Drug Class: Miscellaneous antihypertensive combinations
Highlights of Prescribing Information
TRIBENZOR (olmesartan medoxomil, amlodipine, hydrochlorothiazide) tablets, for oral use
Initial U.S. Approval: 2010
WARNING: FETAL TOXICITY
See full prescribing information for complete boxed warning.
-
When pregnancy is detected, discontinue Tribenzor as soon as possible (5.1, 8.1).
- Drugs that act directly on the renin-angiotensin system can cause injury and death to the developing fetus (5.1, 8.1).
Indications and Usage for Tribenzor
Tribenzor is a combination of olmesartan medoxomil, an angiotensin II receptor blocker, amlodipine, a dihydropyridine calcium channel blocker, and hydrochlorothiazide, a thiazide diuretic, indicated for the treatment of hypertension, to lower blood pressure. Lowering blood pressure reduces the risk of fatal and nonfatal cardiovascular events, primarily strokes and myocardial infarctions. (1).
Limitations of Use
Tribenzor is not indicated for initial therapy (1).
Tribenzor Dosage and Administration
- Dose once daily. Dosage may be increased after 2 weeks to a maximum dose of 40 /10 /25 mg once daily (2).
- Dose selection should be individualized based on previous therapy (2).
Dosage Forms and Strengths
Tablets: (olmesartan medoxomil/amlodipine/hydrochlorothiazide) 20 /5 /12.5 mg, 40 /5 /12.5 mg, 40 /5 /25 mg, 40 /10 /12.5 mg, 40 /10 /25 mg (3)
Contraindications
- Anuria: Hypersensitivity to sulfonamide-derived drugs (4).
- Do not co-administer aliskiren with Tribenzor in patients with diabetes (4).
Warnings and Precautions
- Hypotension: Correct volume or salt depletion prior to administration. (5.2).
- Monitor renal function and potassium in susceptible patients
- Increased angina or myocardial infarction with calcium channel blockers may occur upon dosage initiation or increase (5.3).
- Observe for signs of fluid or electrolyte imbalance (5.6).
- Exacerbation or activation of systemic lupus erythematosus (5.8).
- Acute angle-closure glaucoma (5.9).
- Sprue-like enteropathy has been reported. Consider discontinuation of Tribenzor in cases where no other etiology is found (5.10).
Adverse Reactions/Side Effects
Most common adverse reactions (incidence ≥2%) are dizziness, peripheral edema, headache, fatigue, nasopharyngitis, muscle spasms, nausea, upper respiratory tract infection, diarrhea, urinary tract infection, and joint swelling (6.1).
To report SUSPECTED ADVERSE REACTIONS, contact Daiichi Sankyo, Inc. at 1-877-437-7763 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
Olmesartan medoxomil (7.1):
- Nonsteroidal anti-inflammatory drugs (NSAIDS): May lead to increased risk of renal impairment and loss of antihypertensive effect.
- Dual inhibition of the renin-angiotensin system: Increased risk of renal impairment, hypotension, and hyperkalemia.
- Colesevelam hydrochloride: Consider administering olmesartan at least 4 hours before colesevelam hydrochloride dose.
- Lithium: Increases in serum lithium concentrations and lithium toxicity.
Amlodipine (7.2):
- Limit simvastatin to 20 mg daily when coadministered.
- Increased exposure to cyclosporin and tacrolimus.
- Increased amlodipine exposure when coadministered with CYP3A inhibitors Hydrochlorothiazide (7.3):
- Antidiabetic drugs: Dosage adjustment of antidiabetic may be required.
- Cholestyramine and colestipol: Reduced absorption of thiazides.
- NSAIDs: Can reduce the diuretic, natriuretic, and antihypertensive effects of diuretics.
Use In Specific Populations
- Lactation: Breastfeeding is not recommended (8.2).
- Renal Impairment: Avoid use in patients with creatinine clearance ≤30 mL/min (8.7).
