Drug Detail:Tecartus (Brexucabtagene autoleucel [ brex-ue-kab-ta-jeen-ah-toe-loo-sel ])
Drug Class: Miscellaneous antineoplastics
Highlights of Prescribing Information
TECARTUS® (brexucabtagene autoleucel) suspension for intravenous infusion
Initial U.S. Approval: 2020
WARNING: CYTOKINE RELEASE SYNDROME AND NEUROLOGIC TOXICITIES
See full prescribing information for complete boxed warning.
- Cytokine Release Syndrome (CRS), including life-threatening reactions, occurred in patients receiving TECARTUS. Do not administer TECARTUS to patients with active infection or inflammatory disorders. Treat severe or life-threatening CRS with tocilizumab or tocilizumab and corticosteroids (2.2, 2.3, 5.1).
- Neurologic toxicities, including life-threatening reactions, occurred in patients receiving TECARTUS, including concurrently with CRS or after CRS resolution. Monitor for neurologic toxicities after treatment with TECARTUS. Provide supportive care and/or corticosteroids, as needed (2.2, 2.3, 5.2).
- TECARTUS is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS) called the YESCARTA and TECARTUS REMS Program (5.3).
Recent Major Changes
| Indications and Usage (1.2) | 10/2021 |
| Dosage and Administration (2.1, 2.2) | 10/2021 |
| Warning and Precautions, Hemophagocytic Lymphohistiocytosis/Macrophage Activation Syndrome (5.4) | 10/2021 |
| Warning and Precautions, Severe Infections (5.6) | 02/2021 |
Indications and Usage for Tecartus
TECARTUS is a CD19-directed genetically modified autologous T cell immunotherapy indicated for the treatment of: (1)
- Adult patients with relapsed or refractory mantle cell lymphoma (MCL).
This indication is approved under accelerated approval based on overall response rate and durability of response. Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial. - Adult patients with relapsed or refractory B-cell precursor acute lymphoblastic leukemia (ALL).
Tecartus Dosage and Administration
For autologous use only. For intravenous use only.
- Do NOT use a leukodepleting filter.
- Administer a lymphodepleting regimen of cyclophosphamide and fludarabine before infusion of TECARTUS. (2.2)
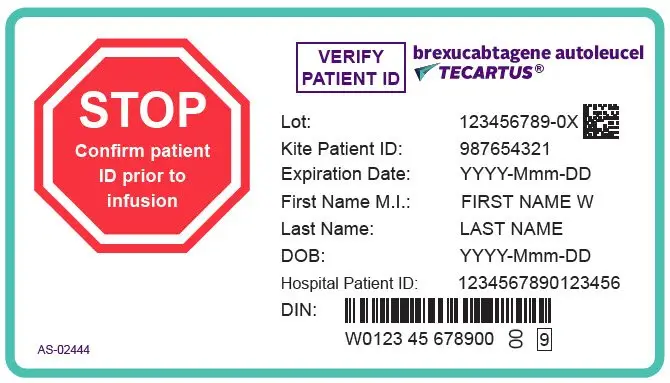
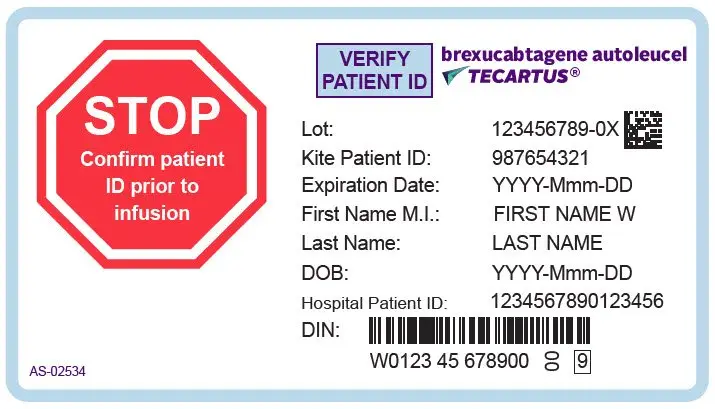
- Verify the patient's identity prior to infusion. (2.2)
- Premedicate with acetaminophen and diphenhydramine. (2.2)
- Confirm availability of tocilizumab prior to infusion. (2.2, 5.1)
- Dosing of TECARTUS is based on the number of chimeric antigen receptor (CAR)-positive viable T cells. (2.1)
- MCL: dose is 2 × 106 CAR-positive viable T cells per kg body weight, with a maximum of 2 × 108 CAR-positive viable T cells. (2.1)
- ALL: dose is 1 × 106 CAR-positive viable T cells per kg body weight, with a maximum of 1 × 108 CAR-positive viable T cells. (2.1)
- Administer TECARTUS in a certified healthcare facility. (2.2, 5.1, 5.2, 5.3)
Dosage Forms and Strengths
- TECARTUS is available as a cell suspension for infusion.
