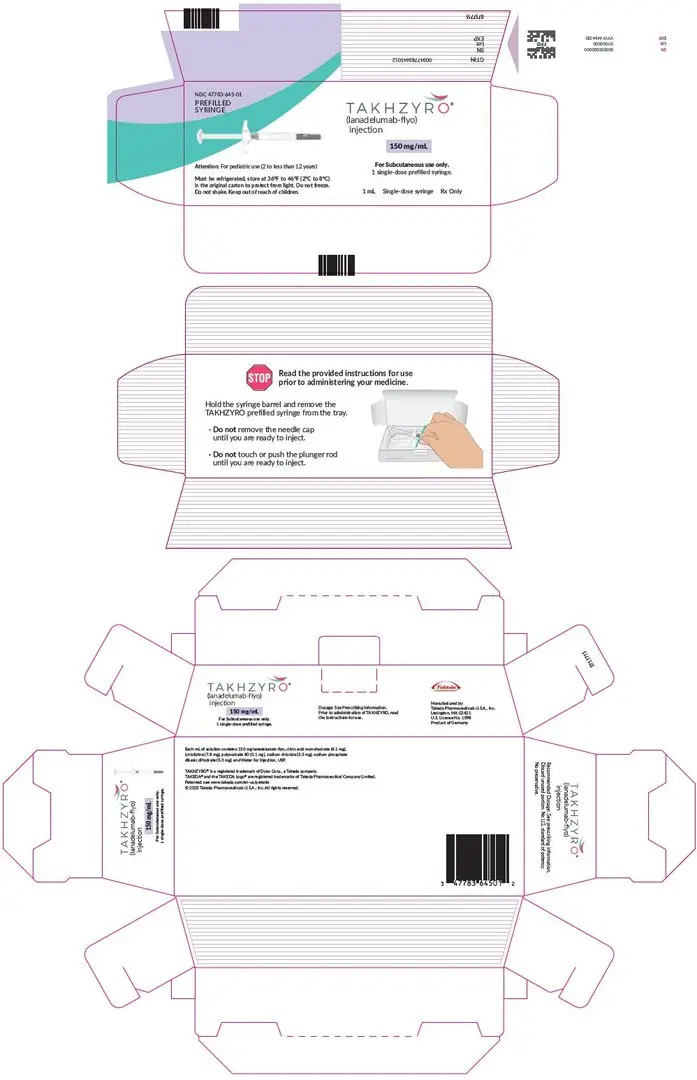
Drug Detail:Takhzyro (Lanadelumab-flyo [ lan-a-del-ue-mab ])
Drug Class: Hereditary angioedema agents
Highlights of Prescribing Information
TAKHZYRO® (lanadelumab-flyo) injection, for subcutaneous use
Initial U.S. Approval: 2018
Recent Major Changes
| Indications and Usage (1) | 02/2023 |
| Dosage and Administration (2) | 02/2023 |
Indications and Usage for Takhzyro
TAKHZYRO is a plasma kallikrein inhibitor (monoclonal antibody) indicated for prophylaxis to prevent attacks of hereditary angioedema (HAE) in adult and pediatric patients 2 years and older. (1)
Takhzyro Dosage and Administration
For subcutaneous use only.
Recommended Dosage:
- Adult and pediatric patients 12 years of age and older: administer 300 mg every 2 weeks by the patient or caregiver. Dosing interval every 4 weeks may be considered in some patients. (2.1)
- Pediatric patients 6 to less than 12 years of age: administer 150 mg every 2 weeks by a healthcare provider or caregiver. Dosing interval every 4 weeks may be considered in some patients. (2.2)
- Pediatric patients 2 to less than 6 years of age: administer 150 mg every 4 weeks by a healthcare provider or caregiver. (2.2)
- See Full Prescribing Information for Administration Instructions. (2.3)
Dosage Forms and Strengths
Injection:
- 150 mg/1 mL (150 mg/mL) solution in a single-dose prefilled syringe. (3)
- 300 mg/2 mL (150 mg/mL) solution in a single-dose prefilled syringe. (3)
- 300 mg/2 mL (150 mg/mL) solution in a single-dose vial. (3)
Contraindications
None. (4)
Warnings and Precautions
Hypersensitivity reactions have been observed. In case of a severe hypersensitivity reaction, discontinue TAKHZYRO administration and institute appropriate treatment. (5.1)
Adverse Reactions/Side Effects
The most common adverse reactions (≥10%) are injection site reactions, upper respiratory infections, headache, rash, dizziness, diarrhea, and myalgia. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Takeda Pharmaceuticals at 1-877-TAKEDA-7 (1-877-825-3327) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
No dedicated drug interaction studies have been conducted. (7)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 2/2023
Related/similar drugs
Cinryze, Berinert, Haegarda, Firazyr, Ruconest, KalbitorFull Prescribing Information
1. Indications and Usage for Takhzyro
TAKHZYRO® is indicated for prophylaxis to prevent attacks of hereditary angioedema (HAE) in adult and pediatric patients aged 2 years and older.
