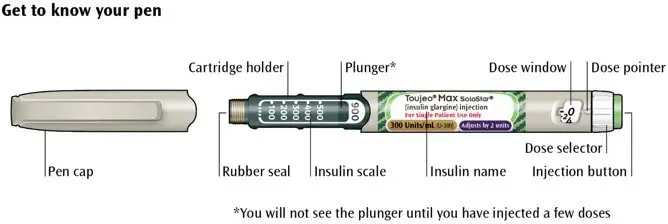
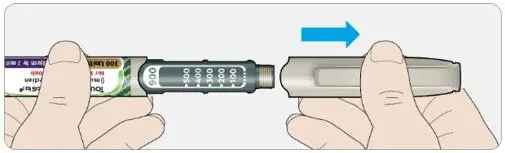
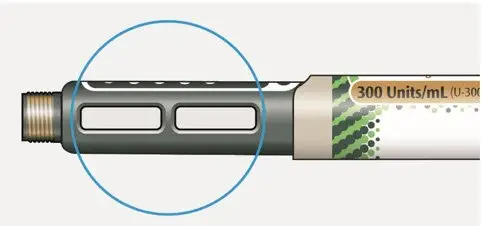
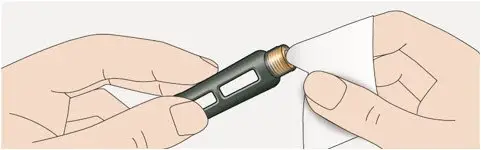
Drug Detail:Toujeo solostar (Insulin glargine [ in-su-lin-glar-gine ])
Drug Class: Insulin
Highlights of Prescribing Information
TOUJEO U-300 (insulin glargine) injection, for subcutaneous use
Initial U.S. Approval: 2015
Indications and Usage for Toujeo
TOUJEO is a long-acting human insulin analog indicated to improve glycemic control in adults and pediatric patients 6 years and older with diabetes mellitus. (1)
Limitations of Use:
Not recommended for the treatment of diabetic ketoacidosis. (1)
Toujeo Dosage and Administration
- Individualize dose based on type of diabetes, metabolic needs, blood glucose monitoring results and glycemic control goal. (2.1, 2.2, 2.3)
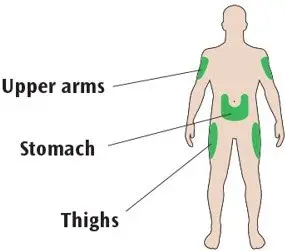
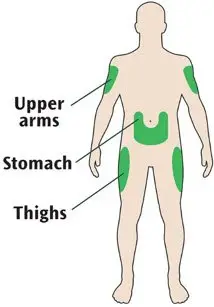
- Administer subcutaneously into the abdominal area, thigh, or deltoid once daily at any time during the day, at the same time every day. (2.1)
- Rotate injection sites to reduce risk of lipodystrophy and localized cutaneous amyloidosis. (2.1)
- Do not dilute or mix with any other insulin or solution. (2.1)
- See Full Prescribing Information for the recommended starting dosage in patients with type 2 diabetes and how to switch to TOUJEO from other insulins (2.3, 2.4)
- Closely monitor glucose when changing switching to TOUJEO and during initial weeks thereafter. (2.4)
Dosage Forms and Strengths
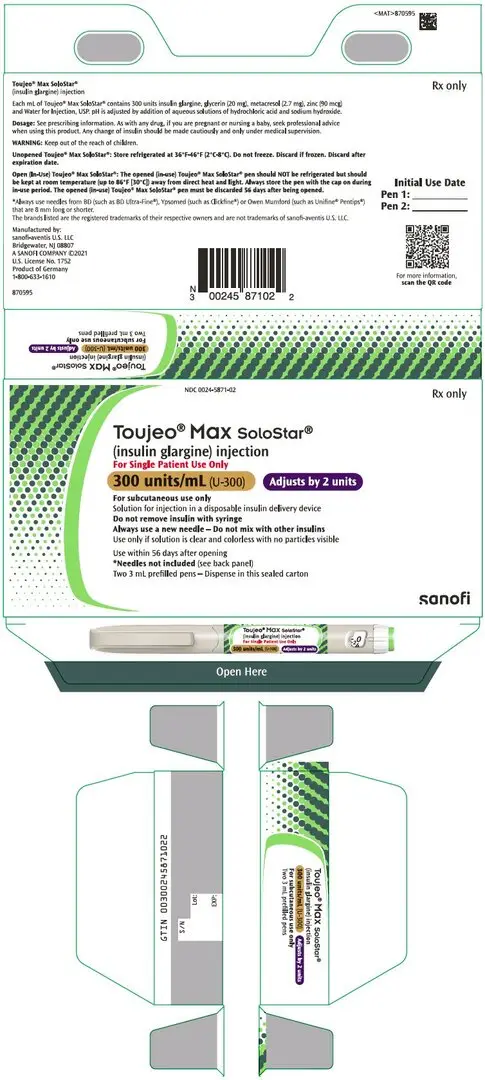
Injection: 300 units/mL (U-300) insulin glargine in:
- 1.5 mL TOUJEO SoloStar single-patient-use prefilled pen (3)
- 3 mL TOUJEO Max SoloStar single-patient-use prefilled pen (3)
Contraindications
- During episodes of hypoglycemia (4)
- Hypersensitivity to insulin glargine or any excipients in TOUJEO (4)
Warnings and Precautions
- Never share a TOUJEO SoloStar or TOUJEO Max SoloStar single-patient-use prefilled pen between patients, even if the needle is changed. (5.1)
- Hyperglycemia or hypoglycemia with changes in insulin regimen: Make changes to a patient's insulin regimen (e.g., insulin strength, manufacturer, type, injection site, or method of administration) under close medical supervision with increased frequency of blood glucose monitoring. (5.2)
