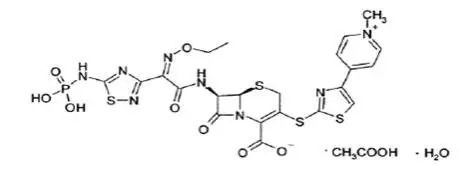
Drug Detail:Teflaro (Ceftaroline [ sef-ta-roe-leen ])
Drug Class: Next generation cephalosporins
Highlights of Prescribing Information
TEFLARO® (ceftaroline fosamil) for injection, for intravenous use
Initial U.S. Approval: 2010
Indications and Usage for Teflaro
Teflaro is a cephalosporin antibacterial indicated in adult and pediatric patients for the treatment of the following infection caused by designated susceptible bacteria:
- Acute bacterial skin and skin structure infections (ABSSSI) in adult and pediatric patients (at least 34 weeks gestational age and 12 days postnatal age) (1.1)
- Community-acquired bacterial pneumonia (CABP) in adult and pediatric patients 2 months of age and older (1.2)
To reduce the development of drug-resistant bacteria and maintain the effectiveness of Teflaro and other antibacterial drugs, Teflaro should be used only to treat or prevent infections that are proven or strongly suspected to be caused by bacteria. (1.3)
Teflaro Dosage and Administration
Dosage of Teflaro by Indication in Adult and Pediatric Patients (2.1, 2.2)
| Indication | Age Range | Dosage | Infusion Time | Duration |
| Acute Bacterial Skin and Skin Structure Infections (ABSSSI) | 18 years and older | 600 mg every 12 hours | 5 to 60 minutes | 5 to 14 days |
| ≥2 years to < 18 years (> 33 kg) | 400 mg every 8 hours OR 600 mg every 12 hours | 5 to 60 minutes | 5 to 14 days | |
| ≥2 years to < 18 years (≤ 33kg) | 12 mg/kg every 8 hours | 5 to 60 minutes | 5 to 14 days | |
| 2 months to < 2 years | 8 mg/kg every 8 hours | 5 to 60 minutes | 5 to 14 days | |
| 0* to < 2 months | 6 mg/kg every 8 hours | 30 to 60 minutes | 5 to 14 days |
*Gestational age 34 weeks and older and postnatal age 12 days and older
| Indication | Age Range | Dosage | Infusion Time | Duration |
| Community Acquired Bacterial Pneumonia (CABP) | 18 years and older | 600 mg every 12 hours | 5 to 60 minutes | 5 to 7 days |
| ≥2 years to < 18 years (> 33 kg) | 400 mg every 8 hours OR 600 mg every 12 hours | 5 to 60 minutes | 5 to 14 days | |
| ≥2 years to < 18 years (≤ 33kg) | 12 mg/kg every 8 hours | 5 to 60 minutes | 5 to 14 days | |
| 2 months to < 2 years | 8 mg/kg every 8 hours | 5 to 60 minutes | 5 to 14 days |
- Dosage adjustment is required in adult patients with creatinine clearance (CrCl) < 50 mL/min and in End-stage Renal Disease (ESRD) including hemodialysis (2.3)
- There is insufficient information to recommend a dosage regimen for pediatric patients with CrCL < 50 mL/min/1.73 m2 (2.3)
Dosage Forms and Strengths
For Injection: 600 mg or 400 mg of sterile ceftaroline fosamil powder in single-dose 20 mL vials. The powder is constituted and further diluted for intravenous injection. (3)
Contraindications
- Known serious hypersensitivity to ceftaroline or other members of the cephalosporin class. (4)
Warnings and Precautions
- Serious hypersensitivity (anaphylactic) reactions have been reported with beta-lactam antibacterial drugs, including Teflaro. If a hypersensitivity reaction occurs, discontinue Teflaro. (5.1)
-
Clostridioides difficile-associated diarrhea (CDAD) has been reported with nearly all systemic antibacterial agents, including Teflaro. Evaluate if diarrhea occurs. (5.2)
- Neurological adverse reactions have been reported in patients treated with cephalosporins, including Teflaro. If neurological adverse reactions occur, consider discontinuing Teflaro or making appropriate dosage adjustments in patients with renal impairment. (2.3, 5.3)
- Direct Coombs’ test seroconversion has been reported with Teflaro. If anemia develops during or after therapy, a diagnostic workup for drug-induced hemolytic anemia should be performed and consideration given to discontinuation of Teflaro. (5.4)
Adverse Reactions/Side Effects