2. Takhzyro Dosage and Administration
2.1 Recommended Dosage for Adult and Pediatric Patients 12 Years of Age and Older
The recommended starting dosage in adult and pediatric patients 12 years of age and older is 300 mg administered subcutaneously every 2 weeks (q2wks). A dosing interval of 300 mg every 4 weeks (q4wks) is also effective and may be considered if the patient is well-controlled (e.g., attack free) for more than 6 months.
2.3 Preparation and Administration Instructions
TAKHZYRO is administered subcutaneously only.
TAKHZYRO is intended for administration by a healthcare provider, patient or caregiver.
The patient or caregiver should be trained in subcutaneous injection technique by a healthcare professional.
- Adult and pediatric patients 12 years of age and older: TAKHZYRO may be administered by the patient or caregiver.
- Pediatric patients 2 to less than 12 years of age: TAKHZYRO should be administered by a healthcare provider or caregiver.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Do not use TAKHZYRO if the solution appears discolored or contains visible particles. TAKHZYRO is a clear to slightly opalescent, colorless to slightly yellow solution.
3. Dosage Forms and Strengths
TAKHZYRO is a sterile, preservative-free, clear to slightly opalescent, colorless to slightly yellow solution available in the following presentations.
- Injection: 150 mg/1 mL (150 mg/mL) solution in a single-dose prefilled syringe
- Injection: 300 mg/2 mL (150 mg/mL) solution in a single-dose prefilled syringe
- Injection: 300 mg/2 mL (150 mg/mL) solution in a single-dose vial
6. Adverse Reactions/Side Effects
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Adult and Pediatric Patients 12 Years of Age and Older
The safety of TAKHZYRO is primarily based on a 26-week, randomized, double-blind, parallel-group and placebo-controlled study (Trial 1) in 125 patients with Type I or II HAE. Eligible patients were also able to participate in an open-label extension study (Trial 2) up to 130 weeks. In Trial 1, a total of 84 patients with HAE aged 12 years and older received at least one dose of TAKHZYRO. Overall, 70% of patients were female and 90% of patients were Caucasian with a mean age of 41 years. The proportion of patients who discontinued study drug prematurely due to adverse events was 1.2% for TAKHZYRO-treated patients and 4.9% for placebo-treated patients. No deaths occurred in the trial.
The safety profile of TAKHZYRO was generally similar across all subgroups of patients, including analysis by age, sex, and geographic region.
Table 1 shows adverse reactions occurring in ≥10% of patients in any TAKHZYRO treatment group that also occurred at a higher rate than in the placebo treatment group in Trial 1.
| Adverse Reaction | Placebo (N=41) | TAKHZYRO | |||
|---|---|---|---|---|---|
| 150 mg q4wks (N=28) | 300 mg q4wks (N=29) | 300 mg q2wks (N=27) | Total (N=84) |
||
| n (%) | n (%) | n (%) | n (%) | n (%) | |
| N= number of patients; n =number of patients experiencing the event; q2wks = every 2 weeks; q4wks = every 4 weeks | |||||
|
|||||
| Injection site reactions* | 14 (34) | 16 (57) | 13 (45) | 15 (56) | 44 (52) |
| Upper respiratory infection† | 13 (32) | 3 (11) | 9 (31) | 12 (44) | 24 (29) |
| Headache‡ | 9 (22) | 3 (11) | 6 (21) | 9 (33) | 18 (21) |
| Rash§ | 2(5) | 2 (7) | 3 (10) | 1 (4) | 6 (7) |
| Dizziness | 0 | 1 (4) | 3 (10) | 1 (4) | 5 (6) |
| Diarrhea | 2 (5) | 3 (11) | 0 | 1 (4) | 4 (5) |
| Myalgia | 0 | 1 (4) | 0 | 3 (11) | 4 (5) |
Injection site reactions primarily consisted mainly of pain, erythema, and bruising at the injection site. There was no meaningful difference in injection site reactions with self-administration.
7. Drug Interactions
No dedicated drug interaction studies have been conducted [see Clinical Pharmacology (12.3)].
8. Use In Specific Populations
8.4 Pediatric Use
The safety and effectiveness of TAKHZYRO for prophylaxis to prevent attacks of hereditary angioedema (HAE) have been established in pediatric patients 2 years of age and older.
Use of TAKHZYRO for this indication in patients 12 years of age and older was supported by a subgroup analysis by age of 10 patients aged 12 to <18 years in Trial 1 (a randomized, double-blind, placebo-controlled parallel-group study in adult and pediatric patients 12 years of age and older with HAE). Results of the subgroup analysis by age were consistent with overall study results. An additional 13 pediatric patients aged 12 to <18 years were enrolled in the open-label extension study [see Adverse Reactions (6.1), Clinical Pharmacology (12.3) and Clinical Studies (14)].