- Hypoglycemia: May be life-threatening. Increase frequency of glucose monitoring with changes to: insulin dosage, concomitant drugs, meal pattern, physical activity, and in patients with renal impairment or hepatic impairment or hypoglycemia unawareness. (5.3, 6.1)
- Hypoglycemia Due to Medication errors: Accidental mix-ups between insulin products can occur. Instruct patients to check insulin labels before injection. (5.4)
- Hypersensitivity reactions: Severe, life-threatening, generalized allergy, including anaphylaxis, can occur. Discontinue TOUJEO, monitor and treat if indicated. (5.5, 6.1)
- Hypokalemia: May be life-threatening. Monitor potassium levels in patients at risk of hypokalemia and treat if indicated. (5.6)
- Fluid retention and heart failure with concomitant use of Thiazolidinediones (TZDs): Observe for signs and symptoms of heart failure; consider dosage reduction or discontinuation if heart failure occurs. (5.7)
Adverse Reactions/Side Effects
Adverse reactions commonly associated with TOUJEO (≥5%) are:
- Hypoglycemia, allergic reactions, injection site reaction, lipodystrophy, pruritus, rash, edema, and weight gain. (6.1, 6.2)
To report SUSPECTED ADVERSE REACTIONS, contact sanofi-aventis at 1-800-633-1610 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
- Drugs that Affect Glucose Metabolism: Adjustment of insulin dosage may be needed. (7)
- Antiadrenergic Drugs (e.g., beta-blockers, clonidine, guanethidine, and reserpine): Signs and symptoms of hypoglycemia may be reduced or absent. (5.3, 7)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 3/2023
Related/similar drugs
metformin, Trulicity, Lantus, Victoza, Levemir, Tresiba, BasaglarFull Prescribing Information
1. Indications and Usage for Toujeo
TOUJEO is indicated to improve glycemic control in adults and pediatric patients 6 years of age and older with diabetes mellitus.
2. Toujeo Dosage and Administration
2.1 Important Administration Instructions
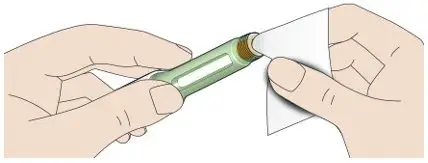
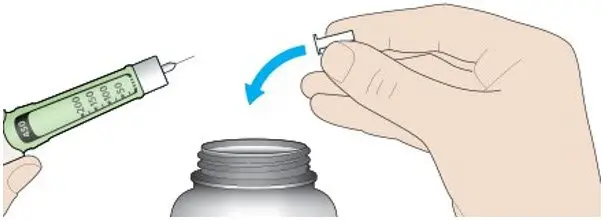
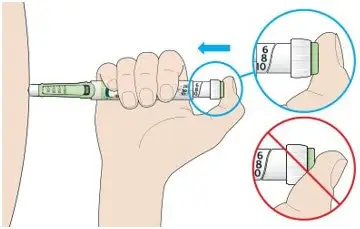
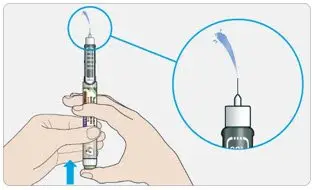
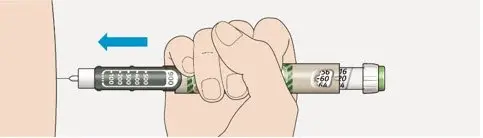
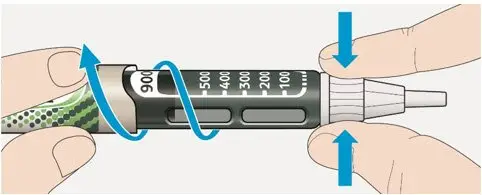
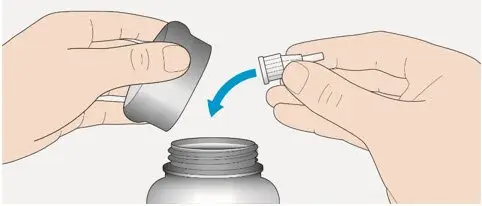
- Always check insulin labels before administration [see Warnings and Precautions (5.4)].
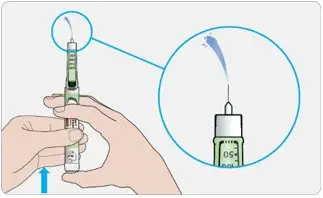
- Visually inspect the TOUJEO solution for particulate matter and discoloration prior to administration and only use if the solution is clear and colorless with no visible particles.