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 10/2020
Full Prescribing Information
WARNING: FETAL TOXICITY
-
When pregnancy is detected, discontinue Tribenzor as soon as possible. (5.1, 8.1)
- Drugs that act directly on the renin-angiotensin system (RAS) can cause injury and death to the developing fetus. (5.1, 8.1)
1. Indications and Usage for Tribenzor
Tribenzor is indicated for the treatment of hypertension, alone or with other antihypertensive agents, to lower blood pressure. Lowering blood pressure reduces the risk of fatal and nonfatal cardiovascular (CV) events, primarily strokes and myocardial infarctions. These benefits have been seen in controlled trials of antihypertensive drugs from a wide variety of pharmacologic classes including the class to which this drug principally belongs. There are no controlled trials demonstrating risk reduction with Tribenzor.
Control of high blood pressure should be part of comprehensive cardiovascular risk management, including, as appropriate, lipid control, diabetes management, antithrombotic therapy, smoking cessation, exercise, and limited sodium intake. Many patients will require more than one drug to achieve blood pressure goals. For specific advice on goals and management, see published guidelines, such as those of the National High Blood Pressure Education Program’s Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC).
Numerous antihypertensive drugs, from a variety of pharmacologic classes and with different mechanisms of action, have been shown in randomized controlled trials to reduce cardiovascular morbidity and mortality, and it can be concluded that it is blood pressure reduction, and not some other pharmacologic property of the drugs, that is largely responsible for those benefits. The largest and most consistent cardiovascular outcome benefit has been a reduction in the risk of stroke, but reductions in myocardial infarction and cardiovascular mortality also have been seen regularly.
Elevated systolic or diastolic pressure causes increased cardiovascular risk, and the absolute risk increase per mmHg is greater at higher blood pressures, so that even modest reductions of severe hypertension can provide substantial benefit. Relative risk reduction from blood pressure reduction is similar across populations with varying absolute risk, so the absolute benefit is greater in patients who are at higher risk independent of their hypertension (for example, patients with diabetes or hyperlipidemia), and such patients would be expected to benefit from more aggressive treatment to a lower blood pressure goal.
Some antihypertensive drugs have smaller blood pressure effects (as monotherapy) in black patients, and many antihypertensive drugs have additional approved indications and effects (e.g., on angina, heart failure, or diabetic kidney disease). These considerations may guide selection of therapy.
Limitations of Use
This fixed combination drug is not indicated for the initial therapy of hypertension.
2. Tribenzor Dosage and Administration
Dose once daily. Dosage may be increased in 2-week intervals, as needed. The maximum recommended dose of Tribenzor is 40/10/25 mg.
Dose selection should be individualized based on previous therapy.
3. Dosage Forms and Strengths
Tribenzor tablets are available in the following strength combinations:
| 20/5/12.5 | 40/5/12.5 | 40/5/25 | 40/10/12.5 | 40/10/25 | |
| Olmesartan medoxomil (mg) | 20 | 40 | 40 | 40 | 40 |
| Amlodipine equivalent (mg) | 5 | 5 | 5 | 10 | 10 |
| Hydrochlorothiazide (mg) | 12.5 | 12.5 | 25 | 12.5 | 25 |
4. Contraindications
Because of the hydrochlorothiazide component, Tribenzor is contraindicated in patients with anuria, hypersensitivity to any component, or hypersensitivity to other sulfonamide-derived drugs.
Do not co-administer aliskiren with Tribenzor in patients with diabetes [See Drug Interactions (7.2)].
5. Warnings and Precautions
5.2 Hypotension in Volume- or Salt-Depleted Patients
Olmesartan medoxomil. In patients with an activated renin-angiotensin system, such as volume- and/or salt-depleted patients (e.g., those being treated with high doses of diuretics) symptomatic hypotension may be anticipated after initiation of treatment with olmesartan medoxomil. Initiate treatment with Tribenzor under close medical supervision. If hypotension does occur, place the patient in the supine position and, if necessary, give an intravenous infusion of normal saline. A transient hypotensive response is not a contraindication to further treatment, which usually can be continued without difficulty once the blood pressure has stabilized.
Amlodipine. Symptomatic hypotension is possible, particularly in patients with severe aortic stenosis. Because of the gradual onset of action, acute hypotension is unlikely.