Use of TAKHZYRO for this indication in patients 2 to less than 12 years of age was supported by extrapolation of efficacy data from Trial 1, an adequate and well controlled study in adult and pediatric (12 to less than 18 years of age) patients, with additional pharmacokinetic analyses showing similar drug exposures between adults (>18 years of age) and pediatric patients (2 to less than 12 years of age), and safety and pharmacodynamic data from an open-label, multicenter study in pediatric patients with HAE aged 2 to less than 12 years that enrolled 21 patients (4 patients were aged 2 to less than 6 years and 17 patients were 6 to less than 12 years of age) [see Adverse Reactions (6.1) and Clinical Pharmacology (12.3)]. The pharmacodynamic response observed in this trial for pediatric patients 2 to less than 12 years of age was similar to that seen in adult and pediatric patients 12 years of age and older [see Clinical Pharmacology (12.2)].
The safety and effectiveness of TAKHZYRO in pediatric patients less than 2 years of age have not been established.
8.5 Geriatric Use
The safety and effectiveness of TAKHZYRO were evaluated in a subgroup of patients (N=5) aged ≥65 years in Trial 1. Results of the subgroup analysis by age were consistent with overall study results [see Adverse Reactions (6.1), Clinical Pharmacology (12.3) and Clinical Studies (14)].
11. Takhzyro Description
Lanadelumab-flyo, a plasma kallikrein inhibitor, is a non-plasma derived, recombinant, fully human, monoclonal antibody (IgG1/κ-light chain) produced in Chinese Hamster Ovary (CHO) cells. Based on the amino acid sequence, the molecular weight of the non-glycosylated lanadelumab-flyo is 146 kDa. The calculated molecular mass of the fully reduced light chain is 23 kDa. The calculated molecular mass of the fully reduced and non-glycosylated heavy chain is 49 kDa.
TAKHZYRO (lanadelumab-flyo) injection is a sterile, preservative-free, clear to slightly opalescent, colorless to slightly yellow solution for subcutaneous use.
Each mL of ready-to-use TAKHZYRO solution contains 150 mg of lanadelumab-flyo, citric acid monohydrate (4.1 mg), L-histidine (7.8 mg), polysorbate 80 (0.1 mg), sodium chloride (5.3 mg), sodium phosphate dibasic dihydrate (5.3 mg), and Water for Injection, USP. The solution has a pH of approximately 6.0.
12. Takhzyro - Clinical Pharmacology
12.1 Mechanism of Action
Lanadelumab-flyo is a fully human monoclonal antibody (IgG1/κ-light chain) that binds plasma kallikrein and inhibits its proteolytic activity. Plasma kallikrein is a protease that cleaves high-molecular-weight-kininogen (HMWK) to generate cleaved HMWK (cHMWK) and bradykinin, a potent vasodilator that increases vascular permeability resulting in swelling and pain associated with HAE. In patients with HAE due to C1-inhibitor (C1-INH) deficiency or dysfunction, normal regulation of plasma kallikrein activity is not present, which leads to uncontrolled increases in plasma kallikrein activity and results in angioedema attacks. Lanadelumab-flyo decreases plasma kallikrein activity to control excess bradykinin generation in patients with HAE.
12.2 Pharmacodynamics
In adult and pediatric (12 to less than 18 years) patients, concentration-dependent inhibition of plasma kallikrein, measured as reduction of cHMWK levels, was demonstrated after subcutaneous administration of TAKHZYRO 150 mg q4wks, 300 mg q4wks or 300 mg q2wks in patients with HAE.
For pediatric patients 2 to less than 6 years (150 mg q4wks) and 6 to less than 12 years (150 mg q2wks), the observed mean percent change from baseline cHMWK levels was similar to that observed in adult and pediatric (12 to less than 18 years) patients (300 mg q2wks or 300 mg q4wks).
12.3 Pharmacokinetics
Following subcutaneous administration, the pharmacokinetics of lanadelumab-flyo was approximately dose-proportional in the therapeutic dose range in patients with HAE (Table 2). The pharmacokinetic properties and exposure (steady state) of lanadelumab-flyo in HAE patients, following subcutaneous administration of 150 mg q4wks, 300 mg q4wks and 300 mg q2wks, are provided in Table 2. Following subcutaneous administration of TAKHZYRO, peak plasma concentrations are reached within 5 days, and terminal elimination half-life is ~2 weeks. The anticipated time to reach steady state concentration was approximately 70 days. At steady-state, the mean accumulation ratio is approximately 1.44, 1.42, and 2.43 for dosing regimen of 150 mg q4wks, 300 mg q4wks and 300 mg q2wks, respectively.