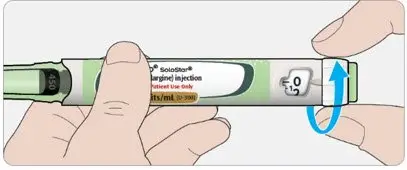
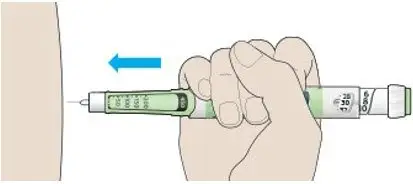
- Inject TOUJEO subcutaneously into the abdominal area, thigh, or deltoid.
- Rotate injection sites within the same region from one injection to the next to reduce the risk of lipodystrophy and localized cutaneous amyloidosis. Do not inject into areas of lipodystrophy or localized cutaneous amyloidosis [see Warnings and Precautions (5.2), Adverse Reactions (6)].
- Use TOUJEO with caution in patients with visual impairment who may rely on audible clicks to dial their dose.
- Do not administer TOUJEO intravenously or in an insulin pump.
- Do not dilute or mix TOUJEO with any other insulin products or solutions.
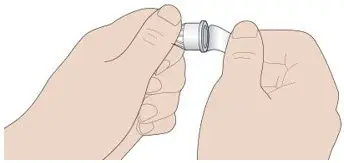
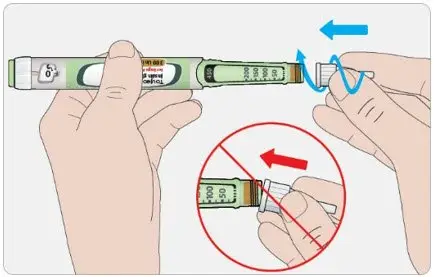
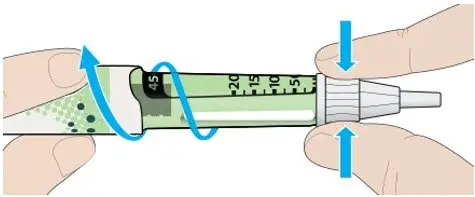
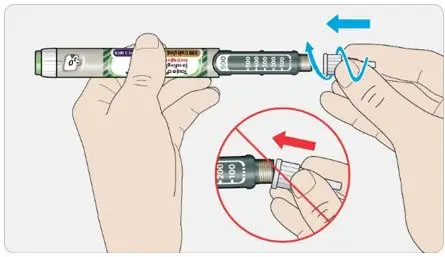
- Never transfer TOUJEO from the cartridges of the TOUJEO SoloStar or TOUJEO Max SoloStar prefilled pen into a syringe for administration [see Warnings and Precautions (5.4)].
2.2 General Dosing Instructions
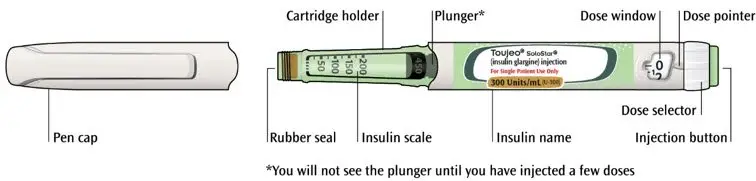
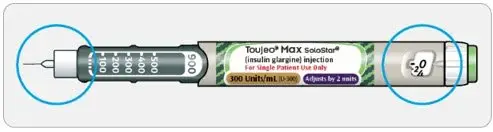
- TOUJEO is available in 2 single-patient-use prefilled pens:
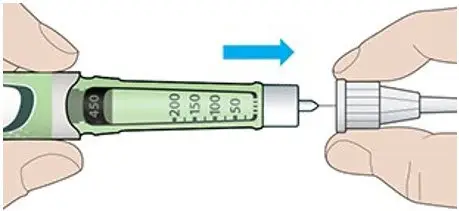
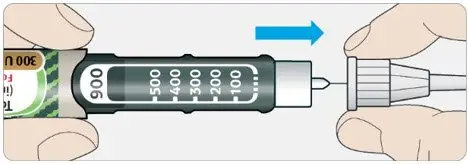
- TOUJEO SoloStar contains 450 units of TOUJEO U-300. It delivers doses in 1-unit increments and can deliver up to 80 units in a single injection.
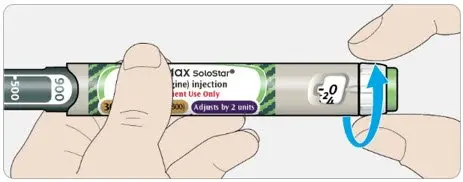
- TOUJEO Max SoloStar contains 900 units of TOUJEO U-300. It delivers doses in 2-unit increments and can deliver up to 160 units in a single injection. It is recommended for patients requiring at least 20 units per day.
- When changing between TOUJEO SoloStar and TOUJEO Max SoloStar, if the patient's previous dose was an odd number, the dose should be increased or decreased by 1 unit to match the dose increments dialable on each prefilled pen.