The most common adverse reactions occurring in >2% of adult patients and ≥3% of pediatric patients are diarrhea, nausea, and rash. Additional adverse reactions that occurred in ≥3% of pediatric patients include vomiting and pyrexia. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Allergan at 1-800-678-1605 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 12/2021
Related/similar drugs
amoxicillin, doxycycline, ciprofloxacin, metronidazole, azithromycin, clindamycin, AugmentinFull Prescribing Information
1. Indications and Usage for Teflaro
1.1 Acute Bacterial Skin and Skin Structure Infections
Teflaro is indicated in adult and pediatric patients (at least 34 weeks gestational age and 12 days postnatal age) for the treatment of acute bacterial skin and skin structure infections (ABSSSI) caused by susceptible isolates of the following Gram-positive and Gram-negative microorganisms: Staphylococcus aureus (including methicillin-susceptible and -resistant isolates), Streptococcus pyogenes, Streptococcus agalactiae, Escherichia coli, Klebsiella pneumoniae, and Klebsiella oxytoca [see Dosage and Administration (2.2) and Use in Specific Populations (8.4)].
1.2 Community-Acquired Bacterial Pneumonia
Teflaro is indicated in adult and pediatric patients 2 months of age and older for the treatment of community-acquired bacterial pneumonia (CABP) caused by susceptible isolates of the following Gram-positive and Gram-negative microorganisms: Streptococcus pneumoniae (including cases with concurrent bacteremia), Staphylococcus aureus (methicillin-susceptible isolates only), Haemophilus influenzae, Klebsiella pneumoniae, Klebsiella oxytoca, and Escherichia coli.
1.3 Usage
To reduce the development of drug-resistant bacteria and maintain the effectiveness of Teflaro and other antibacterial drugs, Teflaro should be used to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. Appropriate specimens for microbiological examination should be obtained in order to isolate and identify the causative pathogens and to determine their susceptibility to ceftaroline. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
2. Teflaro Dosage and Administration
2.1 Recommended Dosage in Adult Patients
The recommended dosage of Teflaro is 600 mg administered every 12 hours by intravenous (IV) infusion over 5 to 60 minutes in patients ≥ 18 years of age. The duration of therapy should be guided by the severity and site of infection and the patient’s clinical and bacteriological progress.
The recommended dosage and administration by infection is described in Table 1.
| Indication | Dosage | Frequency | Infusion Time
| Recommended Duration of Treatment |
| Acute Bacterial Skin and Skin Structure Infections (ABSSSI) | 600 mg | Every 12 hours | 5 to 60 minutes | 5-14 days |
| Community-Acquired Bacterial Pneumonia (CABP) | 600 mg | Every 12 hours | 5 to 60 minutes | 5-7 days |
2.2 Recommended Dosage in Pediatric Patients
The recommended dosage of Teflaro in pediatric patients is based on the age and weight of the child. The duration of therapy should be guided by the severity, site of infection and the patient’s clinical and bacteriological progress.
Pediatric Patients 2 Months of Age and Older
- For pediatric patients 2 months of age and older, Teflaro is administered every 8 hours by intravenous infusion over 5 to 60 minutes.
- Teflaro dosing regimen is dependent on the type of infection (ABSSSI, CABP). See dosing Table 2 below.
| Indication | Age Range | Dosage and Frequency | Infusion time | Recommended Duration of Treatment |
| Acute Bacterial Skin and Skin Structure Infections (ABSSSI) OR Community-Acquired Bacterial Pneumonia (CABP) | 2 months to < 2 years | 8 mg/kg every 8 hours | 5 to 60 minutes | 5-14 days |
| > 2 years to < 18 years (< 33 kg) | 12 mg/kg every 8 hours | |||
| > 2 years to < 18 years (> 33 kg) | 400 mg every 8 hours OR 600 mg every 12 hours |
Pediatric Patients Less Than 2 Months of Age
- Teflaro is administered every 8 hours by intravenous infusion over 30 to 60 minutes for patients less than 2 months of age.