5.3 Increased Angina and/or Myocardial Infarction
Amlodipine. Patients, particularly those with severe obstructive coronary artery disease, may develop increased frequency, duration, or severity of angina or acute myocardial infarction upon starting calcium channel blocker therapy or at the time of dosage increase. The mechanism of this effect has not been elucidated.
5.5 Patients with Hepatic Impairment
Amlodipine. Since amlodipine is extensively metabolized by the liver and the plasma elimination half-life (t1/2) is 56 hours in patients with severely impaired hepatic function, titrate slowly when administering to patients with severe hepatic impairment.
5.6 Electrolyte and Metabolic Imbalances
Tribenzor contains hydrochlorothiazide which can cause hypokalemia, hyponatremia and hypomagnesemia. Hypomagnesemia can result in hypokalemia which may be difficult to treat despite potassium repletion. Tribenzor also contains olmesartan, a drug that affects the RAS. Drugs that inhibit the RAS can also cause hyperkalemia.
Hydrochlorothiazide may alter glucose tolerance and raise serum levels of cholesterol and triglycerides.
Hyperuricemia may occur or frank gout may be precipitated in patients receiving thiazide therapy.
Hydrochlorothiazide decreases urinary calcium excretion and may cause elevations of serum calcium. Monitor calcium levels.
5.8 Systemic Lupus Erythematosus
Hydrochlorothiazide. Thiazide diuretics have been reported to cause exacerbation or activation of systemic lupus erythematosus.
5.9 Acute Myopia and Secondary Angle-Closure Glaucoma
Hydrochlorothiazide, a sulfonamide, can cause an idiosyncratic reaction, resulting in acute transient myopia and acute angle-closure glaucoma. Symptoms include acute onset of decreased visual acuity or ocular pain and typically occur within hours to weeks of drug initiation. Untreated acute angle-closure glaucoma can lead to permanent vision loss. The primary treatment is to discontinue hydrochlorothiazide as rapidly as possible. Prompt medical or surgical treatments may need to be considered if the intraocular pressure remains uncontrolled. Risk factors for developing acute angle-closure glaucoma may include a history of sulfonamide or penicillin allergy.
5.10 Sprue-like Enteropathy
Olmesartan medoxomil. Severe, chronic diarrhea with substantial weight loss has been reported in patients taking olmesartan months to years after drug initiation. Intestinal biopsies of patients often demonstrated villous atrophy. If a patient develops these symptoms during treatment with olmesartan, exclude other etiologies. Consider discontinuation of Tribenzor in cases where no other etiology is identified.
6. Adverse Reactions/Side Effects
6.1 Clinical Trials Experience
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to rates in the clinical studies of another drug and may not reflect the rates observed in practice.
Tribenzor
In the controlled trial of Tribenzor, patients were randomized to Tribenzor (olmesartan medoxomil/amlodipine/hydrochlorothiazide 40/10/25 mg), olmesartan medoxomil/amlodipine 40/10 mg, olmesartan medoxomil/hydrochlorothiazide 40/25 mg, or amlodipine/hydrochlorothiazide 10/25 mg. Subjects who received triple combination therapy were treated between two and four weeks with one of the three dual combination therapies. Safety data from this study were obtained in 574 patients with hypertension who received Tribenzor for 8 weeks.
The frequency of adverse reactions was similar between men and women, patients <65 years of age and patients ≥65 years of age, patients with and without diabetes, and Black and non-Black patients. Discontinuations because of adverse events occurred in 4% of patients treated with Tribenzor 40/10/25 mg compared to 1% of patients treated with olmesartan medoxomil/amlodipine 40/10 mg, 2% of patients treated with olmesartan medoxomil/hydrochlorothiazide 40/25 mg, and 2% of patients treated with amlodipine/hydrochlorothiazide 10/25 mg. The most common reason for discontinuation with Tribenzor was dizziness (1%).
Dizziness was one of the most frequently reported adverse reactions with incidence of 1.4% to 3.6% in subjects continuing on dual combination therapy compared to 5.8% to 8.9% in subjects who switched to Tribenzor.