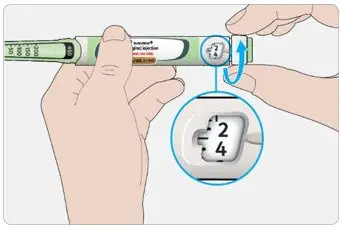
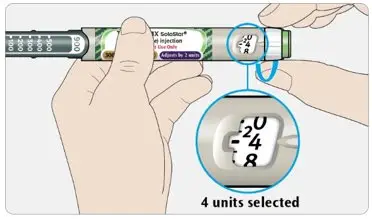
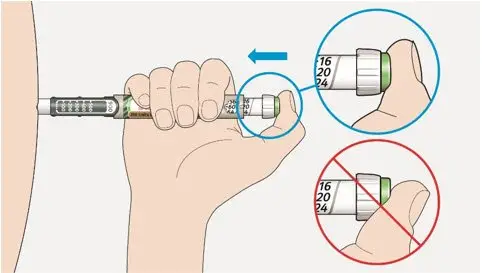
- The dose counter of the TOUJEO SoloStar or TOUJEO Max SoloStar prefilled pen shows the number of units of TOUJEO to be injected and no conversion is required.
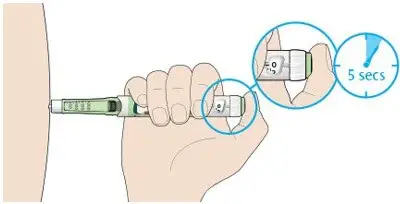
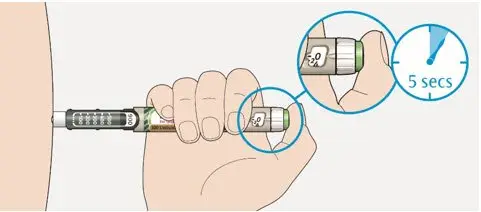
- Inject TOUJEO subcutaneously once a day at the same time of day.
- During changes to a patient's insulin regimen, increase the frequency of blood glucose monitoring [see Warnings and Precautions (5.2)].
- Individualize and titrate the dosage of TOUJEO based on the patient's metabolic needs, blood glucose monitoring results, and glycemic control goal.
- Titrate the dose of TOUJEO no more frequently than every 3 to 4 days.
- Dosage adjustments may be needed with changes in physical activity, changes in meal patterns (i.e., macronutrient content or timing of food intake), changes in renal or hepatic function or during acute illness to minimize the risk of hypoglycemia or hyperglycemia [see Warnings and Precautions (5.2) and Use in Specific Populations (8.6, 8.7)].
2.4 Starting Dose in Pediatric and Adult Patients with Either Type 1 or Type 2 Diabetes Already on Insulin Therapy
Dosage adjustments are recommended to lower the risk of hypoglycemia when switching patients to TOUJEO from another insulin therapy [see Warnings and Precautions (5.3)].
- For patients currently on once-daily long or intermediate-acting insulin, start TOUJEO at the same unit dose as the once-daily long-acting insulin dose. For patients controlled on LANTUS (insulin glargine, 100 units/mL), expect that a higher daily dose of TOUJEO will be needed to maintain the same level of glycemic control [see Clinical Pharmacology (12.2) and Clinical Studies (14.1)].
- For patients currently on twice-daily long or intermediate-acting insulin, start TOUJEO at 80% of the total daily NPH or insulin detemir twice-daily dosage.
- When switching patients to TOUJEO, monitor glucose frequently in the first weeks of therapy [see Warnings and Precautions (5.2) and Clinical Pharmacology (12.2)].
3. Dosage Forms and Strengths
Injection: 300 units/mL (U-300) of insulin glargine available as a clear, colorless, solution in:
- 1.5 mL TOUJEO SoloStar single-patient-use prefilled pen (450 units per 1.5 mL pen)
- 3 mL TOUJEO Max SoloStar single-patient-use prefilled pen (900 units per 3 mL pen)
4. Contraindications
TOUJEO is contraindicated:
- During episodes of hypoglycemia [see Warnings and Precautions (5.3)].
- In patients with hypersensitivity to insulin glargine or any excipients in TOUJEO [see Warnings and Precautions (5.5)].
5. Warnings and Precautions
5.1 Never Share a TOUJEO SoloStar or TOUJEO Max SoloStar Pen Between Patients
TOUJEO SoloStar or TOUJEO Max SoloStar single-patient-use prefilled pens must never be shared between patients, even if the needle is changed. Pen sharing poses a risk for transmission of blood-borne pathogens.
5.3 Hypoglycemia
Hypoglycemia is the most common adverse reaction associated with insulin, including TOUJEO. Severe hypoglycemia can cause seizures, may be life-threatening, or cause death. Hypoglycemia can impair concentration ability and reaction time; this may place the patient and others at risk in situations where these abilities are important (e.g., driving, or operating other machinery). Hypoglycemia can happen suddenly, and symptoms may differ in each patient and change over time in the same patient. Symptomatic awareness of hypoglycemia may be less pronounced in patients with longstanding diabetes, in patients with diabetic neuropathy, in patients using drugs that block the sympathetic nervous system (e.g., beta-blockers) [see Drug Interactions (7)], or who experience recurrent hypoglycemia.