- MCL: Comprises a suspension of 2 × 106 CAR-positive viable T cells per kg of body weight, with a maximum of 2 × 108 CAR-positive viable T cells in approximately 68 mL. (3)
- ALL: Comprises a suspension of 1 × 106 CAR-positive viable T cells per kg of body weight, with a maximum of 1 × 108 CAR-positive viable T cells in approximately 68 mL. (3)
Contraindications
- None. (4)
Warnings and Precautions
- Hemophagocytic Lymphohistiocytosis/Macrophage Activation Syndrome: Administer treatment per institutional standards. (5.4)
- Hypersensitivity Reactions: Monitor for hypersensitivity reactions during infusion. (5.5)
- Severe Infections: Monitor patients for signs and symptoms of infection; treat appropriately. (5.6)
- Prolonged Cytopenias: Patients may exhibit Grade 3 or higher cytopenias for several weeks following TECARTUS infusion. Monitor complete blood counts. (5.7)
- Hypogammaglobulinemia: Monitor and provide replacement therapy. (5.8)
- Secondary Malignancies: In the event that a secondary malignancy occurs after treatment with TECARTUS, contact Kite at 1-844-454-KITE (5483). (5.9)
- Effects on Ability to Drive and Use Machines: Advise patients to refrain from driving and engaging in hazardous occupations or activities, such as operating heavy or potentially dangerous machinery, for at least 8 weeks after receiving TECARTUS. (5.10)
Adverse Reactions/Side Effects
The most common non-laboratory adverse reactions (incidence greater than or equal to 20%) are:
- MCL: fever, CRS, hypotension, encephalopathy, fatigue, tachycardia, arrhythmia, infection with pathogen unspecified, chills, hypoxia, cough, tremor, musculoskeletal pain, headache, nausea, edema, motor dysfunction, constipation, diarrhea, decreased appetite, dyspnea, rash, insomnia, pleural effusion, and aphasia. (6.1)
- ALL: fever, CRS, hypotension, encephalopathy, tachycardia, nausea, chills, headache, fatigue, febrile neutropenia, diarrhea, musculoskeletal pain, hypoxia, rash, edema, tremor, infection with pathogen unspecified, constipation, decreased appetite, and vomiting. (6.1).
To report SUSPECTED ADVERSE REACTIONS, contact Kite at 1-844-454-KITE (5483) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 10/2021
Full Prescribing Information
WARNING: CYTOKINE RELEASE SYNDROME and NEUROLOGIC TOXICITIES
- Cytokine Release Syndrome (CRS), including life-threatening reactions, occurred in patients receiving TECARTUS. Do not administer TECARTUS to patients with active infection or inflammatory disorders. Treat severe or life-threatening CRS with tocilizumab or tocilizumab and corticosteroids [see Dosage and Administration (2.2, 2.3), Warnings and Precautions (5.1)].
- Neurologic toxicities, including life-threatening reactions, occurred in patients receiving TECARTUS, including concurrently with CRS or after CRS resolution. Monitor for neurologic toxicities after treatment with TECARTUS. Provide supportive care and/or corticosteroids, as needed [see Dosage and Administration (2.2, 2.3), Warnings and Precautions (5.2)].
- TECARTUS is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS) called the YESCARTA and TECARTUS REMS Program [see Warnings and Precautions (5.3)].
1. Indications and Usage for Tecartus
TECARTUS is a CD19-directed genetically modified autologous T cell immunotherapy indicated for the treatment of:
1.1 Mantle Cell Lymphoma
Adult patients with relapsed or refractory mantle cell lymphoma (MCL).
This indication is approved under accelerated approval based on overall response rate and durability of response [see Clinical Studies (14)]. Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial.
2. Tecartus Dosage and Administration
For autologous use only. For intravenous use only.
Each single infusion bag of TECARTUS contains a suspension of chimeric antigen receptor (CAR)-positive T cells in approximately 68 mL.
2.2 Administration
TECARTUS is for autologous use only. The patient's identity must match the patient identifiers on the TECARTUS cassette and infusion bag. Do not infuse TECARTUS if the information on the patient-specific label does not match the intended patient.
3. Dosage Forms and Strengths
TECARTUS is available as a cell suspension for infusion.
- MCL: A single dose of TECARTUS contains 2 × 106 CAR-positive viable T cells per kg of body weight [maximum of 2 × 108 CAR-positive viable T cells (for patients 100 kg and above)] in approximately 68 mL suspension in an infusion bag [see How Supplied/Storage and Handling (16)].
