dose combination tablets co-packaged with ivacaftor 75 mg tablets
- Tezacaftor 50 mg/ivacaftor 75 mg tablets are white, capsule-shaped, and debossed with "V50" on one side and plain on the other.
- Ivacaftor 75 mg tablets are light blue, capsule-shaped, and printed with "V 75" in black ink on one side and plain on the other.
Tablets: Tezacaftor 100 mg/ivacaftor 150 mg fixed-dose combination tablets co-packaged with ivacaftor 150 mg tablets
- Tezacaftor 100 mg/ivacaftor 150 mg tablets are yellow, capsule-shaped, and debossed with "V100" on one side and plain on the other.
- Ivacaftor 150 mg tablets are light blue, capsule-shaped, and printed with "V 150" in black ink on one side and plain on the other.
5. Warnings and Precautions
5.1 Transaminase (AST/ALT) Elevations
Elevated transaminases have been observed in patients with CF treated with SYMDEKO, as well as with ivacaftor monotherapy. Assessments of transaminases (ALT and AST) are recommended for all patients prior to initiating SYMDEKO, every 3 months during the first year of treatment, and annually thereafter. For patients with a history of transaminase elevations more frequent monitoring should be considered. In the event of significant elevations of transaminases, e.g., patients with ALT or AST >5 × upper limit of normal (ULN), or ALT or AST >3 × ULN with bilirubin >2 × ULN, dosing should be interrupted and laboratory tests closely followed until the abnormalities resolve. Following the resolution of transaminase elevations consider the benefits and risks of resuming treatment [see Adverse Reactions (6)].
5.2 Hypersensitivity Reactions, Including Anaphylaxis
Hypersensitivity reactions, including cases of anaphylaxis, have been reported in the postmarketing setting [see Adverse Reactions (6.2)]. If signs or symptoms of serious hypersensitivity reactions develop during treatment, discontinue SYMDEKO and institute appropriate therapy. Consider the benefits and risks for the individual patient to determine whether to resume treatment with SYMDEKO.
5.3 Concomitant Use with CYP3A Inducers
Exposure to ivacaftor is significantly decreased and exposure to tezacaftor may be reduced by the concomitant use of CYP3A inducers, which may reduce the therapeutic effectiveness of SYMDEKO. Therefore, co-administration with strong CYP3A inducers is not recommended [see Drug Interactions (7.1), Clinical Pharmacology (12.3), and Patient Counseling Information (17)].
5.4 Cataracts
Cases of non-congenital lens opacities have been reported in pediatric patients treated with SYMDEKO, as well as with ivacaftor monotherapy. Although other risk factors were present in some cases (such as corticosteroid use, exposure to radiation), a possible risk attributable to treatment with SYMDEKO cannot be excluded. Baseline and follow-up ophthalmological examinations are recommended in pediatric patients initiating treatment with SYMDEKO [see Use in Specific Populations (8.4) and Patient Counseling Information (17)].
6. Adverse Reactions/Side Effects
The following adverse reactions are discussed in greater detail in other sections of the label:
- Transaminase Elevations [see Warnings and Precautions (5.1)]
- Hypersensitivity Reactions, Including Anaphylaxis [see Warnings and Precautions (5.2)]
- Cataracts [see Warnings and Precautions (5.4)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
The overall safety profile of SYMDEKO is based on data from 1001 patients in three double-blind, placebo-controlled, clinical trials: two parallel-group trials of 12 and 24 week duration and one cross-over design trial of 8 weeks duration. Eligible patients were also able to participate in an open-label extension safety study (up to 96 weeks of SYMDEKO). In the three placebo-controlled trials (Trials 1, 2, and 3), a total of 496 patients with CF age 12 years and older received at least one dose of SYMDEKO. The proportion of patients who discontinued study drug prematurely due to adverse reactions was 1.6% for SYMDEKO-treated patients and 2.0% for placebo-treated patients. Serious adverse reactions, whether considered drug-related or not by the investigators, that occurred more frequently in SYMDEKO-treated patients compared to placebo included distal intestinal obstruction syndrome, 3 (0.6%) SYMDEKO-treated patients vs. 0 placebo. There were no deaths in the placebo-controlled trials, and one death in the open label extension study due to respiratory failure and influenza infection in a patient who had discontinued SYMDEKO seven weeks prior.
The safety profile of SYMDEKO was generally similar across all subgroups of patients, including analysis by age, sex, baseline percent predicted FEV1 (ppFEV1), and geographic regions.
Table 5 shows adverse reactions occurring in ≥3% of SYMDEKO-treated patients that also occurred at a higher rate than in the placebo-treated patients in the 12- and 24-week placebo controlled, parallel-group trials (Trials 1 and 3).
| Adverse Reactions (Preferred Term) | SYMDEKO N=334 n (%) | Placebo N=343 n (%) |
|---|---|---|
| Headache | 49 (15) | 44 (13) |
| Nausea | 29 (9) | 24 (7) |
| Sinus congestion | 13 (4) | 6 (2) |
| Dizziness | 12 (4) | 8 (2) |
The safety data from the following trials are similar to that observed in Trials 1 and 3:
- an 8-week randomized, double-blind, placebo-controlled crossover study in 244 patients with CF age 12 years and older who were heterozygous for the F508del mutation and a second mutation predicted to be responsive to tezacaftor/ivacaftor (Trial 2).