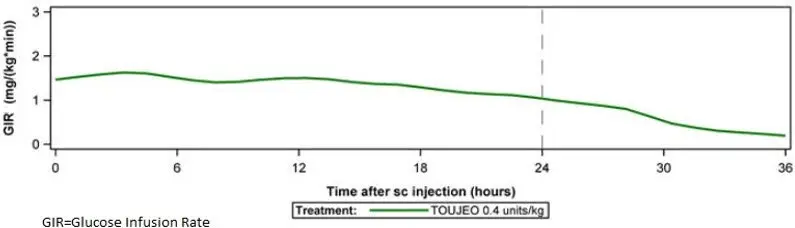
The long-acting effect of TOUJEO may delay recovery from hypoglycemia compared to shorter-acting insulins.
5.4 Hypoglycemia Due to Medication Errors
Accidental mix-ups between insulin products have been reported. To avoid medication errors between TOUJEO and other insulins, instruct patients to always check the insulin label before each injection.
To avoid dosing errors and potential overdose, never use a syringe to remove TOUJEO from the TOUJEO SoloStar or TOUJEO Max SoloStar prefilled pen into a syringe [see Dosage and Administration (2.4) and Warnings and Precautions (5.3)].
5.5 Hypersensitivity Reactions
Severe, life-threatening, generalized allergy, including anaphylaxis, can occur with insulins, including TOUJEO. If hypersensitivity reactions occur, discontinue TOUJEO; treat per standard of care and monitor until symptoms and signs resolve [see Adverse Reactions (6)]. TOUJEO is contraindicated in patients who have had hypersensitivity reactions to insulin glargine or any of the excipients in TOUJEO.
5.6 Hypokalemia
All insulins, including TOUJEO, cause a shift in potassium from the extracellular to intracellular space, possibly leading to hypokalemia. Untreated hypokalemia may cause respiratory paralysis, ventricular arrhythmia, and death. Monitor potassium levels in patients at risk for hypokalemia, if indicated (e.g., patients using potassium-lowering medications, patients taking medications sensitive to serum potassium concentrations).
5.7 Fluid Retention and Heart Failure with Concomitant Use of PPAR-gamma Agonists
Thiazolidinediones (TZDs), which are peroxisome proliferator-activated receptor (PPAR)-gamma agonists, can cause dose-related fluid retention, when used in combination with insulin. Fluid retention may lead to or exacerbate heart failure. Patients treated with insulin, including TOUJEO, and a PPAR-gamma agonist should be observed for signs and symptoms of heart failure. If heart failure develops, it should be managed according to current standards of care, and discontinuation or dose reduction of the PPAR-gamma agonist must be considered.
6. Adverse Reactions/Side Effects
The following adverse reactions are discussed elsewhere:
- Hypoglycemia [see Warnings and Precautions (5.3)]
- Hypoglycemia due to medication errors [see Warnings and Precautions (5.4)]
- Hypersensitivity reactions [see Warnings and Precautions (5.5)]
- Hypokalemia [see Warnings and Precautions (5.6)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates actually observed in clinical practice.
The data in Table 1 reflect the exposure of 304 patients with type 1 diabetes to TOUJEO with mean exposure duration of 23 weeks. The type 1 diabetes population had the following characteristics: Mean age was 46 years and mean duration of diabetes was 21 years. Fifty-five percent were male, 86% were Caucasian, 5% were Black or African American, and 5% were Hispanic. At baseline, the mean eGFR was 82 mL/min/1.73 m2 and 35% of patients had eGFR ≥90 mL/min/1.73 m2. The mean BMI was 28 kg/m2. HbA1c at baseline was greater than or equal to 8% in 58% of patients.
The data in Table 2 reflect the exposure of 1242 patients with type 2 diabetes to TOUJEO with mean exposure duration of 25 weeks. The type 2 diabetes population had the following characteristics: Mean age was 59 years and mean duration of diabetes was 13 years. Fifty-three percent were male, 88% were Caucasian, 7% were Black or African American, and 17% were Hispanic. At baseline, mean eGFR was 79 mL/min/1.73 m2 and 27% of patients had an eGFR ≥90 mL/min/1.73 m2. The mean BMI was 35 kg/m2. HbA1c at baseline was greater than or equal to 8% in 66% of patients.
TOUJEO was studied in 233 pediatric patients (6–17 years of age) with type 1 diabetes for a mean duration of 26 weeks [see Clinical Studies (14.1)].
Common adverse reactions (occurring ≥5%) in TOUJEO-treated subjects during clinical trials in adult patients with type 1 diabetes mellitus and type 2 diabetes mellitus are listed in Table 1 and Table 2, respectively. Common adverse reactions for TOUJEO-treated pediatric subjects with type 1 diabetes mellitus were similar to the adverse reactions listed in Table 1. Hypoglycemia is discussed in a dedicated subsection below.
| TOUJEO + Mealtime Insulin*, % (n=304) |
|
|---|---|
|
|
| Nasopharyngitis | 12.8 |
| Upper respiratory tract infection | 9.5 |
| TOUJEO*, % (n=1242) |
|
|---|---|
|
|
| Nasopharyngitis | 7.1 |
| Upper respiratory tract infection | 5.7 |
6.2 Immunogenicity
As with all therapeutic proteins, there is potential for immunogenicity.