- ALL: A single dose of TECARTUS contains 1 × 106 CAR-positive viable T cells per kg of body weight [maximum of 1 × 108 CAR-positive viable T cells (for patients 100 kg and above)] in approximately 68 mL suspension in an infusion bag [see How Supplied/Storage and Handling (16)].
5. Warnings and Precautions
5.1 Cytokine Release Syndrome
CRS, including fatal or life-threatening reactions, occurred following treatment with TECARTUS. CRS occurred in 91% (75/82) of patients with MCL, including ≥ Grade 3 (Lee grading system1) CRS in 18% of patients. Among the patients with MCL who died after receiving TECARTUS, one patient had a fatal CRS event. The median time to onset of CRS was three days (range: 1 to 13 days) and the median duration of CRS was ten days (range: 1 to 50 days) for patients with MCL. CRS occurred in 92% (72/78) of patients with ALL, including ≥ Grade 3 (Lee grading system1) CRS in 26% of patients. Three patients with ALL had ongoing CRS events at the time of death. The median time to onset of CRS was five days (range: 1 to 12 days) and the median duration of CRS was eight days (range: 2 to 63 days) for patients with ALL.
The incidence of CRS (first occurrence) within the first seven days after TECARTUS infusion was 83% (68/82) in patients with MCL and 90% (70/78) in patients with ALL. In all patients combined (MCL/ALL), the incidence of first CRS (first occurrence) within the first seven days after TECARTUS infusion was 86% (138/160).
Among patients with CRS, the key manifestations (>10%) were similar in MCL and ALL and included fever (93%), hypotension (62%), tachycardia (59%), chills (32%), hypoxia (31%), headache (21%), fatigue (20%), and nausea (13%). Serious events associated with CRS in MCL and ALL combined (≥ 2%) included hypotension, fever, hypoxia, tachycardia, and dyspnea [see Adverse Reactions (6)].
Ensure that a minimum of two doses of tocilizumab are available for each patient prior to infusion of TECARTUS. Monitor patients daily for at least seven days for patients with MCL and at least 14 days for patients with ALL at the certified healthcare facility following infusion for signs and symptoms of CRS. Monitor patients for signs or symptoms of CRS for four weeks after infusion. Counsel patients to seek immediate medical attention should signs or symptoms of CRS occur at any time [see Patient Counseling Information (17)]. At the first sign of CRS, institute treatment with supportive care, tocilizumab, or tocilizumab and corticosteroids as indicated [see Dosage and Administration (2.3)].
5.2 Neurologic Toxicities
Neurologic events, including those that were fatal or life-threatening, occurred following treatment with TECARTUS. Neurologic events occurred in 81% (66/82) of patients with MCL, including ≥ Grade 3 in 37% of patients. The median time to onset for neurologic events was six days (range: 1 to 32 days) with a median duration of 21 days (range: 2 to 454 days) in patients with MCL. Neurologic events occurred in 87% (68/78) of patients with ALL, including ≥ Grade 3 in 35% of patients. The median time to onset for neurologic events was seven days (range: 1 to 51 days) with a median duration of 15 days (range: 1 to 397 days) in patients with ALL. For patients with MCL, 54 (66%) patients experienced CRS before the onset of neurological events. Five (6%) patients did not experience CRS with neurologic events and eight patients (10%) developed neurological events after the resolution of CRS. Neurologic events resolved for 119 out of 134 (89%) patients treated with TECARTUS. Nine patients (three patients with MCL and six patients with ALL) had ongoing neurologic events at the time of death. For patients with ALL, neurologic events occurred before, during, and after CRS in 4 (5%), 57 (73%), and 8 (10%) of patients; respectively. Three patients (4%) had neurologic events without CRS. The onset of neurologic events can be concurrent with CRS, following resolution of CRS or in the absence of CRS.
The incidence of neurologic events (first occurrence) within the first seven days after TECARTUS infusion was 56% (46/82) in patients with MCL and 55% (43/78) in patients with ALL. In all patients combined (MCL/ALL) the incidence of neurologic events (first occurrence) within the first seven days after TECARTUS infusion was 56% (89/160). Ninety-one percent of all treated patients experienced the first CRS or neurological event within the first seven days after TECARTUS infusion.
The most common neurologic events (>10%) were similar in MCL and ALL and included encephalopathy (57%), headache (37%), tremor (34%), confusional state (26%), aphasia (23%), delirium (17%), dizziness (15%), anxiety (14%), and agitation (12%). Serious events (≥ 2%) including encephalopathy, aphasia, confusional state, and seizures occurred after treatment with TECARTUS.