The other most frequent adverse reactions that occurred in at least 2% of subjects are presented in the table below:
| Adverse Reaction | OM40/ AML10/ HCTZ25 mg (N = 574) n (%) | OM40/ AML10 mg (N = 596) n (%) | OM40/ HCTZ25mg (N = 580) n (%) | AML10/ HCTZ25 mg (N = 552) n (%) |
| Edema peripheral | 44 (7.7) | 42 (7.0) | 6 (1.0) | 46 (8.3) |
| Headache | 37 (6.4) | 42 (7.0) | 38 (6.6) | 33 (6.0) |
| Fatigue | 24 (4.2) | 34 (5.7) | 31 (5.3) | 36 (6.5) |
| Nasopharyngitis | 20 (3.5) | 11 (1.8) | 20 (3.4) | 16 (2.9) |
| Muscle spasms | 18 (3.1) | 12 (2.0) | 14 (2.4) | 13 (2.4) |
| Nausea | 17 (3.0) | 12 (2.0) | 22 (3.8) | 12 (2.2) |
| Upper respiratory tract infection | 16 (2.8) | 26 (4.4) | 18 (3.1) | 14 (2.5) |
| Diarrhea | 15 (2.6) | 14 (2.3) | 12 (2.1) | 9 (1.6) |
| Urinary tract infection | 14 (2.4) | 8 (1.3) | 6 (1.0) | 7 (1.3) |
| Joint swelling | 12 (2.1) | 17 (2.9) | 2 (0.3) | 16 (2.9) |
Syncope was reported by 1% of Tribenzor subjects compared to 0.5% or less for the other treatment groups.
Olmesartan medoxomil
Olmesartan medoxomil has been evaluated for safety in more than 3825 patients/subjects, including more than 3275 patients treated for hypertension in controlled trials. This experience included about 900 patients treated for at least 6 months and more than 525 treated for at least 1 year. Treatment with olmesartan medoxomil was well tolerated, with an incidence of adverse reactions similar to that seen with placebo. Adverse reactions were generally mild, transient, and without relationship to the dose of olmesartan medoxomil.
Amlodipine
Amlodipine has been evaluated for safety in more than 11,000 patients in U.S. and foreign clinical trials.
6.2 Post-Marketing Experience
The following adverse reactions have been identified during post-approval use of the individual components of Tribenzor. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Olmesartan medoxomil. The following adverse reactions have been reported in post-marketing experience:
Body as a Whole: asthenia, angioedema, anaphylactic reactions, peripheral edema
Gastrointestinal: vomiting, diarrhea, sprue-like enteropathy [see Warnings and Precautions (5.10)]
Metabolic and Nutritional Disorders: hyperkalemia
Musculoskeletal: rhabdomyolysis
Urogenital System: acute renal failure, increased blood creatinine
Skin and Appendages: alopecia, pruritus, urticaria
Data from one controlled trial and an epidemiologic study have suggested that high-dose olmesartan may increase cardiovascular (CV) risk in diabetic patients, but the overall data are not conclusive. The randomized, placebo-controlled, double-blind ROADMAP trial (Randomized Olmesartan And Diabetes MicroAlbuminuria Prevention trial, n=4447) examined the use of olmesartan, 40 mg daily, vs. placebo in patients with type 2 diabetes mellitus, normoalbuminuria, and at least one additional risk factor for CV disease. The trial met its primary endpoint, delayed onset of microalbuminuria, but olmesartan had no beneficial effect on decline in glomerular filtration rate (GFR). There was a finding of increased CV mortality (adjudicated sudden cardiac death, fatal myocardial infarction, fatal stroke, revascularization death) in the olmesartan group compared to the placebo group (15 olmesartan vs. 3 placebo, HR 4.9, 95% confidence interval [CI], 1.4, 17), but the risk of non-fatal myocardial infarction was lower with olmesartan (HR 0.64, 95% CI 0.35, 1.18).
The epidemiologic study included patients 65 years and older with overall exposure of > 300,000 patient-years. In the sub-group of diabetic patients receiving high-dose olmesartan (40 mg/d) for > 6 months, there appeared to be an increased risk of death (HR 2.0, 95% CI 1.1, 3.8) compared to similar patients taking other angiotensin receptor blockers. In contrast, high-dose olmesartan use in non-diabetic patients appeared to be associated with a decreased risk of death (HR 0.46, 95% CI 0.24, 0.86) compared to similar patients taking other angiotensin receptor blockers. No differences were observed between the groups receiving lower doses of olmesartan compared to other angiotensin blockers or those receiving therapy for < 6 months.