- Teflaro dosing regimen is only recommended for patients with ABSSSI. See dosing Table 3 below.
- Concentrations of Teflaro in the cerebrospinal fluid have not been evaluated [see Use in Specific Populations (8.4)].
- There is no information for dosing Teflaro in infants less than 34 weeks gestational age and less than 12 days postnatal age.
| Indication | Age Range | Dosage and Frequency | Infusion time | Recommended Duration of Treatment |
| Acute Bacterial Skin and Skin Structure Infections (ABSSSI) | 0* to < 2 months | 6 mg/kg every 8 hours | 30 to 60 minutes | 5-14 days |
*Gestational age 34 weeks and older and postnatal age 12 days and older.
2.3 Dosage Adjustments in Patients with Renal Impairment
Adults: No dosage adjustment is required in adult patients with CrCL > 50 mL/min. The dose in adult patients should be adjusted when creatinine clearance (CrCL) is < 50 mL/min as shown below (see Table 4).
| Estimated CrCla (mL/min) | Recommended Dosage Regimen for Teflaro |
| > 50 | No dosage adjustment necessary |
| > 30 to ≤ 50 | 400 mg IV (over 5 to 60 minutes) every 12 hours |
| ≥ 15 to ≤ 30 | 300 mg IV (over 5 to 60 minutes) every 12 hours |
| End-stage renal disease, including hemodialysisb | 200 mg IV (over 5 to 60 minutes) every 12 hoursc |
a Creatinine clearance (CrCl) estimated using the Cockcroft-Gault formula.
b End-stage renal disease is defined as CrCl < 15 mL/min.
c Teflaro is hemodialyzable; thus Teflaro should be administered after hemodialysis on hemodialysis days.
Pediatrics: No dosage adjustment is required in pediatric patients with CrCL > 50 mL/min/1.73 m2, estimated using the Schwartz equation. There is insufficient information to recommend a dosage regimen for pediatric patients with CrCL < 50 mL/min/1.73 m2.
3. Dosage Forms and Strengths
For Injection: Teflaro is supplied as 600 mg or 400 mg of pale yellowish-white to light yellow sterile ceftaroline fosamil (equivalent to 668 mg and 446 mg, respectively, of ceftaroline fosamil monoacetate monohydrate) powder in single-dose, 20 mL clear glass vials. The powder is constituted and further diluted for intravenous injection.
4. Contraindications
Teflaro is contraindicated in patients with known serious hypersensitivity to ceftaroline or other members of the cephalosporin class. Anaphylaxis has been reported with ceftaroline.
5. Warnings and Precautions
5.1 Hypersensitivity Reactions
Serious and occasionally fatal hypersensitivity (anaphylactic) reactions and serious skin reactions have been reported in patients receiving beta-lactam antibacterial drugs. Before therapy with Teflaro is instituted, careful inquiry about previous hypersensitivity reactions to other cephalosporins, penicillins, or carbapenems should be made. Maintain clinical supervision if this product is to be given to a penicillin- or other beta-lactam-allergic patient, because cross sensitivity among beta-lactam antibacterial agents has been clearly established.
If an allergic reaction to Teflaro occurs, discontinue Teflaro and institute appropriate treatment and supportive measures.
5.2 Clostridioides difficile-Associated Diarrhea
Clostridioides difficile-associated diarrhea (CDAD) has been reported for nearly all systemic antibacterial agents, including Teflaro, and may range in severity from mild diarrhea to fatal colitis.
Treatment with antibacterial agents alters the normal flora of the colon and may permit overgrowth of C. difficile.
C. difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin-producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibiotic use. Careful medical history is necessary because CDAD has been reported to occur more than 2 months after the administration of antibacterial agents.