In a 6-month study of type 1 diabetes patients, 79% of patients who received TOUJEO once daily were positive for anti-insulin antibodies (AIA) at least once during the study, including 62% that were positive at baseline and 44% of patients who developed antidrug antibody (i.e., anti-insulin glargine antibody [ADA]) during the study. Eighty percent of the AIA-positive patients on TOUJEO with antibody test at baseline remained AIA positive at month 6.
In two 6-month studies in type 2 diabetes patients, 25% of patients who received TOUJEO once daily were positive for AIA at least once during the study, including 42% who were positive at baseline and 20% of patients who developed ADA during the study. Ninety percent of the AIA-positive patients on TOUJEO with antibody test at baseline, remained AIA positive at month 6.
The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay and may be influenced by several factors such as: assay methodology, sample handling, timing of sample collection, concomitant medication, and underlying disease. For these reasons, comparison of the incidence of antibodies to TOUJEO with the incidence of antibodies in other studies or to other products may be misleading.
6.3 Postmarketing Experience
The following additional adverse reactions have been identified during postapproval use of TOUJEO. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Localized cutaneous amyloidosis at the injection site has occurred. Hyperglycemia has been reported with repeated insulin injections into areas of localized cutaneous amyloidosis; hypoglycemia has been reported with a sudden change to an unaffected injection site.
7. Drug Interactions
Table 3 includes clinically significant drug interactions with TOUJEO.
| Drugs That May Increase the Risk of Hypoglycemia | |
| Drugs: | Antidiabetic agents, ACE inhibitors, angiotensin II receptor blocking agents, disopyramide, fibrates, fluoxetine, monoamine oxidase inhibitors, pentoxifylline, pramlintide, salicylates, somatostatin analogs (e.g., octreotide), and sulfonamide antibiotics, GLP-1 receptor agonists, DPP-4 inhibitors, and SGLT-2 inhibitors. |
| Intervention: | Dosage reductions and increased frequency of glucose monitoring may be required when TOUJEO is coadministered with these drugs. |
| Drugs That May Decrease the Blood Glucose Lowering Effect of TOUJEO | |
| Drugs: | Atypical antipsychotics (e.g., olanzapine and clozapine), corticosteroids, danazol, diuretics, estrogens, glucagon, isoniazid, niacin, oral contraceptives, phenothiazines, progestogens (e.g., in oral contraceptives), protease inhibitors, somatropin, sympathomimetic agents (e.g., albuterol, epinephrine, terbutaline), and thyroid hormones. |
| Intervention: | Dosage increases and increased frequency of glucose monitoring may be required when TOUJEO is coadministered with these drugs. |
| Drugs That May Increase or Decrease the Blood Glucose Lowering Effect of TOUJEO | |
| Drugs: | Alcohol, beta-blockers, clonidine, and lithium salts. Pentamidine may cause hypoglycemia, which may sometimes be followed by hyperglycemia. |
| Intervention: | Dosage adjustment and increased frequency of glucose monitoring may be required when TOUJEO is coadministered with these drugs. |
| Drugs That May Blunt Signs and Symptoms of Hypoglycemia | |
| Drugs: | Beta-blockers, clonidine, guanethidine, and reserpine. |
| Intervention: | Increased frequency of glucose monitoring may be required when TOUJEO is coadministered with these drugs. |
8. Use In Specific Populations
8.4 Pediatric Use
The safety and effectiveness of TOUJEO to improve glycemic control in pediatric patients 6 years of age and older with diabetes mellitus have been established.
The use of TOUJEO for this indication is supported by evidence from an adequate and well-controlled study in 463 pediatric patients 6 to 17 years of age with type 1 diabetes mellitus [see Clinical Studies (14.2)] and from studies in adults with diabetes mellitus [see Clinical Pharmacology (12.3), Clinical Studies (14.3)].
The safety and effectiveness of TOUJEO have not been established in pediatric patients less than 6 years of age.
8.5 Geriatric Use
In controlled clinical studies, 30 of 304 (9.8%) TOUJEO-treated patients with type 1 diabetes and 327 of 1242 (26.3%) TOUJEO-treated patients with type 2 diabetes were ≥65 years of age, among them 2.0% of the patients with type 1 and 3.0% of the patients with type 2 diabetes were ≥75 years of age. No overall differences in safety or effectiveness of TOUJEO have been observed between patients 65 years of age and older and younger adult patients.
Nevertheless, caution should be exercised when TOUJEO is administered to geriatric patients. In geriatric patients with diabetes, the initial dosing, dose increments, and maintenance dosage should be conservative to avoid hypoglycemia [see Warnings and Precautions (5.3), Adverse Reactions (6), and Clinical Studies (14)].
8.6 Renal Impairment
The effect of kidney impairment on the pharmacokinetics of TOUJEO has not been studied. Some studies with human insulin have shown increased circulating levels of insulin in patients with kidney failure. Frequent glucose monitoring and dose adjustment may be necessary for TOUJEO in patients with kidney impairment [see Warnings and Precautions (5.3)].
10. Overdosage
Excess insulin administration may cause hypoglycemia and hypokalemia [see Warnings and Precautions (5.3, 5.6)]. Mild episodes of hypoglycemia can be treated with oral glucose. Lowering the insulin dosage, and adjustments in meal patterns, or physical activity level may be needed. More severe episodes of hypoglycemia with coma, seizure, or neurologic impairment may be treated with glucagon for emergency use or concentrated intravenous glucose. Sustained carbohydrate intake and observation may be necessary because hypoglycemia may recur after apparent clinical recovery. Hypokalemia must be corrected appropriately.