Overall, these data raise a concern of a possible increased CV risk associated with the use of high-dose olmesartan in diabetic patients. There are, however, concerns with the credibility of the finding of increased CV risk, notably the observation in the large epidemiologic study for a survival benefit in non-diabetics of a magnitude similar to the adverse finding in diabetics.
Amlodipine. The following post-marketing event has been reported infrequently where a causal relationship is uncertain: gynecomastia. In post-marketing experience, jaundice and hepatic enzyme elevations (mostly consistent with cholestasis or hepatitis), in some cases severe enough to require hospitalization, have been reported in association with use of amlodipine. Postmarketing reporting has also revealed a possible association between extrapyramidal disorder and amlodipine.
7. Drug Interactions
7.1 Drug Interactions with Olmesartan Medoxomil
Non-Steroidal Anti-Inflammatory Agents including Selective Cyclooxygenase-2 Inhibitors (COX-2 Inhibitors): In patients who are elderly, volume-depleted (including those on diuretic therapy), or with compromised renal function, co-administration of NSAIDs, including selective COX-2 inhibitors, with angiotensin II receptor antagonists, including olmesartan medoxomil, may result in deterioration of renal function, including possible acute renal failure. These effects are usually reversible. Monitor renal function periodically in patients receiving olmesartan medoxomil and NSAID therapy.
The antihypertensive effect of angiotensin II receptor antagonists, including olmesartan medoxomil may be attenuated by NSAIDs including selective COX-2 inhibitors.
Dual Blockade of the Renin-Angiotensin System (RAS): Dual blockade of the RAS with angiotensin receptor blockers, ACE inhibitors, or aliskiren is associated with increased risks of hypotension, hyperkalemia, and changes in renal function (including acute renal failure) compared to monotherapy. Most patients receiving the combination of two RAS inhibitors do not obtain any additional benefit compared to monotherapy. In general, avoid combined use of RAS inhibitors. Closely monitor blood pressure, renal function and electrolytes in patients on Tribenzor and other agents that affect the RAS.
Do not co-administer aliskiren with Tribenzor in patients with diabetes [See Contraindications (4)]. Avoid use of aliskiren with Tribenzor in patients with renal impairment (GFR <60 ml/min).
Use with Colesevelam Hydrochloride: Concurrent administration of bile acid sequestering agent colesevelam hydrochloride reduces the systemic exposure and peak plasma concentration of olmesartan. Administration of olmesartan at least 4 hours prior to colesevelam hydrochloride decreased the drug interaction effect. Consider administering olmesartan at least 4 hours before the colesevelam hydrochloride dose [see Clinical Pharmacology (12.3)].
Lithium: Increases in serum lithium concentrations and lithium toxicity have been reported with concomitant use of olmesartan or thiazide diuretics. Monitor lithium levels in patients receiving Tribenzor and lithium.
7.2 Drug Interactions with Amlodipine
Simvastatin: Co-administration of simvastatin with amlodipine increases the systemic exposure of simvastatin. Limit the dose of simvastatin in patients on amlodipine to 20 mg daily. [see Clinical Pharmacology (12.3)].
Immunosuppressants: Amlodipine may increase the systemic exposure of cyclosporine or tacrolimus when co-administered. Frequent monitoring of trough blood levels of cyclosporine and tacrolimus is recommended and adjust the dose when appropriate [see Clinical Pharmacology (12.3)].
CYP3A Inhibitors: Co-administration of amlodipine with CYP3A inhibitors (moderate and strong) results in increased systemic exposure to amlodipine and may require dose reduction. Monitor for symptoms of hypotension and edema when amlodipine is co-administered with CYP3A inhibitors to determine the need for dose adjustment.
CYP3A Inducers: No information is available on the quantitative effects of CYP3A inducers on amlodipine. Blood pressure should be closely monitored when amlodipine is co-administered with CYP3A inducers.