- a 24-week open-label study in 70 patients with CF age 6 to less than 12 years who were either homozygous for the F508del mutation or heterozygous for the F508del mutation and a second mutation predicted to be responsive to tezacaftor/ivacaftor (Trial 4).
6.2 Postmarketing Experience
The following adverse reactions have been identified during post approval use of SYMDEKO. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Immune System Disorders: anaphylaxis
Skin: rash
7. Drug Interactions
Potential for other drugs to affect tezacaftor/ivacaftor
7.1 Inducers of CYP3A
Tezacaftor and ivacaftor are substrates of CYP3A (ivacaftor is a sensitive substrate of CYP3A). Concomitant use of CYP3A inducers may result in reduced exposures and thus reduced SYMDEKO efficacy. Co-administration of ivacaftor with rifampin, a strong CYP3A inducer, significantly decreased ivacaftor exposure (area under the curve [AUC]) by 89%. Tezacaftor exposures can also be expected to decrease significantly during co-administration with strong CYP3A inducers. Therefore, co-administration of SYMDEKO with strong CYP3A inducers is not recommended [see Warnings and Precautions (5.3), Clinical Pharmacology (12.3), and Patient Counseling Information (17)].
Examples of strong CYP3A inducers include:
- rifampin, rifabutin, phenobarbital, carbamazepine, phenytoin, and St. John's wort (Hypericum perforatum)
7.2 Inhibitors of CYP3A
Co-administration with itraconazole, a strong CYP3A inhibitor, increased tezacaftor exposure (AUC) by 4.0-fold and ivacaftor by 15.6-fold. When co-administered with strong CYP3A inhibitors, the dosing regimen of SYMDEKO should be adjusted [see Dosage and Administration (2.4), Clinical Pharmacology (12.3), and Patient Counseling Information (17)].
Examples of strong CYP3A inhibitors include:
- ketoconazole, itraconazole, posaconazole, and voriconazole
- telithromycin and clarithromycin
Co-administration of fluconazole increased ivacaftor exposure (AUC) by 3.0-fold. Simulation suggested co-administration with fluconazole, a moderate CYP3A inhibitor, may increase tezacaftor exposure (AUC) by approximately 2.0-fold. When co-administered with moderate CYP3A inhibitors, the dosing regimen of SYMDEKO should be adjusted [see Dosage and Administration (2.4), Clinical Pharmacology (12.3), and Patient Counseling Information (17)].
Examples of moderate CYP3A inhibitors include:
- fluconazole
- erythromycin
Co-administration of SYMDEKO with grapefruit juice, which contains one or more components that moderately inhibit CYP3A, may increase exposure of tezacaftor and ivacaftor; therefore, food or drink containing grapefruit should be avoided during treatment with SYMDEKO [see Dosage and Administration (2.4), Clinical Pharmacology (12.3), and Patient Counseling Information (17)].
7.3 Ciprofloxacin
Co-administration of SYMDEKO with ciprofloxacin had no significant effect on the exposure of tezacaftor or ivacaftor. Therefore, no dose adjustment is necessary during concomitant administration of SYMDEKO with ciprofloxacin [see Clinical Pharmacology (12.3)].
Potential for tezacaftor/ivacaftor to affect other drugs
7.4 CYP3A Substrates
Co-administration of SYMDEKO with midazolam (oral), a sensitive CYP3A substrate, did not affect midazolam exposure. No dose adjustment of CYP3A substrates is required when co-administered with SYMDEKO [see Clinical Pharmacology (12.3)].
7.5 CYP2C9 Substrates
Ivacaftor may inhibit CYP2C9; therefore, monitoring of the international normalized ratio (INR) during co administration of SYMDEKO with warfarin is recommended. Other medicinal products for which exposure may be increased by SYMDEKO include glimepiride and glipizide; these medicinal products should be used with caution [see Clinical Pharmacology (12.3)].
7.6 Digoxin and Other P-gp Substrates
Co-administration of SYMDEKO with digoxin, a sensitive P-gp substrate, increased digoxin exposure by 1.3-fold consistent with weak inhibition of P-gp by ivacaftor. Administration of SYMDEKO may increase systemic exposure of medicinal products that are sensitive substrates of P-gp, which may increase or prolong their therapeutic effect and adverse reactions. When used concomitantly with digoxin or other substrates of P-gp with a narrow therapeutic index such as cyclosporine, everolimus, sirolimus, and tacrolimus, caution and appropriate monitoring should be used [see Clinical Pharmacology (12.3)].
7.7 Hormonal Contraceptives
SYMDEKO has been studied with an ethinyl estradiol/norethindrone oral contraceptive and was found to have no significant effect on the exposures of the hormonal contraceptive. SYMDEKO is not expected to modify the efficacy of hormonal contraceptives [see Clinical Pharmacology (12.3)].
8. Use In Specific Populations
8.4 Pediatric Use
The safety and effectiveness of SYMDEKO for the treatment of CF have been established in pediatric patients age 6 to less than 18 years who are homozygous for the F508del mutation or who have at least one mutation in the CFTR gene that is responsive to tezacaftor/ivacaftor based on in vitro data and/or clinical evidence [see Clinical Pharmacology (12.1) and Clinical Studies (14)].
Clinical trials included the following patients with CF:
- 12 to less than 18 years of age who are homozygous for the F508del mutation [see Adverse Reactions (6) and Clinical Studies (14)].