Monitor patients daily for at least seven days for patients with MCL and at least 14 days for patients with ALL at the certified healthcare facility following infusion for signs and symptoms of neurologic toxicities. Monitor patients for signs or symptoms of neurologic toxicities for four weeks after infusion and treat promptly [see Dosage and Administration (2.3)].
5.3 YESCARTA and TECARTUS REMS Program
Because of the risk of CRS and neurologic toxicities, TECARTUS is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS) called the YESCARTA and TECARTUS REMS Program [see Boxed Warning and Warnings and Precautions (5.1 and 5.2)]. The required components of the YESCARTA and TECARTUS REMS Program are:
- Healthcare facilities that dispense and administer TECARTUS must be enrolled and comply with the REMS requirements. Certified healthcare facilities must have on-site, immediate access to tocilizumab, and ensure that a minimum of two doses of tocilizumab are available for each patient for infusion within two hours after TECARTUS infusion, if needed for treatment of CRS.
- Certified healthcare facilities must ensure that healthcare providers who prescribe, dispense, or administer TECARTUS are trained in the management of CRS and neurologic toxicities.
Further information is available at www.YescartaTecartusREMS.com or 1-844-454-KITE (5483).
5.4 Hemophagocytic Lymphohistiocytosis/Macrophage Activation Syndrome
Hemophagocytic Lymphohistiocytosis/Macrophage Activation Syndrome (HLH/MAS), including life-threatening reactions, occurred following treatment with TECARTUS. HLH/MAS occurred in 4% (3/78) of patients with ALL. Two patients experienced Grade 3 events and 1 patient experienced a Grade 4 event. The median time to onset for HLH/MAS was 8 days (range: 6 to 9 days) with a median duration of 5 days (range: 2 to 8 days).
All three patients with HLH/MAS had concurrent CRS symptoms and neurologic events after TECARTUS infusion. Treatment of HLH/MAS should be administered per institutional standards.
5.5 Hypersensitivity Reactions
Serious hypersensitivity reactions, including anaphylaxis, may occur due to dimethyl sulfoxide (DMSO) or residual gentamicin in TECARTUS.
5.6 Severe Infections
Severe or life-threatening infections occurred in patients after TECARTUS infusion. Infections (all grades) occurred in 56% (46/82) of patients with MCL and 44% (34/78) of patients with ALL. Grade 3 or higher infections, including bacterial, viral, and fungal infections, occurred in 30% of patients with ALL and MCL. TECARTUS should not be administered to patients with clinically significant active systemic infections. Monitor patients for signs and symptoms of infection before and after TECARTUS infusion and treat appropriately. Administer prophylactic antimicrobials according to local guidelines.
Febrile neutropenia was observed in 6% of patients with MCL and 35% of patients with ALL after TECARTUS infusion and may be concurrent with CRS. The febrile neutropenia in 27 (35%) of patients with ALL includes events of "febrile neutropenia" (11 (14%)) plus the concurrent events of "fever" and "neutropenia" (16 (21%)). In the event of febrile neutropenia, evaluate for infection and manage with broad spectrum antibiotics, fluids, and other supportive care as medically indicated.
In immunosuppressed patients, life-threatening and fatal opportunistic infections have been reported. The possibility of rare infectious etiologies (e.g., fungal and viral infections such as HHV-6 and progressive multifocal leukoencephalopathy) should be considered in patients with neurologic events and appropriate diagnostic evaluations should be performed.
5.7 Prolonged Cytopenias
Patients may exhibit cytopenias for several weeks following lymphodepleting chemotherapy and TECARTUS infusion. In patients with MCL, Grade 3 or higher cytopenias not resolved by Day 30 following TECARTUS infusion occurred in 55% (45/82) of patients and included thrombocytopenia (38%), neutropenia (37%), and anemia (17%). In patients with ALL who were responders to TECARTUS treatment, Grade 3 or higher cytopenias not resolved by Day 30 following TECARTUS infusion occurred in 20% (7/35) of the patients and included neutropenia (12%) and thrombocytopenia (12%); Grade 3 or higher cytopenias not resolved by Day 60 following TECARTUS infusion occurred in 11% (4/35) of the patients and included neutropenia (9%) and thrombocytopenia (6%). Monitor blood counts after TECARTUS infusion.
5.8 Hypogammaglobulinemia
B cell aplasia and hypogammaglobulinemia can occur in patients receiving treatment with TECARTUS. Hypogammaglobulinemia was reported in 16% (13/82) of patients with MCL and 9% (7/78) of patients with ALL. Monitor immunoglobulin levels after treatment with TECARTUS and manage using infection precautions, antibiotic prophylaxis, and immunoglobulin replacement.