7.3 Drug Interactions with Hydrochlorothiazide
When administered concurrently the following drugs may interact with thiazide diuretics:
Antidiabetic Drugs (oral agents and insulin): Dosage adjustment of the antidiabetic drug may be required.
Cholestyramine and Colestipol Resins: Absorption of hydrochlorothiazide is impaired in the presence of anionic exchange resins. Single dose of either cholestyramine or colestipol resins bind the hydrochlorothiazide and reduce its absorption from the gastrointestinal tract by up to 85% and 43%, respectively.
Corticosteroids, ACTH: Intensified electrolyte depletion, particularly hypokalemia.
Non-steroidal Anti-inflammatory Drugs: In some patients the administration of a non-steroidal anti-inflammatory agent can reduce the diuretic, natriuretic, and antihypertensive effects of loop, potassium-sparing and thiazide diuretics. Therefore, when hydrochlorothiazide tablets and non-steroidal anti-inflammatory agents are used concomitantly, the patients should be observed closely to determine if the desired effect of the diuretic is obtained.
8. Use In Specific Populations
8.4 Pediatric Use
The safety and effectiveness of Tribenzor in pediatric patients have not been established.
8.7 Renal Impairment
There are no studies of Tribenzor in patients with renal impairment. Avoid use in patients with severe renal impairment (creatinine clearance <30 mL/min).
Olmesartan medoxomil. Patients with renal insufficiency have elevated serum concentrations of olmesartan compared with patients with normal renal function. After repeated dosing, AUC was approximately tripled in patients with severe renal impairment (creatinine clearance <20 mL/min). No initial dosage adjustment is recommended for patients with moderate to marked renal impairment (creatinine clearance <40 mL/min). The pharmacokinetics of olmesartan in patients undergoing hemodialysis has not been studied.
Amlodipine. The pharmacokinetics of amlodipine are not significantly influenced by renal impairment.
Hydrochlorothiazide. Thiazide should be used with caution in patients with severe renal disease. In patients with renal disease, thiazides may precipitate azotemia. Cumulative effects of the drug may develop in patients with impaired renal function.
12. Tribenzor - Clinical Pharmacology
12.3 Pharmacokinetics
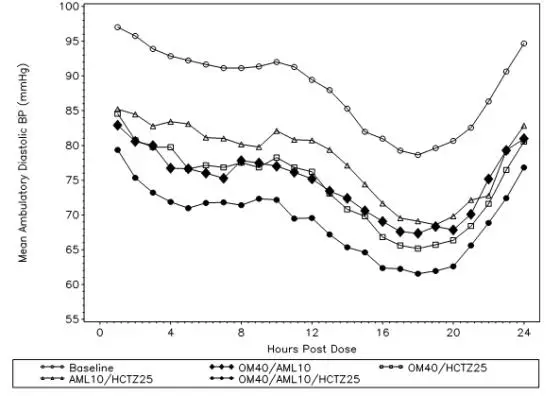
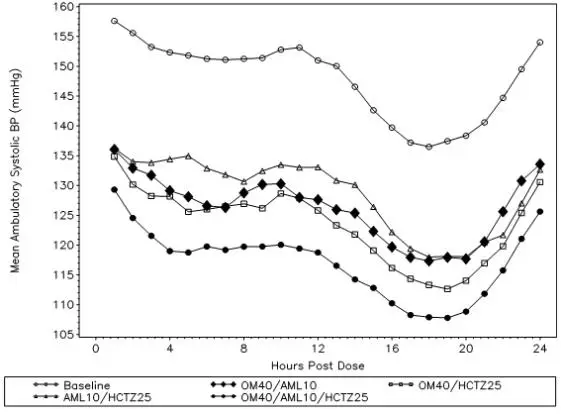
Tribenzor. After oral administration of Tribenzor in normal healthy adults, peak plasma concentrations of olmesartan, amlodipine, and hydrochlorothiazide are reached in about 1.5 to 3 hours, 6 to 8 hours, and 1.5 to 2 hours, respectively. The rate and extent of absorption of olmesartan medoxomil, amlodipine, and hydrochlorothiazide from Tribenzor are the same as when administered as individual dosage forms. Food does not affect the bioavailability of Tribenzor.