- 12 to less than 18 years of age who are heterozygous for the F508del mutation and a second mutation predicted to be responsive to tezacaftor/ivacaftor [see Adverse Reactions (6) and Clinical Studies (14)].
- 6 to less than 12 years of age who are either homozygous for the F508del mutation or heterozygous for the F508del mutation and a second mutation predicted to be responsive to tezacaftor/ivacaftor [see Adverse Reactions (6) and Clinical Pharmacology (12)]
The effectiveness of SYMDEKO in patients age 6 to less than 12 years was extrapolated from patients age 12 years and older with support from population pharmacokinetic analyses showing similar tezacaftor and ivacaftor exposure levels in patients age 6 to less than 12 years and in patients age 12 years and older [see Clinical Pharmacology (12.3)]. Safety of SYMDEKO in this population was derived from a 24-week, open-label, clinical trial in 70 patients age 6 to less than 12 years (mean age at screening 8.1 years) administered either tezacaftor 50 mg/ivacaftor 75 mg and ivacaftor 75 mg or tezacaftor 100 mg/ivacaftor 150 mg and ivacaftor 150 mg, 12 hours apart (Trial 4). The safety profile for patients in this trial was similar to that observed in Trials 1 and 3 [see Adverse Reactions (6.1)].
The safety and effectiveness of SYMDEKO in patients with CF younger than 6 years of age have not been studied.
8.5 Geriatric Use
Clinical trials of SYMDEKO did not include sufficient numbers of patients 65 years of age and over to determine whether they respond differently from younger patients.
8.6 Hepatic Impairment
No dose adjustment is necessary for patients with mild hepatic impairment (Child-Pugh Class A). A reduced dose of SYMDEKO is recommended in patients with moderate hepatic impairment (Child-Pugh Class B). There is no experience in patients with severe hepatic impairment (Child-Pugh Class C), but tezacaftor/ivacaftor exposure is expected to be higher than in patients with moderate hepatic impairment. Therefore, use with caution at a reduced dose in patients with severe hepatic impairment after weighing the risks and benefits of treatment [see Dosage and Administration (2.3), Clinical Pharmacology (12.3), and Patient Counseling Information (17)].
8.7 Renal Impairment
SYMDEKO has not been studied in patients with moderate or severe renal impairment or in patients with end-stage renal disease. No dose adjustment is recommended for mild and moderate renal impairment. Caution is recommended in patients with severe renal impairment or end-stage renal disease [Clinical Pharmacology (12.3)].
8.8 Patients with Severe Lung Dysfunction
Trial 1 and Trial 2 included a total of 39 SYMDEKO-treated patients with ppFEV1 <40 at baseline (range 30-40); 23 patients in Trial 1 and 16 patients in Trial 2. There were 24 placebo-treated patients in Trial 1, and 15 placebo- and 13 ivacaftor-treated patients in Trial 2, with ppFEV1 <40 at baseline. The safety and efficacy in this subgroup were comparable to the overall results observed in both Trials 1 and 2.
10. Overdosage
No specific antidote is available for overdose with SYMDEKO. Treatment of overdosage consists of general supportive measures including monitoring of vital signs and observation of the clinical status of the patient.
11. Symdeko Description
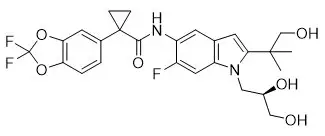
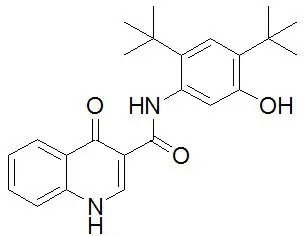
SYMDEKO is co-packaged as a tezacaftor/ivacaftor fixed-dose combination tablet and an ivacaftor tablet. Both tablets are for oral administration.
12. Symdeko - Clinical Pharmacology
12.1 Mechanism of Action
Tezacaftor facilitates the cellular processing and trafficking of select mutant forms of CFTR (including F508del-CFTR) to increase the amount of mature CFTR protein delivered to the cell surface. Ivacaftor is a CFTR potentiator that facilitates increased chloride transport by potentiating the channel-open probability (or gating) of the CFTR protein at the cell surface. For ivacaftor to function CFTR protein must be present at the cell surface. Ivacaftor can potentiate the CFTR protein delivered to the cell surface by tezacaftor, leading to a further enhancement of chloride transport than either agent alone. The combined effect of tezacaftor and ivacaftor is increased quantity and function of CFTR at the cell surface, resulting in increases in chloride transport.
CFTR Chloride Transport Assay in Fischer Rat Thyroid (FRT) cells expressing mutant CFTR
The chloride transport response of mutant CFTR protein to tezacaftor/ivacaftor was determined in Ussing chamber electrophysiology studies using a panel of FRT cell lines transfected with individual CFTR mutations. Tezacaftor/ivacaftor increased chloride transport in FRT cells expressing CFTR mutations that result in CFTR protein being delivered to the cell surface.
The in vitro chloride transport response threshold was designated as a net increase of at least 10% of normal over baseline because it is predictive or reasonably expected to predict clinical benefit. For individual mutations, the magnitude of the net change over baseline in CFTR-mediated chloride transport in vitro is not correlated with the magnitude of clinical response.
Note that splice site mutations cannot be studied in the FRT assay.