| Pharmacokinetic Parameters | Lanadelumab-flyo | ||
|---|---|---|---|
| 150 mg q4wks (N=28) | 300 mg q4wks (N=29) | 300 mg q2wks (N=27) |
|
| CL/F: apparent clearance; Vc/F: apparent volume of distribution; AUCtau,ss: area under the curve over the dosing interval at steady-state; Cmax,ss: maximum concentration at steady-state; Cmin,ss: minimum concentration at steady state; Tmax: time to maximum concentration; t1/2 terminal elimination half-life. | |||
| CL/F (L/day) | 0.667 (0.162) | 0.742 (0.239) | 0.809 (0.370) |
| Vc/F (L) | 14.1 (2.93) | 14.9 (4.45) | 16.6 (4.79) |
| AUCtau,ss
(µg*day/mL) | 233 (56.6) | 441(137) | 408 (138) |
| Cmax,ss
(µg/mL) | 12.0 (3.01) | 23.3 (7.94) | 34.4 (11.2) |
| Cmin,ss
(µg/mL) | 4.81 (1.40) | 8.77 (2.80) | 25.4 (9.18) |
| tmax
(day) | 5.17 (1.09) | 5.17 (1.12) | 4.11 (0.377) |
| t1/2
(day) | 14.9 (2.00) | 14.2 (1.89) | 15.0 (2.48) |
12.6 Immunogenicity
The observed incidence of anti-drug antibodies is highly dependent on the sensitivity and specificity of the assay. Differences in assay methods preclude meaningful comparisons of the incidence of anti-drug antibodies in the studies described below with the incidence of anti-drug antibodies in other studies, including those of lanadelumab-flyo or of other lanadelumab products.
In Trial 1 with adult and pediatric patients 12 years of age and older, 10 (12%) lanadelumab-flyo-treated and 2 (5%) placebo-treated patients had at least 1 anti-drug antibody (ADA)-positive sample during the 26-week treatment period; antibody titers were low (range: 20 to 1280). The ADA response observed was transient in 2/10 lanadelumab-flyo and 1/2 placebo-treated patients. Pre-existing low titer antibodies were observed in 3 lanadelumab-flyo-treated patients and 1 placebo-treated patient with ADAs. Two patients receiving 150 mg q4wks had low titer antibodies classified as neutralizing.
In an open-label, multicenter study in pediatric patients 2 to less than 12 years of age, 3/20 (15%) lanadelumab-treated patients who completed the study developed ADAs during the 52-week treatment period; all of whom were in the 6 to less than 12 years age group. The ADA response observed was transient in all 3 patients. None of these patients had pre-existing antibodies. One patient had neutralizing antibodies.
The development of ADA including neutralizing antibodies against lanadelumab-flyo did not appear to adversely affect pharmacokinetics (PK), pharmacodynamics (PD), safety or clinical response.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Animal studies have not been conducted to evaluate the carcinogenic potential of lanadelumab-flyo. Published literature supports bradykinin, which is elevated in HAE, as a pro-tumorigenic molecule. However, the malignancy risk in humans from an antibody that inhibits plasma kallikrein activity, such as lanadelumab-flyo, which lowers bradykinin levels, is currently unknown.
Male and female fertility were unaffected based upon no observed adverse histopathological findings in the reproductive organs from sexually mature cynomolgus monkeys that received lanadelumab-flyo for 13 weeks at subcutaneous doses up to 50 mg/kg/week (resulting in approximately 22 times the exposure at the MRHD on an AUC basis).
16. How is Takhzyro supplied
17. Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
INSTRUCTIONS FOR USE
TAKHZYRO® (tak-ZYE-roe)
(lanadelumab-flyo)
injection, for subcutaneous use
single-dose 1 mL prefilled syringe
This Instructions for Use contains information on how to inject TAKHZYRO. Please make sure to read, understand, and follow the Instructions for Use before injecting TAKHZYRO.
A healthcare provider should show you how to prepare and inject TAKHZYRO properly before you use it for the first time. Contact your healthcare provider if you have any questions.