The safety of immunization with live viral vaccines during or following TECARTUS treatment has not been studied. Vaccination with live virus vaccines is not recommended for at least six weeks prior to the start of lymphodepleting chemotherapy, during TECARTUS treatment, and until immune recovery following treatment with TECARTUS.
5.9 Secondary Malignancies
Patients treated with TECARTUS may develop secondary malignancies. Monitor life-long for secondary malignancies. In the event that a secondary malignancy occurs, contact Kite at 1-844-454-KITE (5483) to obtain instructions on patient samples to collect for testing.
5.10 Effects on Ability to Drive and Use Machines
Due to the potential for neurologic events, including altered mental status or seizures, patients receiving TECARTUS are at risk for altered or decreased consciousness or coordination in the eight weeks following TECARTUS infusion. Advise patients to refrain from driving and engaging in hazardous occupations or activities, such as operating heavy or potentially dangerous machinery, during this initial period.
6. Adverse Reactions/Side Effects
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Cytokine Release Syndrome [see Warnings and Precautions (5.1)]
- Neurologic Toxicities [see Warnings and Precautions (5.2)]
- Hemophagocytic Lymphohistiocytosis/Macrophage Activation Syndrome [see Warnings and Precautions (5.4)]
- Hypersensitivity Reactions [see Warnings and Precautions (5.5)]
- Severe Infections [see Warnings and Precautions (5.6)]
- Prolonged Cytopenias [see Warnings and Precautions (5.7)]
- Hypogammaglobulinemia [see Warnings and Precautions (5.8)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
6.2 Immunogenicity
TECARTUS has the potential to induce anti-product antibodies, which has been evaluated using an enzyme-linked immunosorbent assay (ELISA) for the detection of binding antibodies against FMC63, the originating antibody of the anti-CD19 CAR. To date, no anti-CAR T-cell antibody immunogenicity has been observed in ZUMA-2. Based on an initial screening assay in ZUMA-2, 17 of 82 patients tested positive for antibodies at any time point; however, a confirmatory orthogonal cell-based assay demonstrated that all 17 patients were antibody negative at all time points tested. Based on an initial screening assay in ZUMA-3, 16 of 100 patients tested positive for antibodies at any timepoint. Among patients with evaluable samples for confirmatory testing, two patients were confirmed to be antibody-positive after treatment. One of the two patients had a confirmed positive antibody result at Month 6. The second patient had a confirmed antibody result at retreatment Day 28 and Month 3. There is no evidence that the kinetics of initial expansion and persistence of TECARTUS, or the safety or effectiveness of TECARTUS, were altered in these patients.
8. Use In Specific Populations
8.4 Pediatric Use
The safety and efficacy of TECARTUS have not been established in pediatric patients.
8.5 Geriatric Use
Of the 82 patients treated with TECARTUS for MCL, 42 (51%) were 65 years of age and over. Of the 78 patients treated with TECARTUS for ALL, 12 (15%) were 65 years of age and over. No overall differences in safety or effectiveness were observed between these patients and younger patients.
11. Tecartus Description
TECARTUS (brexucabtagene autoleucel) is a CD19-directed genetically modified autologous T cell immunotherapy. To prepare TECARTUS, a patient's own T cells are harvested and genetically modified ex vivo by retroviral transduction to express a chimeric antigen receptor (CAR) comprising a murine anti-CD19 single-chain variable fragment (scFv) linked to CD28 and CD3-zeta co-stimulatory domains. The anti-CD19 CAR T cells are expanded and infused back into the patient, where they can recognize and eliminate CD19-expressing target cells.
TECARTUS is prepared from the patient's peripheral blood mononuclear cells, which are obtained via a standard leukapheresis procedure. The mononuclear cells are enriched for T cells and activated with anti-CD3 and anti-CD28 antibodies in the presence of IL-2, then transduced with a replication-incompetent retroviral vector containing the anti-CD19 CAR transgene. The transduced T cells are expanded in cell culture, washed, formulated into a suspension, and cryopreserved. The manufacture of TECARTUS includes a T cell enrichment step that may reduce the likelihood of circulating CD19-expressing tumor cells in patients' leukapheresis material driving the activation, expansion, and exhaustion of the anti-CD19 CAR T cells during the ex vivo manufacturing process. The product must pass a sterility test before release for shipping as a frozen suspension in a patient-specific infusion bag. The product is thawed prior to infusion [see Dosage and Administration (2.2), How Supplied/Storage and Handling (16)].
In addition to T cells, TECARTUS may contain natural killer (NK) cells. The formulation contains CryoStor (dimethyl sulfoxide [DMSO], final concentration, 5%), sodium chloride (NaCl), and Human Serum Albumin (HSA).