Table 6 lists responsive CFTR mutations based on (1) a clinical FEV1 response and/or (2) in vitro data in FRT cells, indicating that tezacaftor/ivacaftor increases chloride transport to at least 10% of normal over baseline. CFTR gene mutations that are not responsive to ivacaftor alone are not expected to respond to SYMDEKO except for F508del homozygotes.
|
|||||
| 546insCTA | E92K | G576A | L346P | R117G | S589N |
| 711+3A→G* | E116K | G576A;R668C † | L967S | R117H | S737F |
| 2789+5G→A* | E193K | G622D | L997F | R117L | S912L |
| 3272-26A→G* | E403D | G970D | L1324P | R117P | S945L * |
| 3849+10kbC→T * | E588V | G1069R | L1335P | R170H | S977F* |
| A120T | E822K | G1244E | L1480P | R258G | S1159F |
| A234D | E831X | G1249R | M152V | R334L | S1159P |
| A349V | F191V | G1349D | M265R | R334Q | S1251N |
| A455E * | F311del | H939R | M952I | R347H * | S1255P |
| A554E | F311L | H1054D | M952T | R347L | T338I |
| A1006E | F508C | H1375P | P5L | R347P | T1036N |
| A1067T | F508C;S1251N † | I148T | P67L * | R352Q * | T1053I |
| D110E | F508del ‡ | I175V | P205S | R352W | V201M |
| D110H * | F575Y | I336K | Q98R | R553Q | V232D |
| D192G | F1016S | I601F | Q237E | R668C | V562I |
| D443Y | F1052V | I618T | Q237H | R751L | V754M |
| D443Y;G576A;R668C † | F1074L | I807M | Q359R | R792G | V1153E |
| D579G * | F1099L | I980K | Q1291R | R933G | V1240G |
| D614G | G126D | I1027T | R31L | R1066H | V1293G |
| D836Y | G178E | I1139V | R74Q | R1070Q | W1282R |
| D924N | G178R | I1269N | R74W | R1070W * | Y109N |
| D979V | G194R | I1366N | R74W;D1270N † | R1162L | Y161S |
| D1152H * | G194V | K1060T | R74W;V201M † | R1283M | Y1014C |
| D1270N | G314E | L15P | R74W;V201M;D1270N † | R1283S | Y1032C |
| E56K | G551D | L206W * | R75Q | S549N | |
| E60K | G551S | L320V | R117C * | S549R | |
12.2 Pharmacodynamics
Effects on Sweat Chloride
In Trial 1 (patients age 12 years and older who were homozygous for the F508del mutation), the treatment difference between SYMDEKO and placebo in mean absolute change from baseline in sweat chloride through Week 24 was -10.1 mmol/L (95% CI: -11.4, -8.8).
In Trial 2 (patients age 12 years and older who were heterozygous for the F508del mutation and a second mutation predicted to be responsive to tezacaftor/ivacaftor), the treatment difference in mean absolute change from baseline in sweat chloride through Week 8 was -9.5 mmol/L (95% CI: -11.7, -7.3) between SYMDEKO and placebo, and -4.5 mmol/L (95% CI: -6.7, -2.3) between ivacaftor and placebo.
In Trial 4 (patients age 6 to less than 12 years) a reduction in sweat chloride was observed from baseline through Week 4 and sustained throughout the 24-week treatment period. Mean absolute change in sweat chloride from baseline through Week 24 was -14.5 mmol/L (95% CI: -17.4, -11.6).
12.3 Pharmacokinetics
The pharmacokinetics of tezacaftor and ivacaftor are similar between healthy adult volunteers and patients with CF. Following once-daily dosing of tezacaftor and twice-daily dosing of ivacaftor in patients with CF, plasma concentrations of tezacaftor and ivacaftor reach steady-state within 8 days and within 3 to 5 days, respectively, after starting treatment. At steady-state, the accumulation ratio is approximately 1.5 for tezacaftor and 2.2 for ivacaftor. Exposures of tezacaftor (administered alone or in combination with ivacaftor) increase in an approximately dose-proportional manner with increasing doses from 10 mg to 300 mg once daily.
Key pharmacokinetic parameters for tezacaftor and ivacaftor at steady state are shown in Table 7.
| Drug | Cmax
(mcg/mL) | Effective t½
(h) | AUC0-24h or AUC0-12h
(mcg∙h/mL)* |
|
|---|---|---|---|---|
|
||||
| Tezacaftor 100 mg once daily/ivacaftor 150 mg every 12 hours | Tezacaftor | 5.95 (1.50) | 15.0 (3.44) | 84.5 (27.8) |
| Ivacaftor | 1.17 (0.424) | 13.7 (6.06) | 11.3 (4.60) | |
Specific Populations
Based on population PK analyses, the PK exposure parameters of tezacaftor/ivacaftor in children and adolescents (ages 6 to <18 years) are similar to the AUCss range observed in adults when given in combination.
Pediatric patients age 6 to less than 12 years
| Age Group | Dose | tezacaftor AUCss mcg∙h/mL* | ivacaftor AUCss mcg∙h/mL* |
|---|---|---|---|
|
|||
| 6 to <12 years † | 71.3 (28.3) | 8.5 (3.34) | |
| 6 to <12 years (<30 kg) | tezacaftor 50 mg/ ivacaftor 75 mg | 56.7 (22.3) | 6.92 (2.07) |
| 6 to <12 years (≥30 kg) † | tezacaftor 100 mg/ ivacaftor 150 mg | 92.7 (21.9) | 10.8 (3.52) |
Potential for Other Drugs to Affect Tezacaftor/Ivacaftor
In vitro studies showed that ivacaftor and tezacaftor were substrates of CYP3A enzymes (i.e., CYP3A4 and CYP3A5). Exposure to ivacaftor and tezacaftor will be reduced by concomitant CYP3A inducers and increased by concomitant CYP3A inhibitors.