| Important information: | |
|
|
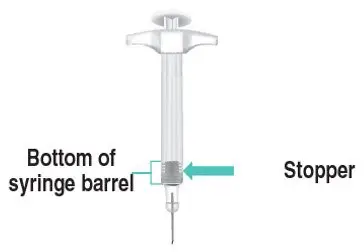
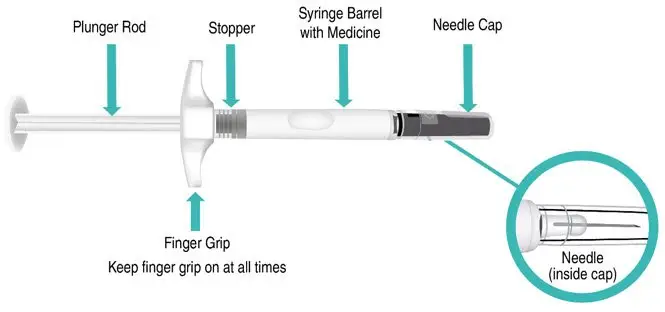 Figure A: TAKHZYRO prefilled syringe |
|
| Storing TAKHZYRO: | |
|
Step 1: Prepare for your injection
|
|
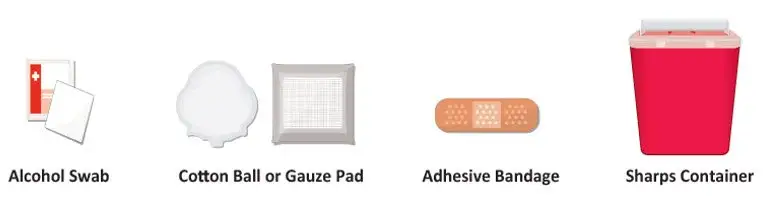 Figure B: Supplies |
|
|  |
|  Figure C: Remove prefilled syringe |
|  Figure D: Wash hands |
| 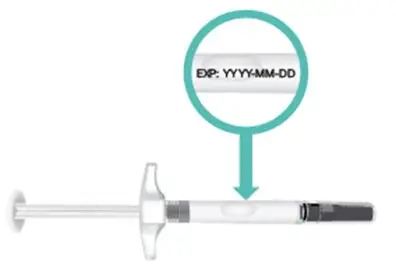 Figure E: Location of expiration date |
| 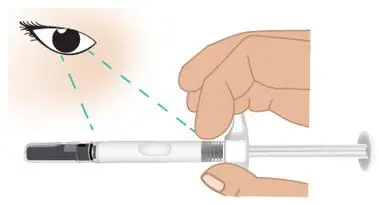 Figure F: Inspect the prefilled syringe |
| Step 2: Select and prepare injection site | |
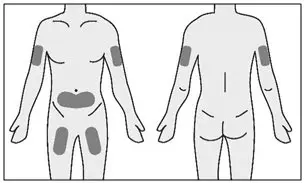
Rotate injection sites to keep skin healthy. Each new injection should be given at least 1 inch from the last site you used. | 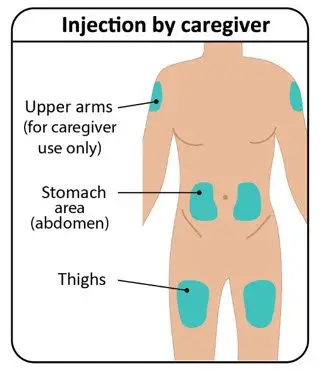 Figure G: Injection sites |
| 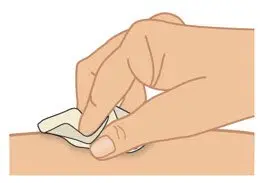 Figure H: Clean injection site |
| 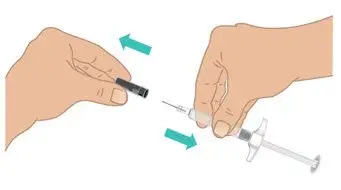 Figure I: Remove needle cap |
| |
Step 3: Inject TAKHZYRO
| 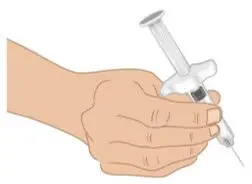 Figure J: Grip prefilled syringe |
|
|  Figure K: Pinch a 1-inch fold of skin |
|
| 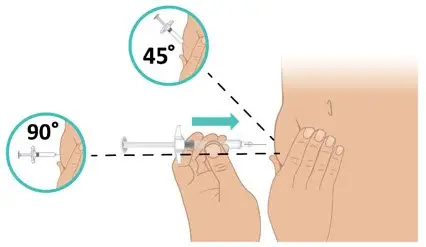 Figure L: Insert the needle Figure L: Insert the needle |
|
| 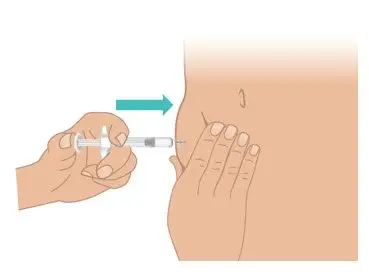 Figure M: Push the plunger rod all the way down |
|
|
||
|
||
Step 4: Throw away (dispose of) TAKHZYRO prefilled syringe
FDA-cleared sharps containers are generally available through pharmacies, medical supply companies, healthcare providers, and online. | 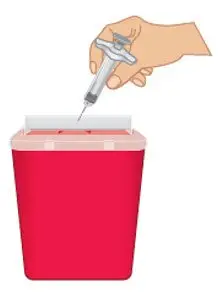 Figure O: Dispose in a sharps container |
|
|
Questions?
For product or service-related questions, call 1-877-TAKEDA-7 (1-877-825-3327) or go to www.TAKHZYRO.com.
Manufactured by:
Takeda Pharmaceuticals U.S.A., Inc.
Lexington, MA 02421
U.S. License No. 1898
TAKHZYRO® is a registered trademark of Dyax Corp., a Takeda company. TAKEDA® and the TAKEDA Logo® are registered trademarks of Takeda Pharmaceutical Company Limited.