12. Tecartus - Clinical Pharmacology
12.1 Mechanism of Action
TECARTUS, a CD19-directed genetically modified autologous T cell immunotherapy, binds to CD19-expressing cancer cells and normal B cells. Studies demonstrated that following anti-CD19 CAR T cell engagement with CD19-expressing target cells, the CD28 and CD3-zeta co-stimulatory domains activate downstream signaling cascades that lead to T cell activation, proliferation, acquisition of effector functions, and secretion of inflammatory cytokines and chemokines. This sequence of events leads to killing of CD19-expressing cells.
12.2 Pharmacodynamics
After TECARTUS infusion, pharmacodynamic responses were evaluated over a four-week interval by measuring transient elevation of cytokines, chemokines, and other molecules in blood. Levels of cytokines and chemokines such as IL-6, IL-8, IL-10, IL-15, TNF-α, IFN-γ, and sIL2Rα were analyzed. Peak elevation was generally observed within 8 days after infusion, and levels generally returned to baseline within 28 days.
Due to the on-target effect of TECARTUS, a period of B cell aplasia is expected.
12.3 Pharmacokinetics
Following infusion (target dose of 2 × 106 anti-CD19 CAR T cells/kg) of TECARTUS in ZUMA-2, anti-CD19 CAR T cells exhibited an initial rapid expansion followed by a decline to near baseline levels by three months. Peak levels of anti-CD19 CAR T cells occurred within the first 15 days after TECARTUS infusion. Following infusion (target dose of 1 × 106 anti-CD19 CAR T cells/kg) of TECARTUS in ZUMA-3 (Phase 2), anti-CD19 CAR T cells exhibited an initial rapid expansion followed by a decline to near baseline levels by 6 months. Median anti- CD19 CAR T-cell time to peak was 15 days after TECARTUS infusion.
14. Clinical Studies
14.1 Relapsed or Refractory Mantle Cell Lymphoma
A single-arm, open-label, multicenter trial (ZUMA-2; NCT02601313) evaluated the efficacy and safety of a single infusion of TECARTUS in adult patients with relapsed or refractory mantle cell lymphoma (MCL) who had previously received anthracycline- or bendamustine-containing chemotherapy, an anti-CD20 antibody, and a Bruton tyrosine kinase inhibitor (BTKi; ibrutinib or acalabrutinib). Eligible patients also had disease progression after their last regimen or refractory disease to their most recent therapy. The study excluded patients with active or serious infections, prior allogeneic hematopoietic stem cell transplant (HSCT), detectable cerebrospinal fluid malignant cells or brain metastases, and any history of central nervous system (CNS) lymphoma or CNS disorders.
Seventy-four patients were leukapheresed, five (7%) of whom did not begin conditioning chemotherapy or receive TECARTUS: three (4%) experienced manufacturing failure, one (1%) died of progressive disease, and one (1%) withdrew from the study. One patient (1%) received lymphodepleting chemotherapy but did not receive TECARTUS due to ongoing active atrial fibrillation. Sixty-eight of the patients who were leukapheresed received a single infusion of TECARTUS, and 60 of these patients were followed for at least six months after their first objective disease response, qualifying them as efficacy-evaluable. Among the 60 efficacy-evaluable patients, 2 × 106 CAR-positive viable T cells/kg were administered to 54 (90%). The remaining six (10%) patients received doses of 1.0, 1.6, 1.8, 1.8, 1.9, and 1.9 × 106 CAR-positive viable T cells/kg.
Of the 60 efficacy-evaluable patients, the median age was 65 years (range: 38 to 79 years), 51 (85%) were male, and 56 (93%) were white. Most (50 patients; 83%) had stage IV disease. Twenty patients (33% of 60) had baseline bone marrow examinations performed per protocol; of these, ten (50%) were negative, eight (40%) were positive, and two (10%) were indeterminate. The median number of prior therapies among all 60 efficacy-evaluable patients was three (range: two to five). Twenty-six (43%) of the patients had relapsed after or were refractory to autologous HSCT. Twenty-one (35%) had relapsed after their last therapy for MCL, while 36 (60%) were refractory to their last therapy for MCL. Among the 60 efficacy-evaluable patients, 14 (23%) had blastoid MCL. Following leukapheresis and prior to administration of TECARTUS, 21 (35%) of the 60 patients received bridging therapy. Sixteen (27%) were treated with a BTKi, 9 (15%) with a corticosteroid, and 4 (7%) with both a BTKi and a corticosteroid.