Olmesartan medoxomil. Olmesartan medoxomil is rapidly and completely bioactivated by ester hydrolysis to olmesartan during absorption from the gastrointestinal tract. The absolute bioavailability of olmesartan medoxomil is approximately 26%. After oral administration, the Cmax of olmesartan is reached after 1 to 2 hours. Food does not affect the bioavailability of olmesartan medoxomil.
Amlodipine. After oral administration of therapeutic doses of amlodipine, absorption produces peak plasma concentrations between 6 and 12 hours. Absolute bioavailability is estimated between 64% and 90%.
Hydrochlorothiazide. When plasma levels have been followed for at least 24 hours, the plasma half-life has been observed to vary between 5.6 and 14.8 hours.
Distribution
Olmesartan medoxomil. The volume of distribution of olmesartan is approximately 17 L. Olmesartan is highly bound to plasma proteins (99%) and does not penetrate red blood cells. The protein binding is constant at plasma olmesartan concentrations well above the range achieved with recommended doses.
In rats, olmesartan crossed the blood-brain barrier poorly, if at all. Olmesartan passed across the placental barrier in rats and was distributed to the fetus. Olmesartan was distributed to milk at low levels in rats.
Amlodipine. Ex vivo studies have shown that approximately 93% of the circulating drug is bound to plasma proteins in hypertensive patients. Steady-state plasma levels of amlodipine are reached after 7 to 8 days of consecutive daily dosing.
Hydrochlorothiazide. Hydrochlorothiazide crosses the placental but not the blood-brain barrier and is excreted in breast milk.
Metabolism and Excretion
Olmesartan medoxomil. Following the rapid and complete conversion of olmesartan medoxomil to olmesartan during absorption, there is virtually no further metabolism of olmesartan. Total plasma clearance of olmesartan is 1.3 L/h, with a renal clearance of 0.6 L/h. Approximately 35% to 50% of the absorbed dose is recovered in urine while the remainder is eliminated in feces via the bile.
Olmesartan appears to be eliminated in a biphasic manner with a terminal elimination half-life of approximately 13 hours. Olmesartan shows linear pharmacokinetics following single oral doses of up to 320 mg and multiple oral doses of up to 80 mg. Steady-state levels of olmesartan are achieved within 3 to 5 days and no accumulation in plasma occurs with once-daily dosing.
Amlodipine. Amlodipine is extensively (about 90%) converted to inactive metabolites via hepatic metabolism. Elimination from the plasma is biphasic with a terminal elimination half-life of about 30 to 50 hours. Ten percent of the parent compound and 60% of the metabolites are excreted in the urine.
Hydrochlorothiazide. Hydrochlorothiazide is not metabolized but is eliminated rapidly by the kidney. At least 61% of the oral dose is eliminated unchanged within 24 hours.
Specific Populations
Geriatric Patients
Olmesartan medoxomil. The pharmacokinetics of olmesartan medoxomil were studied in the elderly (≥65 years). Overall, maximum plasma concentrations of olmesartan were similar in young adults and the elderly. Modest accumulation of olmesartan was observed in the elderly with repeated dosing; AUCѕѕ, τ was 33% higher in elderly patients, corresponding to an approximate 30% reduction in CLR.
Amlodipine. Elderly patients have decreased clearance of amlodipine with a resulting increase in AUC of approximately 40% to 60%, and a lower initial dose may be required.
Male and Female Patients
Population pharmacokinetic analysis indicated that gender had no effect on the clearance of olmesartan and amlodipine. Female patients had approximately 20% smaller clearances of hydrochlorothiazide than male patients.
Olmesartan medoxomil. Minor differences were observed in the pharmacokinetics of olmesartan medoxomil in women compared to men. Area under the curve and Cmax were 10% to 15% higher in women than in men.
Patients with Renal Impairment
Olmesartan medoxomil. In patients with renal insufficiency, serum concentrations of olmesartan were elevated compared to subjects with normal renal function. After repeated dosing, the AUC was approximately tripled in patients with severe renal impairment (creatinine clearance <20 mL/min). The pharmacokinetics of olmesartan medoxomil in patients undergoing hemodialysis has not been studied.