©2023 Takeda Pharmaceuticals U.S.A., Inc. All rights reserved.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Approved: 02/2023
INSTRUCTIONS FOR USE
TAKHZYRO® (tak-ZYE-roe)
(lanadelumab-flyo)
injection, for subcutaneous use
single-dose prefilled syringe
This Instructions for Use contains information on how to inject TAKHZYRO. Please make sure to read, understand, and follow the Instructions for Use before injecting TAKHZYRO.
A healthcare provider should show you how to prepare and inject TAKHZYRO properly before you use it for the first time. Contact your healthcare provider if you have any questions.
| Important information: | |
|
|
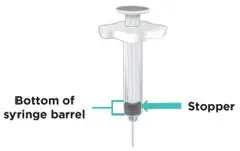
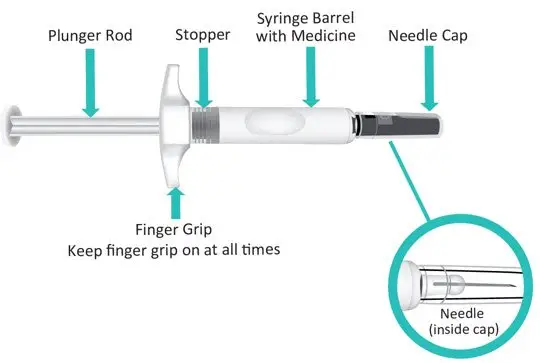 Figure A: TAKHZYRO prefilled syringe |
|
| Storing TAKHZYRO: | |
|
Step 1: Prepare for your injection
|
|
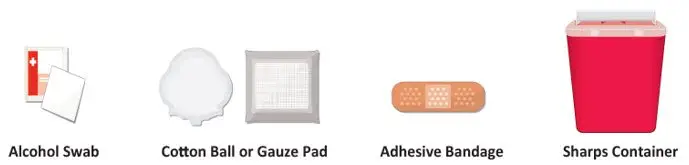 Figure B: Supplies |
|
|  |
| 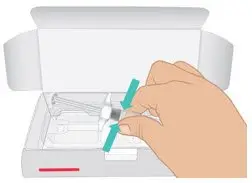 Figure C: Remove prefilled syringe |
| 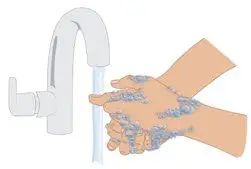 Figure D: Wash hands |
| 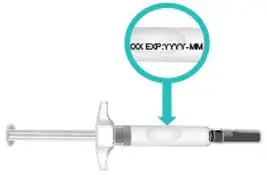 Figure E: Location of expiration date |
| 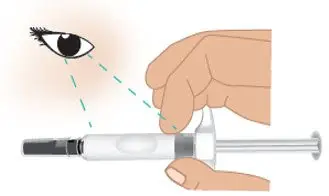 Figure F: Inspect the prefilled syringe |
| Step 2: Select and prepare injection site | |
| 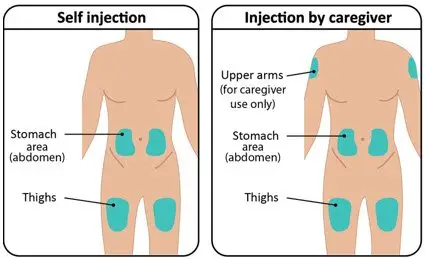 Figure G: Injection sites |
| 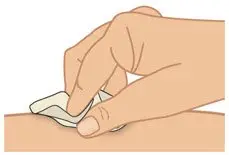 Figure H: Clean injection site |
| 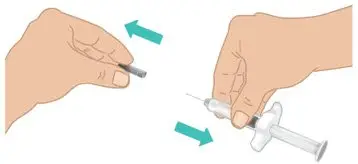 Figure I: Remove needle cap |
| |
Step 3: Inject TAKHZYRO
| 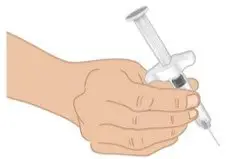 Figure J: Grip prefilled syringe |
|
| 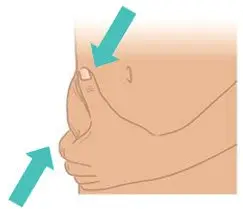 Figure K: Pinch a 1-inch fold of skin |
|
| 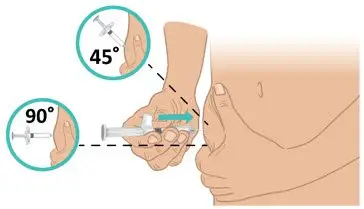 Figure L: Insert the needle Figure L: Insert the needle |
|
| 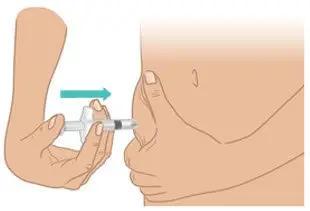 Figure M: Push the plunger rod all the way down |
|
|
||
|
||
Step 4: Throw away (dispose of) TAKHZYRO prefilled syringe
FDA-cleared sharps containers are generally available through pharmacies, medical supply companies, healthcare providers, and online. | 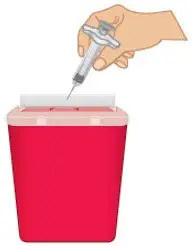 Figure O: Dispose in a sharps container |
|
|
Questions?