If CDAD is suspected or confirmed, antibacterials not directed against C. difficile should be discontinued, if possible. Appropriate fluid and electrolyte management, protein supplementation, antibiotic treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated [see Adverse Reactions (6.1)].
5.3 Neurological Adverse Reactions
Neurological adverse reactions have been reported during postmarketing surveillance in patients treated with cephalosporins, including Teflaro. These reactions include encephalopathy and seizures [see Adverse Reactions (6.2)]. Most cases occurred in patients with renal impairment who did not receive appropriate dosage adjustment. The neurological adverse reactions were reversible and resolved after discontinuation of Teflaro or after hemodialysis. If neurological adverse reactions associated with Teflaro therapy occur, consider discontinuing Teflaro or making appropriate dosage adjustments in patients with renal impairment [see Dosage and Administration (2.3)].
5.4 Direct Coombs’ Test Seroconversion
Seroconversion from a negative to a positive direct Coombs’ test result occurred in 120/1114 (10.8%) of adult patients receiving Teflaro and 49/1116 (4.4%) of patients receiving comparator drugs in the four pooled adult Phase 3 trials.
In the pooled adult Phase 3 CABP trials, 51/520 (9.8%) of Teflaro-treated patients compared to 24/534 (4.5%) of ceftriaxone-treated patients seroconverted from a negative to a positive direct Coombs’ test result. No adverse reactions representing hemolytic anemia were reported in any treatment group.
Seroconversion from a negative to a positive direct Coombs’ test result occurred in 42/234 (17.9%) of children receiving Teflaro and 3/93 (3.2%) of patients receiving comparator drugs in the three pooled pediatric trials. No adverse reactions representing hemolytic anemia were reported in any treatment group.
If anemia develops during or after treatment with Teflaro, drug-induced hemolytic anemia should be considered. Diagnostic studies including a direct Coombs’ test, should be performed. If drug-induced hemolytic anemia is suspected, discontinuation of Teflaro should be considered and supportive care should be administered to the patient (i.e. transfusion) if clinically indicated.
6. Adverse Reactions/Side Effects
The following serious adverse reactions are described in greater detail in the Warnings and Precautions section
- Hypersensitivity Reactions [see Warnings and Precautions (5.1)]
-
Clostridioides difficile-Associated diarrhea [see Warnings and Precautions (5.2)]
- Neurological Adverse Reactions [see Warnings and Precautions (5.3)]
- Direct Coombs’ Test Seroconversion [see Warnings and Precautions (5.4)]
6.2 Postmarketing Experience
The following adverse reactions have been identified during post approval use of Teflaro in adult patients. Because these adverse reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Blood and lymphatic system disorders: Agranulocytosis, leukopenia, eosinophilic pneumonia.
Nervous system disorders: Encephalopathy, seizures [see Warnings and Precautions (5.3)]
8. Use In Specific Populations
8.4 Pediatric Use
The safety and effectiveness of Teflaro in the treatment of ABSSSI have been established in pediatric patients (at least 34 weeks gestational age and 12 days postnatal age).
The safety and effectiveness of Teflaro in the treatment of CABP have been established in the age groups 2 months to less than 18 years old.
Use of Teflaro in these age groups is supported by evidence from adequate and well-controlled studies of Teflaro in adults with additional pharmacokinetic and safety data in pediatric patients 2 months of age and older with ABSSSI or CABP [see Clinical Studies (14.1 and 14.2)]. Use of Teflaro in pediatric patients less than 2 months of age was supported by pharmacokinetic and safety data in 11 infants at least 34 weeks gestational age and 12 days postnatal age. In these infants, concentrations of Teflaro in the cerebrospinal fluid were not evaluated [see Adverse Reactions (6.1), Clinical Pharmacology (12.3) and Clinical Studies (14.2)].
Results from the clinical studies in pediatric patients show that Teflaro demonstrated a safety profile that was comparable with treatment of ABSSSI and CABP in adults at the clinical dosages studied.
Safety and effectiveness of Teflaro in pediatric patients less than 34 weeks gestational age and less than 12 days postnatal age for the treatment of ABSSSI have not been established.