In vitro studies showed that tezacaftor is a substrate for the uptake transporter OATP1B1, and efflux transporters P-gp and BCRP. Tezacaftor is not a substrate for OATP1B3. In vitro studies showed that ivacaftor is not a substrate for OATP1B1, OATP1B3, or P-gp.
The effects of co-administered drugs on the exposure of tezacaftor and ivacaftor (or ivacaftor alone) are shown in Table 10 [see Dosage and Administration (2.4) and Drug Interactions (7)].
| Dose and Schedule | Mean Ratio (90% CI) of Other Drugs No Effect=1.0 |
||||
|---|---|---|---|---|---|
| Drug | Dose | TEZ/IVA or IVA | Effect on Drug PK | AUC | Cmax |
| ↑ = increase, ↓ = decrease, ↔ = no change. CI = Confidence interval; TEZ = tezacaftor; IVA = ivacaftor; PK = Pharmacokinetics | |||||
|
|||||
| Midazolam | 2 mg single oral dose | TEZ 100 mg/IVA 150 mg every morning + IVA 150 mg every evening | ↔ Midazolam | 1.12 (1.01, 1.25) | 1.13 (1.01, 1.25) |
| Digoxin | 0.5 mg single dose | TEZ 100 mg/IVA 150 mg every morning + IVA 150 mg every evening | ↑ Digoxin | 1.30 (1.17, 1.45) | 1.32 (1.07, 1.64) |
| Oral Contraceptive | Ethinyl estradiol/ Norethindrone 0.035 mg/1.0 mg once daily | TEZ 100 mg/IVA 150 mg every morning + IVA 150 mg every evening | ↔ Ethinyl estradiol | 1.12 (1.03, 1.22) | 1.15 (0.99, 1.33) |
| ↔ Norethindrone | 1.05 (0.98, 1.12) | 1.01 (0.87, 1.19) |
|||
| Pitavastatin | 2 mg single dose | TEZ 100 mg/IVA 150 mg every morning + IVA 150 mg every evening | ↑ Pitavastatin* | 1.24 (1.17, 1.31) | 0.977 (0.841, 1.14) |
| Rosiglitazone | 4 mg single oral dose | IVA 150 mg twice daily | ↔ Rosiglitazone | 0.975 (0.897, 1.06) | 0.928 (0.858, 1.00) |
| Desipramine | 50 mg single dose | IVA 150 mg twice daily | ↔ Desipramine | 1.04 (0.985, 1.10) | 1.00 (0.939; 1.07) |
| Dose and Schedule | Mean Ratio (90% CI) of Tezacaftor and Ivacaftor No Effect = 1.0 |
||||
|---|---|---|---|---|---|
| Drug | Dose | TEZ/IVA or IVA | Effect on TEZ/IVA PK | AUC | Cmax |
| ↑ = increase, ↓ = decrease, ↔ = no change. CI = Confidence interval; TEZ = tezacaftor; IVA = ivacaftor; PK = Pharmacokinetics | |||||
|
|||||
| Itraconazole | 200 mg twice a day on Day 1, followed by 200 mg once daily | TEZ 25 mg + IVA 50 mg once daily | ↑ Tezacaftor | 4.02 (3.71, 4.63) | 2.83 (2.62, 3.07) |
| ↑ Ivacaftor | 15.6 (13.4, 18.1) | 8.60 (7.41, 9.98) |
|||
| Ciprofloxacin | 750 mg twice daily | TEZ 50 mg + IVA 150 mg twice daily | ↔ Tezacaftor | 1.08 (1.03, 1.13) | 1.05 (0.99, 1.11) |
| ↑ Ivacaftor* | 1.17 (1.06, 1.30) | 1.18 (1.06, 1.31) |
|||
| Oral Contraceptive | Norethindrone/ethinyl estradiol 1.0 mg/0.035 mg once daily | TEZ 100 mg/IVA 150 mg every morning + IVA 150 mg every evening | ↔ Tezacaftor | 1.01 (0.963, 1.05) | 1.01 (0.933, 1.09) |
| ↔ Ivacaftor | 1.03 (0.960, 1.11) | 1.03 (0.941, 1.14) |
|||
| Rifampin | 600 mg once daily | IVA 150 mg single dose | ↓ Ivacaftor | 0.114 (0.097, 0.136) | 0.200 (0.168, 0.239) |
| Fluconazole | 400 mg single dose on Day 1, followed by 200 mg once daily | IVA 150 mg twice daily | ↑ Ivacaftor | 2.95 (2.27, 3.82) | 2.47 (1.93, 3.17) |
13. Nonclinical Toxicology
14. Clinical Studies
14.1 Trial in Patients with CF Who Were Homozygous for the F508del Mutation in the CFTR Gene (Trial 1)
Trial 1 evaluated 504 patients (248 SYMDEKO, 256 placebo) with CF age 12 years and older (mean age 26.3 years). The mean ppFEV1 at baseline was 60.0% (range: 27.8% to 96.2%). The primary efficacy endpoint was change in lung function as determined by absolute change from baseline in ppFEV1 through Week 24. Treatment with SYMDEKO resulted in a statistically significant improvement in ppFEV1. The treatment difference between SYMDEKO and placebo for the mean absolute change in ppFEV1 from baseline through Week 24 was 4.0 percentage points (95% CI: 3.1, 4.8; P<0.0001). These changes persisted throughout the 24-week treatment period (Figure 2). Improvements in ppFEV1 were observed regardless of age, sex, baseline ppFEV1, colonization with Pseudomonas, concomitant use of standard-of-care medications for CF, and geographic region.