For product or service-related questions, call 1-877-TAKEDA-7 (1-877-825-3327) or go to www.TAKHZYRO.com.
Manufactured by:
Takeda Pharmaceuticals U.S.A., Inc.
Lexington, MA 02421
U.S. License No. 1898
TAKHZYRO® is a registered trademark of Dyax Corp., a Takeda company. TAKEDA® and the TAKEDA Logo® are registered trademarks of Takeda Pharmaceutical Company Limited.
©2022 Takeda Pharmaceuticals U.S.A., Inc. All rights reserved.
This Instructions for Use has been approved by the U.S Food and Drug Administration.
Approved: 02/2022
INSTRUCTIONS FOR USETAKHZYRO® (tak-ZYE-roe)(lanadelumab-flyo)injection, for subcutaneous use
Be sure that you read, understand, and follow the Instructions for Use before injecting TAKHZYRO. A healthcare provider should show you how to prepare and inject TAKHZYRO properly before you use it for the first time. Contact your healthcare provider if you have any questions.
Important information:
- TAKHZYRO is a ready-to-use solution for injection under the skin (subcutaneous). It is supplied in a single-dose, glass vial.
- Your healthcare provider will prescribe the dose that you should take.
- Only use the syringes, transfer needles, and injection needles that your healthcare provider prescribes.
- Only use the syringes, transfer needles and injection needles 1 time. Throw away (dispose of) any used syringes and needles.
Storing TAKHZYRO:
- Store TAKHZYRO in the refrigerator at 36°F to 46°F (2°C to 8°C). Do not freeze.
- Store TAKHZYRO in the original carton to protect the vial from light.
- Do not shake TAKHZYRO.
- Keep TAKHZYRO and all medicines out of the reach of children.
Supplies needed for your TAKHZYRO Injection
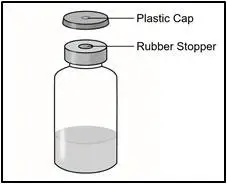 | 1 Vial containing TAKHZYRO |
 | TAKHZYRO Instructions for Use |
 | 2 Alcohol wipes |
 | 1 Empty 3 mL syringe |
 | 18G Transfer needle (longer needle) Do not use the transfer needle to inject TAKHZYRO. |
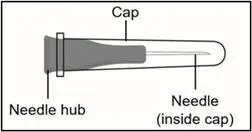 | 27G ½-inch Injection needle (shorter needle). Do not use the injection needle to withdraw TAKHYZRO from the vial. |
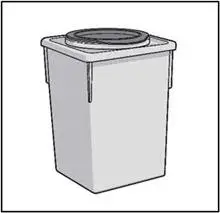 | Sharps disposal container. See "STEP 6" at the end of this Instructions for Use. |
STEP 1: Prepare for your injection
- Gather all supplies and place them on a well-lighted flat work surface.
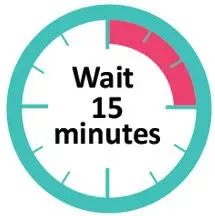
- Take the vial out of the refrigerator 15 minutes before use and allow it to reach room temperature before preparing an injection.
- Check the expiration date on the box and vial label of TAKHZYRO. Do not use if the expiration date has passed.
- Check the supplies for damage. Do not use if they appear damaged.
- Clean your work area and wash your hands before preparing your dose. Do not touch any surface or body part, especially your face, after washing your hands before injection.
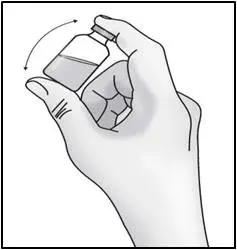
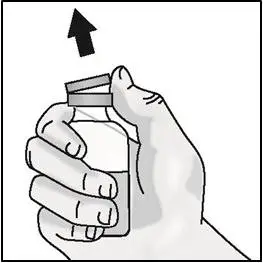
- Remove the vial from the packaging. Do not use the vial if the plastic cap covering is missing.