Safety and effectiveness of Teflaro in pediatric patients below the age of 2 months for the treatment of CABP have not been established as no data are available.
12. Teflaro - Clinical Pharmacology
12.3 Pharmacokinetics
The mean pharmacokinetic parameters of ceftaroline in healthy adults (n=6) with normal renal function after single and multiple 1-hour IV infusions of 600 mg ceftaroline fosamil administered every 12 hours are summarized in Table 8. Pharmacokinetic parameters were similar for single and multiple dose administration.
| Parameter | Single 600 mg Dose Administered as a 1-Hour Infusion (n=6) | Multiple 600 mg Doses Administered Every 12 Hours as 1-Hour Infusions for 14 Days (n=6) |
| Cmax (mcg/mL) | 19.0 (0.71) | 21.3 (4.10) |
| Tmax (h)a | 1.00 (0.92-1.25) | 0.92 (0.92-1.08) |
| AUC (mcg•h/mL) b | 56.8 (9.31) | 56.3 (8.90) |
| T1/2 (h) | 1.60 (0.38) | 2.66 (0.40) |
| CL (L/h) | 9.58 (1.85) | 9.60 (1.40) |
| a Reported as median (range) b AUC0-∞, for single-dose administration; AUC0-tau, for multiple-dose administration; Cmax, maximum observed concentration; Tmax, time of Cmax; AUC0-∞, area under concentration-time curve from time 0 to infinity; AUC0-tau, area under concentration-time curve over dosing interval (0-12 hours); T1/2, terminal elimination half-life; CL, plasma clearance |
||
The Cmax and AUC of ceftaroline increase approximately in proportion to dose within the single dose range of 50 to 1000 mg. No appreciable accumulation of ceftaroline is observed following multiple IV infusions of 600 mg administered every 12 hours for up to 14 days in healthy adults with normal renal function.
The systemic exposure (AUC), T1/2, and clearance of ceftaroline were similar following administration of 600 mg ceftaroline fosamil in a volume of 50 mL to healthy subjects every 8 hours for 5 days as a 5-minute or 60-minute infusion, and the Tmax of ceftaroline occurred about 5 minutes after the end of the ceftaroline fosamil infusion for both infusion durations. The mean (SD) Cmax of ceftaroline was 32.5 (4.82) mcg/mL for the 5-minute infusion duration (n=11) and 17.4 (3.87) mcg/mL for the 60-minute infusion duration (n=12).
Distribution
The average binding of ceftaroline to human plasma proteins is approximately 20% and decreases slightly with increasing concentrations over 1-50 mcg/mL (14.5-28.0%). The median (range) steady-state volume of distribution of ceftaroline in healthy adult males (n=6) following a single 600 mg IV dose of radiolabeled ceftaroline fosamil was 20.3 L (18.3-21.6 L), similar to extracellular fluid volume.
Elimination
Metabolism
Ceftaroline fosamil is the water-soluble prodrug of the bioactive ceftaroline. Ceftaroline fosamil is converted into bioactive ceftaroline in plasma by a phosphatase enzyme and concentrations of the prodrug are measurable in plasma primarily during IV infusion. Hydrolysis of the beta-lactam ring of ceftaroline occurs to form the microbiologically inactive, open-ring metabolite ceftaroline M-1. The mean (SD) plasma ceftaroline M-1 to ceftaroline AUC0-∞ ratio following a single 600 mg IV infusion of ceftaroline fosamil in healthy adults (n=6) with normal renal function is 28% (3.1%).
When incubated with pooled human liver microsomes, ceftaroline was metabolically stable (< 12% metabolic turnover), indicating that ceftaroline is not a substrate for hepatic CYP450 enzymes.
Excretion
Ceftaroline and its metabolites are primarily eliminated by the kidneys. Following administration of a single 600 mg IV dose of radiolabeled ceftaroline fosamil to healthy male adults (n=6), approximately 88% of radioactivity was recovered in urine and 6% in feces within 48 hours. Of the radioactivity recovered in urine approximately 64% was excreted as ceftaroline and approximately 2% as ceftaroline M-1. The mean (SD) renal clearance of ceftaroline was 5.56 (0.20) L/h, suggesting that ceftaroline is predominantly eliminated by glomerular filtration.