Key secondary efficacy variables included relative change from baseline in ppFEV1 through Week 24; number of pulmonary exacerbations from baseline through Week 24; absolute change in BMI from baseline at Week 24, and absolute change in CFQ-R Respiratory Domain Score (a measure of respiratory symptoms relevant to patients with CF, such as cough, sputum production, and difficulty breathing) from baseline through Week 24. For the purposes of this trial, a pulmonary exacerbation was defined as a change in antibiotic therapy (IV, inhaled, or oral) as a result of 4 or more of 12 pre-specified sino-pulmonary signs/symptoms. See Table 11 for a summary of key secondary outcomes in Trial 1.
| Placebo N=256 | SYMDEKO N=248 |
||
|---|---|---|---|
| BMI: body mass index; CI: confidence interval; CFQ-R: Cystic Fibrosis Questionnaire-Revised; IVA: ivacaftor; NA: not applicable; ppFEV1: percent predicted forced expiratory volume in 1 second; | |||
|
|||
| Relative change in ppFEV1 from baseline through Week 24 (%) | Treatment difference (95% CI) | - | 6.8 (5.3, 8.3) |
| P value | NA | P<0.0001† | |
| Number of pulmonary exacerbations from baseline through Week 24 | Number of events (event rate per year‡) Rate ratio (95% CI) | 122 (0.99) | 78 (0.64) 0.65 (0.48, 0.88) |
| P value | NA | P=0.0054† | |
| Absolute change in BMI from baseline at Week 24 (kg/m2) | Treatment difference (95% CI) | - | 0.06 (-0.08, 0.19) |
| Absolute change in CFQ-R Respiratory Domain Score from baseline through Week 24 (points) | Treatment difference (95% CI) | - | 5.1 (3.2, 7.0) |
Figure 2: Absolute Change From Baseline in Percent Predicted FEV1 at Each Visit in Trial 1
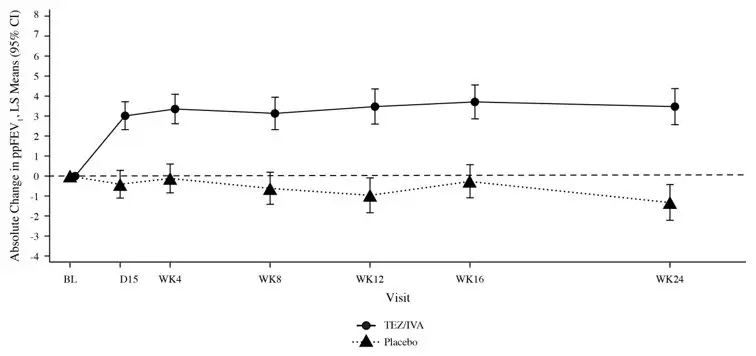
14.2 Trial in Patients with CF Who Were Heterozygous for the F508del Mutation and a Second Mutation Predicted to be Responsive to Tezacaftor/Ivacaftor (Trial 2)
Trial 2 evaluated 244 patients with CF age 12 years and older (mean age 34.8 years). The mean ppFEV1 at baseline was 62.3% (range: 34.6 to 93.5). Of the 244 patients included in the efficacy analysis, 146 patients had a splice mutation and 98 patients had a missense mutation as the second allele. 161 patients received SYMDEKO, 156 patients received ivacaftor, and 161 patients received placebo. The primary efficacy endpoint was the mean absolute change from study baseline in percent predicted FEV1 averaged at Weeks 4 and 8 of treatment. The key secondary efficacy endpoint was absolute change in CFQ-R Respiratory Domain Score from study baseline averaged at Weeks 4 and 8 of treatment. For the overall population, treatment with SYMDEKO compared to placebo resulted in significant improvement in ppFEV1 (6.8 percentage points [95% CI: 5.7, 7.8]; P<0.0001) and CFQ-R Respiratory Domain Score (11.1 points (95% CI 8.7, 13.6); P<0.0001). Treatment difference for ppFEV1 between ivacaftor- and placebo-treated patients was 4.7 percentage points (95% CI: 3.7, 5.8; P<0.0001) and 2.1 percentage points (95% CI: 1.2, 2.9; P<0.0001) between SYMDEKO- and ivacaftor-treated patients, which were statistically significant. Improvements in ppFEV1 were observed regardless of age, baseline ppFEV1, sex, mutation class, colonization with Pseudomonas, concomitant use of standard-of-care medications for CF, and geographic region. Statistically significant improvements compared to placebo were also observed in the subgroup of patients with splice mutations and missense mutations (Table 12).