Among the 60 efficacy-evaluable patients, the median time from leukapheresis to product delivery was 15 days (range: 11 to 28 days), and the median time from leukapheresis to product infusion was 27 days (range: 19 to 63 days). The protocol-defined lymphodepleting chemotherapy regimen of cyclophosphamide 500 mg/m2 intravenously and fludarabine 30 mg/m2 intravenously, both given on each of the fifth, fourth, and third days before TECARTUS infusion, was administered to 53 (88%) of the 60 efficacy-evaluable patients. The remaining seven patients (12%) either received lymphodepletion over four or more days or received TECARTUS four or more days after completing lymphodepletion. All treated patients received TECARTUS infusion on Day 0 and were hospitalized until at least Day 7.
The primary endpoint of objective response rate (ORR) per the Lugano Classification (2014) in patients treated with TECARTUS as determined by an independent review committee is provided in Table 7. The median time to response was 28 days (range: 24 to 92 days) with a median follow-up time for DOR of 8.6 months.
| Efficacy-Evaluable Patients N = 60 | All Leukapheresed Patients (ITT) N = 74 |
|
|---|---|---|
| CI, confidence interval; CR, complete remission; DOR, duration of response; NE, not estimable; NR, not reached; PR, partial remission. | ||
|
||
| Response Rate | ||
| Objective Response Rate*, n (%) [95% CI] | 52 (87%) [75, 94] | 59 (80%) [69, 88] |
| Complete Remission Rate, n (%) [95% CI] | 37 (62%) [48, 74] | 41 (55%) [43, 67] |
| Partial Remission Rate, n (%) [95% CI] | 15 (25%) [15, 38] | 18 (24%) [15, 36] |
| Duration of Response (DOR) | ||
| Median in months [95% CI] Range† in months | NR [8.6, NE] 0.0+, 29.2+ | NR [8.6, NE] 0.0+, 29.2+ |
| DOR, if best response is CR, median in months [95% CI] Range† in months | NR [13.6, NE] 1.9+, 29.2+ | NR [13.6, NE] 0.0+, 29.2+ |
| DOR, if best response is PR, median in months [95% CI] Range† in months | 2.2 [1.5, 5.1] 0.0+, 22.1+ | 2.2 [1.5, 5.1] 0.0+, 22.1+ |
| Median Follow-up for DOR in months‡ | 8.6 | 8.1 |
14.2 Relapsed or Refractory B-cell precursor Acute Lymphoblastic Leukemia
The efficacy of TECARTUS was evaluated in ZUMA-3 (NCT02614066), an open-label, single-arm, multicenter trial in adult patients with relapsed or refractory B-cell precursor acute lymphoblastic leukemia (ALL). Eligible patients were adults with primary refractory ALL, first relapse following a remission lasting ≤ 12 months, relapsed or refractory ALL after second-line or higher therapy, or relapsed or refractory ALL at least 100 days after allogeneic stem cell transplantation (HSCT). The study excluded patients with active or serious infections, active graft-vs-host disease or taking immunosuppressive medications within 4 weeks prior to enrollment, and any history of CNS disorders, including CNS-2 disease with neurologic changes and CNS-3 disease irrespective of neurological changes. Treatment consisted of lymphodepleting chemotherapy (fludarabine 25 mg/m2 iv daily on Days -4, -3 and -2; cyclophosphamide 900 mg/m2 iv on Day -2) followed by a single intravenous infusion of TECARTUS at a target dose of 1 × 106 anti-CD19 CAR T cells/kg (maximum 1 × 108 cells) on Day 0. All treated patients were hospitalized until at least Day 7.
Seventy-one patients were enrolled and leukapheresed; six of these patients did not receive TECARTUS due to manufacturing failure, eight patients were not treated primarily due to adverse events following leukapheresis, two patients underwent leukapheresis and received lymphodepleting chemotherapy but were not treated with TECARTUS, and one patient treated with TECARTUS was inevaluable for efficacy. Among the remaining 54 efficacy-evaluable patients, the median time from leukapheresis to product delivery was 16 days (range: 11 to 39 days) and the median time from leukapheresis to TECARTUS infusion was 29 days (range: 20 to 60 days).
Of the 54 patients who were efficacy evaluable, the median age was 40 years (range: 19 to 84 years), 61% were male, and 67% were White, 6% were Asian, 2% were Black or African American, and 2% were American Indian or Alaska Native. At enrollment, 46% had refractory relapse, 26% had primary refractory disease, 20% had untreated second or later relapse, and 7% had first untreated relapse. Among prior therapies, 43% of patients were previously treated with allo-SCT, 46% with blinatumomab, and 22% with inotuzumab. Twenty-six percent of patients were Philadelphia chromosome positive (Ph+). Fifty (93%) patients had received bridging therapy between leukapheresis and lymphodepleting chemotherapy to control disease burden.