Specific Populations
Patients with Renal Impairment
Following administration of a single 600 mg IV dose of Teflaro, the geometric mean AUC0-∞ of ceftaroline in subjects with mild (CrCl > 50 to ≤ 80 mL/min, n=6) or moderate (CrCl > 30 to ≤ 50 mL/min, n=6) renal impairment was 19% and 52% higher, respectively, compared to healthy subjects with normal renal function (CrCl > 80 mL/min, n=6). Following administration of a single 400 mg IV dose of Teflaro, the geometric mean AUC0-∞ of ceftaroline in subjects with severe (CrCl ≥ 15 to ≤30 mL/min, n=6) renal impairment was 115% higher compared to healthy subjects with normal renal function (CrCl > 80 mL/min, n=6). Dosage adjustment is recommended in patients with moderate and severe renal impairment [see Dosage and Administration (2.2)].
A single 400 mg dose of Teflaro was administered to subjects with ESRD (n=6) either 4 hours prior to or 1 hour after hemodialysis (HD). The geometric mean ceftaroline AUC0-∞ following the post-HD infusion was 167% higher compared to healthy subjects with normal renal function (CrCl > 80 mL/min, n=6). The mean recovery of ceftaroline in the dialysate following a 4-hour HD session was 76.5 mg, or 21.6% of the administered dose. Dosage adjustment is recommended in patients with ESRD (defined as CrCL < 15 mL/min), including patients on HD [see Dosage and Administration (2.2)].
Patients with Hepatic Impairment
The pharmacokinetics of ceftaroline in patients with hepatic impairment have not been established. As ceftaroline does not appear to undergo significant hepatic metabolism, the systemic clearance of ceftaroline is not expected to be significantly affected by hepatic impairment.
Geriatric Patients
Following administration of a single 600 mg IV dose of Teflaro to healthy elderly subjects (≥ 65 years of age, n=16), the geometric mean AUC0-∞ of ceftaroline was ~33% higher compared to healthy young adult subjects (18-45 years of age, n=16). The difference in AUC0-∞ was mainly attributable to age-related changes in renal function. Dosage adjustment for Teflaro in elderly patients should be based on renal function [see Dosage and Administration (2.2)].
Pediatric Patients
The pharmacokinetics of ceftaroline were evaluated in adolescent patients (ages 12 to 17, n=7) with normal renal function following administration of a single 8 mg/kg IV dose of Teflaro (or 600 mg for subjects weighing > 75 kg). The mean plasma clearance and terminal phase volume of distribution for ceftaroline in adolescent subjects were similar to healthy adults (n=6) with normal renal function in a separate study following administration of a single 600 mg IV dose. However, the mean Cmax and AUC0-∞ for ceftaroline in adolescent subjects who received a single 8 mg/kg dose were 10% and 23% less than in healthy adult subjects who received a single 600 mg IV dose. The population pharmacokinetic analyses demonstrated that the pharmacokinetics of ceftaroline in pediatric patients from 2 months to < 18 years of age were similar to those in adult patients after accounting for weight and maturational changes. No clinically significant differences in ceftaroline AUC were predicted in patients from 12 days to 2 months postnatal age and with ≥34 weeks of gestational age compared to adults and pediatric patients 2 months of age and older when given the approved recommended dosage for each patient population [see Adverse Reactions (6), Use in Specific Populations (8.4) and Clinical Studies (14)].
Gender
Following administration of a single 600 mg IV dose of Teflaro to healthy elderly males (n=10) and females (n=6) and healthy young adult males (n=6) and females (n=10), the mean Cmax and AUC0-∞ for ceftaroline were similar between males and females, although there was a trend for higher Cmax (17%) and AUC0-∞ (6-15%) in female subjects. Population pharmacokinetic analysis did not identify any significant differences in ceftaroline AUC0-tau based on gender in Phase 2/3 patients with ABSSSI or CABP. No dose adjustment is recommended based on gender.