| Mutation (n) | Absolute Change in percent predicted FEV1*† | Absolute Change in CFQ-R Respiratory Domain Score (Points)*‡ | Absolute Change in Sweat Chloride (mmol/L)*‡ |
|---|---|---|---|
| (n=) patient numbers analysed | |||
|
|||
| Splice mutations (n= 93 for TEZ/IVA, n=97 for PBO) Results shown as difference in mean (95% CI) change from study baseline for SYMDEKO vs. placebo-treated patients: |
|||
| 7.4 (6.0, 8.7) | 9.5 (6.3, 12.7) | -5.4 (-8.0, -2.7) | |
| By individual splice mutation (n). Results shown as mean (minimum, maximum) for change from study baseline for SYMDEKO-treated patients | |||
| 2789+5G→A (25 ) | 8.6 (-1.5, 23.4) | 12.0 (-8.3, 38.9) | -3.2 (-16.5, 9.0) |
| 3272-26A→G (23) | 5.7 (-2.1, 25.9) | 5.7 (-22.2, 44.4) | -3.8 (-22.3, 16.5) |
| 3849+10kbC→T (43) | 5.8 (-7.2, 22.3) | 8.2 (-25.0, 47.2) | -5.6 (-27.0, 8.5) |
| 711+3A→G (2) | 4.3 (2.0, 6.7) | -4.2 (-5.6, -2.8) | -15.4 (-21.0, -9.8) |
| E831X§ (0) | NA | NA | NA |
| Missense mutations (n=66 for TEZ/IVA, n=63 for PBO) Results shown as difference in mean (95% CI) change from study baseline for SYMDEKO vs. placebo-treated patients: |
|||
| 5.9 (4.2, 7.5) | 13.4 (9.6, 17.3) | -16.3 (-19.7, -12.9) | |
| By individual missense mutation (n). Results shown as mean (minimum, maximum) for change from study baseline for SYMDEKO-treated patients | |||
| D579G (2) | 8.1 (-0.2, 16.4) | 11.1 (5.6, 16.7) | -23.1 (-24.8, -21.5) |
| D110H (1) | -1.0 (-1.0, -1.0) | -11.1 (-11.1, -11.1) | -22.5 (-22.5, -22.5) |
| D1152H (21) | 3.8 (-2.5, 12.5) | 15.2 (-8.3, 55.6) | -4.1 (-15.0, 11.5) |
| A455E (11) | 8.5 (2.6, 16.1) | 11.6 (-11.1, 44.4) | -0.3 (-8.8, 14.0) |
| L206W (4) | 3.0 (-4.5, 10.2) | 12.5 (-2.8, 38.9) | -36.1 (-44.5, -27.5) |
| P67L (11) | 9.4 (0.0, 31.9) | 11.7 (-12.5, 72.2) | -29.3 (-50.0, 0.8) |
| R1070W (2) | 6.1 (2.0, 10.1) | 29.2 (16.7, 41.7) | -13.8 (-26.8, -0.8) |
| R117C (1) | 2.9 (2.9, 2.9) | 16.7 (16.7, 16.7) | -38.8 (-38.8, -38.8) |
| R347H (2) | -0.5 (-2.8, 1.7) | 5.6 (-5.6, 16.7) | -13.8 (-19.0, -8.5) |
| R352Q (2) | 4.9 (2.6, 7.1) | 8.3 (8.3, 8.3) | -43.3 (-49.8, -36.8) |
| S945L (7) | 9.6 (0.7, 19.5) | 11.3 (-4.2, 25.0) | -29.0 (-42.5, -8.0) |
| S977F (2) | 10.1 (5.5, 14.7) | -1.4 (-8.3, 5.6) | -13.9 (-22.3, -5.5) |
In an analysis of BMI at Week 8, an exploratory endpoint, patients treated with SYMDEKO had a mean improvement of 0.2 kg/m2 (95% CI [0.0, 0.3]), 0.1 kg/m2 (95% CI [-0.1, 0.3]), and 0.3 kg/m2 (95% CI [0.1, 0.5]) versus placebo for the overall, splice, and missense mutation populations of patients, respectively.
14.3 Trial in Patients with CF Who Were Heterozygous for the F508del Mutation and a Second Mutation Not Predicted to be Responsive to Tezacaftor/Ivacaftor (Trial 3)
Trial 3 evaluated 168 patients with CF (83 SYMDEKO and 85 placebo) age 12 years and older (mean age 26.1 years) who were heterozygous for the F508del mutation and had a second CFTR mutation predicted to be unresponsive to tezacaftor/ivacaftor. CF patients with the F508del mutation and one of the following mutations in the CFTR gene were enrolled in the study (listed in decreasing frequency): W1282X, G542X, N1303K, 621+1G>T, 1717-1G>A, 1898+1G>A, CFTRdele2,3, 2183delAA>G, 2184insA, R1162X, R553X, 3659delC, 3905insT, G970R, I507del, R1066C, R347P, 1154insTC, 1811+1.6kbA>G, 2184delA, 405+1G>A, E60X, G85E, L1077P, Q39X, S466X, Y1092X, 1078delT, 1248+1G>A, 1677delTA, 1812-1G>A, 2869INSG, 3120+1G>A, 394delTT, 457TAT>G, 711+1G>T, 711+5G>A, 712-1G>T, G673x, L1065P, Q220X, Q493X, R709X, V520F. The mean ppFEV1 at baseline was 57.5% [range: 31.0 to 96.7]. The primary efficacy endpoint was change from baseline in absolute ppFEV1 through Week 12. The overall treatment difference between SYMDEKO and placebo for the mean absolute change in ppFEV1 from baseline through Week 12 was 1.2 percentage points (95% CI: -0.3, 2.6). This study was terminated following the planned interim analysis because the pre-specified futility criteria were met.