11. Toujeo Description
Insulin glargine is a long-acting human insulin analog produced by recombinant DNA technology utilizing a nonpathogenic laboratory strain of Escherichia coli (K12) as the production organism. Insulin glargine differs from human insulin in that the amino acid asparagine at position A21 is replaced by glycine and two arginines remain at the C-terminus of the B-chain. Insulin glargine has a molecular weight of 6063 Da.
TOUJEO (insulin glargine) injection is a sterile, clear and colorless solution for subcutaneous injection. Each mL of TOUJEO contains 300 units of insulin glargine dissolved in a clear aqueous fluid.
The 1.5 mL TOUJEO SoloStar prefilled pen presentation contains the following inactive ingredients per mL: glycerin (20 mg), metacresol (2.7 mg), zinc (90 mcg), and Water for Injection, USP.
The 3 mL TOUJEO Max SoloStar prefilled pen presentation contains the following inactive ingredients per mL: glycerin (20 mg), metacresol (2.7 mg), zinc (90 mcg), and Water for Injection, USP.
The pH is adjusted by addition of aqueous solutions of hydrochloric acid and sodium hydroxide. TOUJEO has a pH of approximately 4.
12. Toujeo - Clinical Pharmacology
12.1 Mechanism of Action
The primary activity of insulin, including insulin glargine, is regulation of glucose metabolism. Insulin and its analogs lower blood glucose by stimulating peripheral glucose uptake, especially by skeletal muscle and fat, and by inhibiting hepatic glucose production. Insulin inhibits lipolysis and proteolysis and enhances protein synthesis.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
In mice and rats, standard two-year carcinogenicity studies with insulin glargine were performed at doses up to 0.455 mg/kg, which was for the rat approximately 65 times the recommended human subcutaneous starting dosage of 0.2 Units/kg/day (0.007 mg/kg/day). The findings in female mice were not conclusive due to excessive mortality in all dose groups during the study. Histiocytomas were found at injection sites in male rats (statistically significant) and male mice (not statistically significant) in acid vehicle containing groups. These tumors were not found in female animals, in saline control, or insulin comparator groups using a different vehicle. The relevance of these findings to humans is unknown.
Insulin glargine was not mutagenic in tests for detection of gene mutations in bacteria and mammalian cells (Ames and HGPRT test) and in tests for detection of chromosomal aberrations (cytogenetics in vitro in V79 cells and in vivo in Chinese hamsters).
In a combined fertility and prenatal and postnatal study in male and female rats at subcutaneous doses up to 0.36 mg/kg/day, which was approximately 50 times the recommended human subcutaneous starting dose of 0.2 Units/kg/day (0.007 mg/kg/day), maternal toxicity due to dose-dependent hypoglycemia, including some deaths, was observed. Consequently, a reduction of the rearing rate occurred in the high-dose group only. Similar effects were observed with NPH insulin.
14. Clinical Studies
14.1 Overview of Clinical Studies
The safety and effectiveness of TOUJEO given once daily was compared to that of once-daily LANTUS in 26-week, open-label, randomized, active-control, parallel studies of 546 adult patients and 463 pediatric patients with type 1 diabetes mellitus and 2,474 patients with type 2 diabetes mellitus (Tables 4 and 5). At trial end, the reduction in glycated hemoglobin (HbA1c) and fasting plasma glucose with TOUJEO titrated to goal was similar to that with LANTUS titrated to goal. At the end of the trial, depending on the patient population and concomitant therapy, patients were receiving a higher dose of TOUJEO than LANTUS.
14.3 Clinical Studies in Adult Patients with Type 2 Diabetes
In a 26-week open-label, controlled study (Study C, n=804), adults with type 2 diabetes were randomized to once-daily treatment in the evening with either TOUJEO or LANTUS. Short-acting mealtime insulin analogues with or without metformin were also administered. The average age was 60 years. The majority of patients were White (92%) and 53% were male; 20% of patients had GFR >90 mL/min/1.73 m2. The mean BMI was approximately 36.6 kg/m2. At week 26, treatment with TOUJEO provided a mean reduction in HbA1c that met the prespecified noninferiority margin of 0.4% compared to LANTUS (Table 6). Patients treated with TOUJEO used 11% more basal insulin than patients treated with LANTUS. There were no clinically important differences in body weight between treatment groups.
In two open-label, controlled studies (n=1670), adults with type 2 diabetes mellitus were randomized to either TOUJEO or LANTUS once daily for 26 weeks as part of a regimen of combination therapy with noninsulin antidiabetic drugs. At the time of randomization, 808 patients were treated with basal insulin for more than 6 months (Study D) and 862 patients were insulin-naive (Study E).
In Study D, the average age was 58.2 years. The majority of patients were White (94%) and 46% were male; 33% of patients had GFR >90 mL/min/1.73 m2. The mean BMI was approximately 34.8 kg/m2. At week 26, treatment with TOUJEO provided a mean reduction in HbA1c that met the prespecified noninferiority margin of 0.4% compared to LANTUS (Table 6). Patients treated with TOUJEO used 12% more basal insulin than patients treated with LANTUS. There were no clinically important differences in body weight between treatment groups.