Race
A population pharmacokinetic analysis was performed to evaluate the impact of race on the pharmacokinetics of ceftaroline using data from Phase 2/3 adult ABSSSI and CABP trials. No significant differences in ceftaroline AUC0-tau was observed across White (n=35), Hispanic (n=34), and Black (n=17) race groups for ABSSSI patients. Patients enrolled in CABP trials were predominantly categorized as White (n=115); thus there were too few patients of other races to draw any conclusions. No dosage adjustment is recommended based on race.
Drug Interactions Studies
No clinical drug-drug interaction studies have been conducted with Teflaro. There is minimal potential for drug-drug interactions between Teflaro and CYP450 substrates, inhibitors, or inducers; drugs known to undergo active renal secretion; and drugs that may alter renal blood flow.
In vitro studies in human liver microsomes indicate that ceftaroline does not inhibit the major cytochrome P450 isoenzymes CYP1A1, CYP1A2, CYP2A6, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, CYP2E1 and CYP3A4. In vitro studies in human hepatocytes also demonstrate that ceftaroline and its inactive open-ring metabolite are not inducers of CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, or CYP3A4/5. Therefore, Teflaro is not expected to inhibit or induce the clearance of drugs that are metabolized by these metabolic pathways in a clinically relevant manner.
Population pharmacokinetic analysis did not identify any clinically relevant differences in ceftaroline exposure (Cmax and AUC0-tau) in Phase 2/3 patients with ABSSSI or CABP who were taking concomitant medications that are known inhibitors, inducers, or substrates of the cytochrome P450 system; anionic or cationic drugs known to undergo active renal secretion; and vasodilator or vasoconstrictor drugs that may alter renal blood flow.
14. Clinical Studies
14.2 Community-Acquired Bacterial Pneumonia (CABP)
Adult Patients
A total of 1231 adults with a diagnosis of CABP were enrolled in two randomized, multi-center, multinational, double-blind, non-inferiority trials (Trials 1 and 2) comparing Teflaro (600 mg administered IV over 1 hour every 12 hours) with ceftriaxone (1 g ceftriaxone administered IV over 30 minutes every 24 hours). In both treatment groups of CABP Trial 1, two doses of oral clarithromycin (500 mg every 12 hours), were administered as adjunctive therapy starting on Study Day 1. No adjunctive macrolide therapy was used in CABP Trial 2. Patients with known or suspected MRSA were excluded from both trials. Patients with new or progressive pulmonary infiltrate(s) on chest radiography and signs and symptoms consistent with CABP with the need for hospitalization and IV therapy were enrolled in the trials. Treatment duration was 5 to 7 days. A switch to oral therapy was not allowed. Among all subjects who received any amount of study drug in the two CABP trials, the 30-day all-cause mortality rates were 11/609 (1.8%) for the Teflaro group vs. 12/610 (2.0%) for the ceftriaxone group, and the difference in mortality rates was not statistically significant.
To evaluate the treatment effect of ceftaroline, an analysis was conducted in CABP patients for whom the treatment effect of antibacterials may be supported by historical evidence. The analysis endpoint required subjects to meet sign and symptom criteria at Day 4 of therapy: a responder had to both (a) be in stable condition, based on temperature, heart rate, respiratory rate, blood pressure, oxygen saturation, and mental status; (b) show improvement from baseline on at least one symptom of cough, dyspnea, pleuritic chest pain, or sputum production, while not worsening on any of these four symptoms. The analysis used a microbiological intent-to-treat population (mITT population) containing only subjects with a confirmed bacterial pathogen at baseline. Results for this analysis are presented in Table 12.