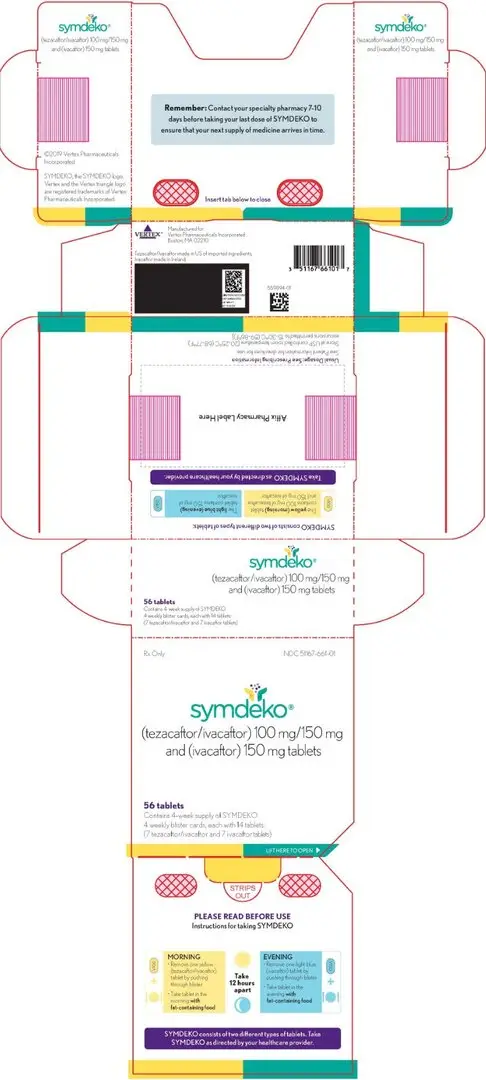
16. How is Symdeko supplied
SYMDEKO (tezacaftor 50 mg/ivacaftor 75 mg fixed-dose combination tablet co-packaged with ivacaftor 75 mg tablet):
- Tezacaftor 50 mg/ivacaftor 75 mg fixed-dose combination tablets are supplied as white, capsule shaped tablets containing 50 mg of tezacaftor and 75 mg of ivacaftor. Each tablet is debossed with "V50" on one side and plain on the other.
- Ivacaftor 75 mg tablets are supplied as light blue, film-coated, capsule-shaped tablets containing 75 mg of ivacaftor. Each tablet is printed with the characters "V 75" on one side and plain on the other.
- 56-count tablet carton containing a 4-week supply (4 weekly wallets, each with 14 tablets) NDC 51167-113-01
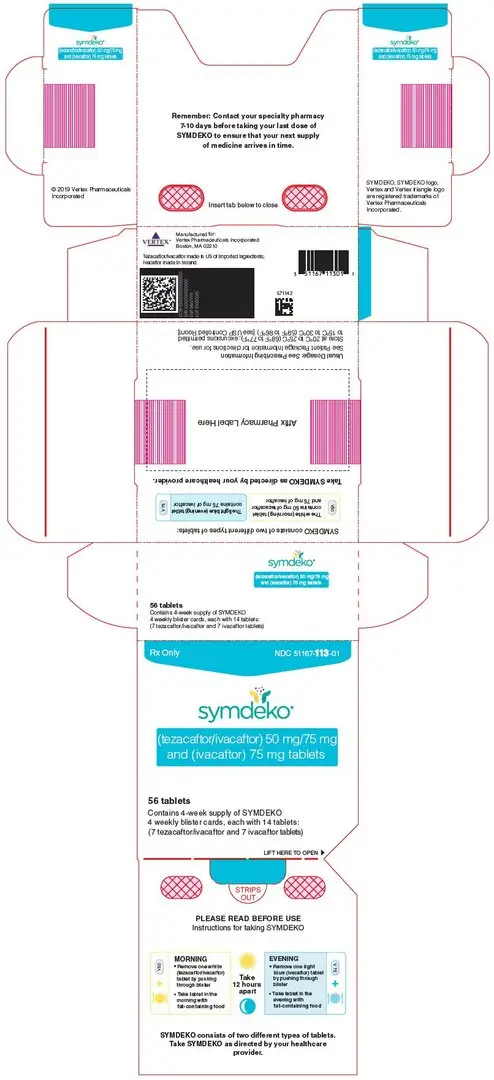
SYMDEKO (tezacaftor 100 mg/ivacaftor 150 mg fixed-dose combination tablets co-packaged with ivacaftor 150 mg tablet):
- Tezacaftor 100 mg/ivacaftor 150 mg fixed-dose combination tablets are supplied as yellow, capsule-shaped tablets containing 100 mg of tezacaftor and 150 mg of ivacaftor. Each tablet is debossed with "V100" on one side and plain on the other.
- Ivacaftor 150 mg tablets are supplied as light blue, film-coated, capsule-shaped tablets containing 150 mg of ivacaftor. Each tablet is printed with the characters "V 150" on one side and plain on the other.
- 56-count tablet carton containing a 4-week supply (4 weekly wallets, each with 14 tablets) NDC 51167-661-01
| SYMDEKO
tezacaftor and ivacaftor kit |
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
| SYMDEKO
tezacaftor and ivacaftor kit |
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
| Labeler - Vertex Pharmaceuticals Incorporated (602478257) |