The efficacy of TECARTUS was established on the basis of complete remission (CR) within 3 months after infusion and the duration of CR (DOCR). Twenty-eight (51.9%) of the 54 evaluable patients achieved CR, and with a median follow-up for responders of 7.1 months, the median DOCR was not reached (Table 8). The median time to CR was 56 days (range: 25 to 86 days). All efficacy evaluable patients had potential follow-up for ≥ 10 months with a median actual follow-up time of 12.3 months (range: 0.3 to 22.1 months).
| Efficacy Evaluable Patients*
N= 54 | All Leukapheresed Patients N = 71 |
|
|---|---|---|
| CI, confidence interval; CR, complete remission; CRi, complete remission with incomplete blood count recovery; DOR, duration of remission; NE, not estimable; NR, not reached, OCR, overall complete remission; NE, not estimable | ||
|
||
| OCR rate (CR + CRi), n (%) [95% CI] | 35 (64.8) [51, 77] | 36 (50.7) [39, 63] |
| CR rate, n (%) [95% CI] | 28 (51.9) [37.8, 65.7] | 29 (40.9) [29.3, 53.2] |
| Duration of Remission, Median in months [95% CI] (Range† in months) | 13.6 [9.4, NE] (0.03+, 16.07+) | 13.6 [8.7, NE] (0.03+, 16.07+) |
| DOR, if best response is CR, median in months [95% CI] (Range in months) | NR [9.6, NE] (0.03+, 16.07+) | 13.6 [9.4, NE] (0.03+, 16.07+) |
| DOR, if best response is CRi, median in months [95% CI] (Range in months) | 8.7 [1.0, NE] (0.03+, 10.15+) | 8.7 [1.0, NE] (0.03+, 10.15+) |
| Median Follow-up for CR in months | 7.1 (0.03+, 16.1+) | 5.0 (0.03+, 16.1+) |
15. References
- Lee DW et al (2014). Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014 Jul 10; 124(2): 188–195.
16. How is Tecartus supplied
TECARTUS is supplied in an infusion bag containing approximately 68 mL of frozen suspension of genetically modified autologous T cells in 5% DMSO and human serum albumin.
Each TECARTUS infusion bag is individually packed in a metal cassette. TECARTUS is stored in the vapor phase of liquid nitrogen and supplied in a liquid nitrogen dry shipper.
| Indication | Infusion Bag NDC number | Metal Cassette NDC number |
|---|---|---|
| MCL | 71287-219-01 | 71287-219-02 |
| ALL | 71287-220-01 | 71287-220-02 |
17. Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Ensure that patients understand the risk of manufacturing failure (4% in clinical trial). In case of a manufacturing failure, a second manufacturing of TECARTUS may be attempted. In addition, while the patient awaits the product, additional chemotherapy (not the lymphodepletion) may be necessary and may increase the risk of adverse events during the pre-infusion period.
Advise patients to seek immediate attention for any of the following:
- Cytokine Release Syndrome (CRS) - Signs or symptoms associated with CRS, including fever, chills, fatigue, tachycardia, nausea, hypoxia, and hypotension [see Warnings and Precautions (5.1) and Adverse Reactions (6)].
- Neurologic Toxicities - Signs or symptoms associated with neurologic events, including encephalopathy, seizures, changes in level of consciousness, speech disorders, tremors, and confusion [see Warnings and Precautions (5.2) and Adverse Reactions (6)].
- Severe Infections - Signs or symptoms associated with infection [see Warnings and Precautions (5.6) and Adverse Reactions (6)].
- Prolonged Cytopenias - Signs or symptoms associated with bone marrow suppression, including neutropenia, anemia, thrombocytopenia, or febrile neutropenia [see Warnings and Precautions (5.7) and Adverse Reactions (6)].
Advise patients of the need to:
- Refrain from driving or operating heavy or potentially dangerous machinery for at least eight weeks after TECARTUS infusion [see Warnings and Precautions (5.10)].
- Have periodic monitoring of blood counts.
- Contact Kite at 1-844-454-KITE (5483) if they are diagnosed with a secondary malignancy [see Warnings and Precautions (5.9)].
| TECARTUS
brexucabtagene autoleucel suspension |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| TECARTUS
brexucabtagene autoleucel suspension |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Kite Pharma, Inc. (963353359) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Kite Pharma, Inc. | 963353359 | MANUFACTURE(71287-219, 71287-220) , LABEL(71287-219, 71287-220) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Kite Pharma, Inc. | 116931311 | ANALYSIS(71287-219, 71287-220) | |