| Teflaro
n/N (%) | Ceftriaxone
n/N (%) | Treatment Difference
(2-sided 95% CI) |
|
| CABP Trial 1 | 48/69 (69.6%) | 42/72 (58.3%) | 11.2 (-4.6,26.5) |
| CABP Trial 2 | 58/84 (69.0%) | 51/83 (61.4%) | 7.6 (-6.8,21.8) |
The protocol-specified analyses included clinical cure rates at the TOC (8 to 15 days after the end of therapy) in the co-primary Modified Intent-to-Treat Efficacy (MITTE) and CE populations (Table 13) and clinical cure rates at TOC by pathogen in the Microbiologically Evaluable (ME) population (Table 14). However, there are insufficient historical data to establish the magnitude of drug effect for antibacterials drugs compared with placebo at a TOC time point. Therefore, comparisons of Teflaro to ceftriaxone based on clinical response rates at TOC cannot be utilized to establish non-inferiority. Neither trial established that Teflaro was statistically superior to ceftriaxone in terms of clinical response rates. The MITTE population included all patients who received any amount of study drug according to their randomized treatment group and were in PORT (Pneumonia Outcomes Research Team) Risk Class III or IV. The CE population included patients in the MITTE population who demonstrated sufficient adherence to the protocol.
| Teflaro
n/N (%) | Ceftriaxone
n/N (%) | Treatment Difference
(2-sided 95% CI) |
|
| CABP Trial 1 | |||
| CE | 194/224 (86.6%) | 183/234 (78.2%) | 8.4 (1.4, 15.4) |
| MITTE | 244/291 (83.8%) | 233/300 (77.7%) | 6.2 (-0.2, 12.6) |
| CABP Trial 2 | |||
| CE | 191/232 (82.3%) | 165/214 (77.1%) | 5.2 (-2.2, 12.8) |
| MITTE | 231/284 (81.3%) | 203/269 (75.5%) | 5.9 (-1.0, 12.8) |
| Teflaro
n/N (%) | Ceftriaxone
n/N (%) |
|
| Gram-positive:
Streptococcus pneumoniae | 54/63 (85.7%) | 41/59 (69.5%) |
| Staphylococcus aureus
(methicillin-susceptible isolates only) | 18/25 (72.0%) | 14/25 (56.0%) |
| Gram-negative:
Haemophilus influenzae | 15/18 (83.3%) | 17/20 (85.0%) |
| Klebsiella pneumoniae | 12/12 (100%) | 10/12 (83.3%) |
| Klebsiella oxytoca | 5/6 (83.3%) | 7/8 (87.5%) |
| Escherichia coli | 10/12 (83.3%) | 9/12 (75.0%) |
Pediatric Patients
The CABP pediatric trial was a randomized, parallel-group, active controlled trial in pediatric patients 2 months to < 18 years of age.
A total of 161 children with a diagnosis of CABP were enrolled in a randomized, multi-center, multinational, active controlled trial comparing Teflaro with ceftriaxone. Patients with new or progressive pulmonary infiltrate(s) on chest radiography and signs and symptoms consistent with CABP including acute onset or worsening symptoms of cough, tachypnea, sputum production, grunting, chest pain, cyanosis, or increased work of breathing with the need for hospitalization and IV therapy were enrolled in the trial. Treatment duration was 5 to 14 days. A switch to oral therapy with amoxicillin clavulanate was allowed on Study Day 4.
The primary objective was to evaluate the safety and tolerability of Teflaro. The study was not powered for comparative inferential efficacy analysis, and no efficacy endpoint was identified as primary.
To evaluate the treatment effect of Teflaro, an analysis was conducted in 143 patients with CABP in the MITT population. This analysis evaluated responder rates at Study Day 4 based on achieving improvement in at least 2 out of 7 symptoms (cough, dyspnea, chest pain, sputum production, chills, feeling of warmth / feverish and exercise intolerance or lethargy) and have worsening in none of these symptoms.
The clinical response at Study Day 4 was 69.2% (74/107) for Teflaro and 66.7% (24/36) for the comparator, with a treatment difference of 2.5% (95% CI of –13.9, 20.9).
Clinical cure rates at test of cure were 87.9% (94/107) for Teflaro and 88.9% (32/36) for the comparator, with a treatment difference of -1.0 (95% CI –11.5, 14.1).
| TEFLARO
ceftaroline fosamil powder, for solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| TEFLARO
ceftaroline fosamil powder, for solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Allergan, Inc. (144796497) |