 |
|
| Important: Do not shake. | |
 |
|
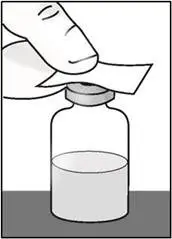 |
|
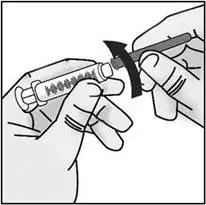
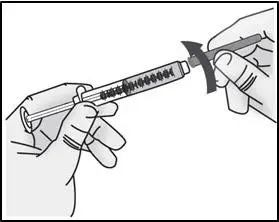
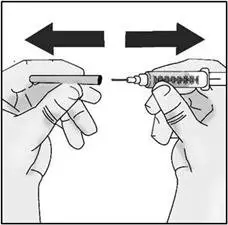
STEP 2: Attach transfer needle to syringe
 |
|
| Important: Do not remove the transfer needle cap from the needle when attaching to the syringe. | |
 |
|
 |
|
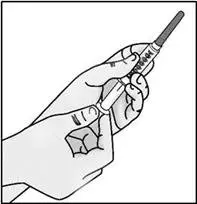
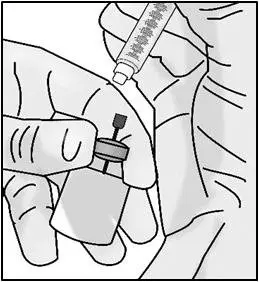
STEP 3: Transfer TAKHZYRO into syringe and switch to the injection needle
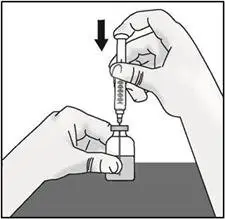 |
|
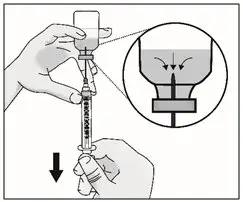 |
|
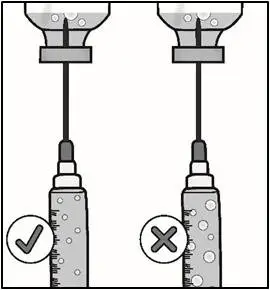
| Important: Be sure to keep the tip of the transfer needle in the medicine to avoid drawing air in as you pull back the plunger. | |
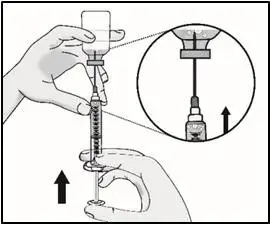  |
|
| Important: Check again to make sure you have the right amount of medicine in your syringe. If you do not have enough medicine, pull back on the plunger again while keeping the needle in the medicine to get your full dose. | |
  |
|
 |
|
 |
|
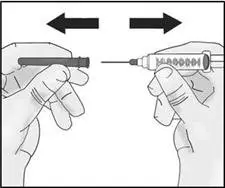
| Important: Do not remove the needle cap from the injection needle when attaching to the syringe. Do not use the transfer needle to inject TAKHZYRO as this may cause harm such as pain and bleeding. |
STEP 4: Select and prepare injection site
 |
|
Important:
|
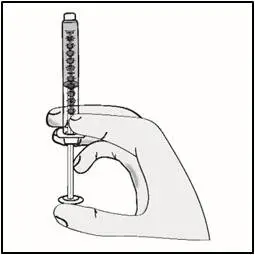
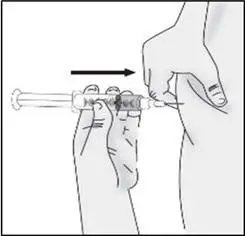
STEP 5: Inject TAKHZYRO
 |
|
 |
|
| Important: Be sure to inject under the skin which is not too shallow (skin layer) or too deep (muscle). | |
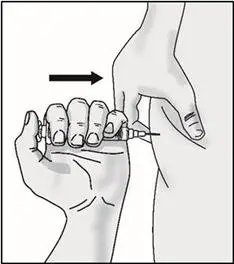 |
|
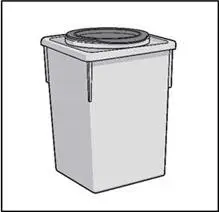
STEP 6: THROW AWAY (DISPOSE OF) NEEDLE AND SYRINGE
 |
|
For more information, visit www.TAKHZYRO.com
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Manufactured by:
Takeda Pharmaceuticals U.S.A., Inc.
Lexington, MA 02421
U.S. License No: 1898
TAKHZYRO® is a registered trademark of Dyax Corp., a Takeda company. TAKEDA® and the TAKEDA Logo® are registered trademarks of Takeda Pharmaceutical Company Limited.
©2021 Takeda Pharmaceuticals U.S.A., Inc. All rights reserved.
Approved: 11/2021
| TAKHZYRO
lanadelumab-flyo solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| TAKHZYRO
lanadelumab-flyo injection, solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| TAKHZYRO
lanadelumab-flyo solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Takeda Pharmaceuticals America, Inc. (039997266) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Rentschler Biopharma SE | 313166479 | API MANUFACTURE(47783-646, 47783-644, 47783-645) , ANALYSIS(47783-646, 47783-644, 47783-645) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Vetter Pharma-Fertigung GmbH & Co. KG | 316126754 | MANUFACTURE(47783-646, 47783-645) , ANALYSIS(47783-646, 47783-645) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Catalent Indiana LLC | 172209277 | MANUFACTURE(47783-644) , ANALYSIS(47783-644) | |