In Study E, the average age was 58 years. The majority of patients were White (78%) and 58% were male; 29% of patients had GFR >90 mL/min/1.73 m2. The mean BMI was approximately 33 kg/m2. At week 26, treatment with TOUJEO provided a mean reduction in HbA1c that met the prespecified noninferiority margin compared to LANTUS (Table 6). Patients treated with TOUJEO used 15% more basal insulin than patients treated with LANTUS. There were no clinically important differences in body weight between treatment groups.
| Study C | Study D | Study E | ||||
|---|---|---|---|---|---|---|
|
||||||
| Treatment duration | 26 weeks | 26 weeks | 26 weeks | |||
| Treatment in combination with | Mealtime insulin analog ± metformin | Noninsulin antidiabetic drugs | ||||
| TOUJEO | LANTUS | TOUJEO | LANTUS | TOUJEO | LANTUS | |
| Number of patients treated* | 404 | 400 | 403 | 405 | 432 | 430 |
| HbA1c (%) | ||||||
| Baseline mean | 8.13 | 8.14 | 8.27 | 8.22 | 8.49 | 8.58 |
| Adjusted mean change from baseline | -0.90 | -0.87 | -0.73 | -0.70 | -1.42 | -1.46 |
| Adjusted mean difference† | -0.03 | -0.03 | 0.04 | |||
| [95% Confidence interval] | [-0.14 to 0.08] | [-0.17 to 0.10] | [-0.09 to 0.17] | |||
| Fasting Plasma Glucose (mg/dL) | ||||||
| Baseline mean | 157 | 160 | 149 | 142 | 179 | 184 |
| Adjusted mean change from baseline | -29 | -30 | -18 | -22 | -61 | -68 |
| Adjusted mean difference† | 0.8 | 3 | 7 | |||
| [95% Confidence interval] | [-5 to 7] | [-3 to 9] | [2 to 12] | |||
16. How is Toujeo supplied
16.1 How Supplied
TOUJEO U-300 (insulin glargine) injection 300 units/mL is a clear and colorless solution and is available as:
| TOUJEO | Total volume | Total units available in presentation | Max dose per injection | Dose increment | NDC number | Package size |
|---|---|---|---|---|---|---|
| SoloStar single-patient-use prefilled pen | 1.5 mL | 450 units | 80 units | 1 unit | 0024-5869-03 | 3 pens/pack |
| Max SoloStar single-patient-use prefilled pen | 3 mL | 900 units | 160 units | 2 units | 0024-5871-02 | 2 pens/pack |
Needles are not included in the packs of TOUJEO SoloStar or TOUJEO Max SoloStar single-patient-use prefilled pen.
BD (such as BD Ultra-Fine®), Ypsomed (such as Clickfine®) or Owen Mumford (such as Unifine® Pentips®) needles‡ can be used in conjunction with TOUJEO SoloStar or TOUJEO Max SoloStar single-patient-use prefilled pen and are sold separately.
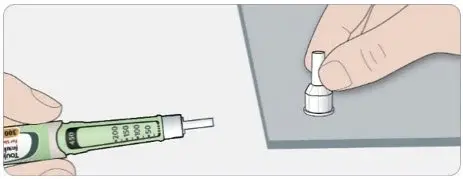
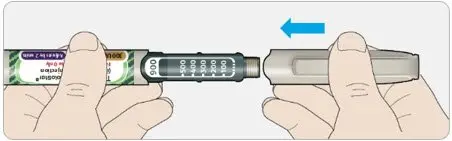
A new sterile needle must be attached before each injection. TOUJEO SoloStar or TOUJEO Max SoloStar single-patient-use prefilled pens must never be shared between patients, even if the needle is changed.
16.2 Storage
Dispense in the original sealed carton with the enclosed Instructions for Use.
TOUJEO SoloStar or TOUJEO Max SoloStar prefilled pen should not be stored in the freezer and should not be allowed to freeze. Discard TOUJEO prefilled pen if it has been frozen. Protect TOUJEO SoloStar/TOUJEO Max SoloStar from direct heat and light.
Storage conditions are summarized in the following table:
| Not in-use (unopened) Refrigerated 36°F–46°F (2°C–8°C) | In-use (opened)*
Room temperature only (Do not refrigerate) up to 86°F (30°C) |
|
|---|---|---|
|
||
| 1.5 mL TOUJEO SoloStar single-patient-use prefilled pen | Until expiration date | 56 days* |
| 3 mL TOUJEO Max SoloStar single-patient-use prefilled pen | Until expiration date | 56 days* |
17. Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use). There are separate Instructions for Use for TOUJEO SoloStar and TOUJEO Max SoloStar.
| TOUJEO
insulin glargine injection, solution |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| TOUJEO MAX
insulin glargine injection, solution |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Sanofi-Aventis U.S. LLC (824676584) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Sanofi-Aventis Deutschland GmbH | 313218430 | MANUFACTURE(0024-5869, 0024-5871) , ANALYSIS(0024-5869, 0024-5871) , LABEL(0024-5869, 0024-5871) , PACK(0024-5869, 0024-5871) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Genzyme Corporation | 050424395 | LABEL(0024-5871) , PACK(0024-5871) | |