Amlodipine. The pharmacokinetics of amlodipine are not significantly influenced by renal impairment.
Patients with Hepatic Impairment
Olmesartan medoxomil. Increases in AUC0-∞ and Cmax were observed in patients with moderate hepatic impairment compared to those in matched controls, with an increase in AUC of about 60%.
Amlodipine. Patients with hepatic insufficiency have decreased clearance of amlodipine with a resulting increase in AUC of approximately 40% to 60%.
Heart Failure
Amlodipine. Patients with heart failure have decreased clearance of amlodipine with a resulting increase in AUC of approximately 40% to 60%.
Drug Interaction Studies
Simvastatin: Co-administration of multiple doses of 10 mg of amlodipine with 80 mg simvastatin resulted in a 77% increase in exposure to simvastatin compared to simvastatin alone. [see Drug Interactions (7.2)].
CYP3A inhibitors: Co-administration of a 180 mg daily dose of diltiazem with 5 mg amlodipine in elderly hypertensive patients resulted in a 60% increase in amlodipine systemic exposure. Erythromycin co-administration in healthy volunteers did not significantly change amlodipine systemic exposure. However, strong inhibitors of CYP3A (e.g., itraconazole, clarithromycin) may increase the plasma concentrations of amlodipine to a greater extent [see Drug Interactions (7.2)].
Cyclosporine: In a prospective study in renal transplant patients, an average 40% increase in trough cyclosporine levels was observed in the presence of amlodipine. [see Drug Interactions (7.2)].
Colesevelam: Concomitant administration of 40 mg olmesartan medoxomil and 3750 mg colesevelam hydrochloride in healthy subjects resulted in 28% reduction in Cmax and 39% reduction in AUC of olmesartan. Lesser effects, 4% and 15% reduction in Cmax and AUC respectively, were observed when olmesartan medoxomil was administered 4 hours prior to colesevelam hydrochloride [see Drug Interactions (7.1)].
Cimetidine: Co-administration of amlodipine with cimetidine did not alter the pharmacokinetics of amlodipine.
Grapefruit juice: Co-administration of 240 mL of grapefruit juice with a single oral dose of amlodipine 10 mg in 20 healthy volunteers had no significant effect on the pharmacokinetics of amlodipine.
Maalox® (antacid): Co-administration of the antacid Maalox® with a single dose of amlodipine had no significant effect on the pharmacokinetics of amlodipine.
Sildenafil: A single 100 mg dose of sildenafil in subjects with essential hypertension had no effect on the pharmacokinetic parameters of amlodipine. When amlodipine and sildenafil were used in combination, each agent independently exerted its own blood pressure lowering effect.
Atorvastatin: Co-administration of multiple 10 mg doses of amlodipine with 80 mg of atorvastatin resulted in no significant change in the steady state pharmacokinetic parameters of atorvastatin.
Digoxin: Co-administration of amlodipine with digoxin did not change serum digoxin levels or digoxin renal clearance in normal volunteers.
No significant drug interactions were reported in studies in which olmesartan medoxomil was coadministered with digoxin in healthy volunteers.
Ethanol (alcohol): Single and multiple 10 mg doses of amlodipine had no significant effect on the pharmacokinetics of ethanol.
Warfarin: Co-administration of amlodipine with warfarin did not change the warfarin prothrombin response time. No significant drug interactions were reported in studies in which olmesartan medoxomil was coadministered with warfarin in healthy volunteers.
Antacids: The bioavailability of olmesartan medoxomil was not significantly altered by the co-administration of antacids [Al(OH)3/Mg(OH)2].
| TRIBENZOR
olmesartan medoxomil / amlodipine besylate / hydrochlorothiazide tablet, film coated |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| TRIBENZOR
olmesartan medoxomil / amlodipine besylate / hydrochlorothiazide tablet, film coated |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| TRIBENZOR
olmesartan medoxomil / amlodipine besylate / hydrochlorothiazide tablet, film coated |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| TRIBENZOR
olmesartan medoxomil / amlodipine besylate / hydrochlorothiazide tablet, film coated |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| TRIBENZOR
olmesartan medoxomil / amlodipine besylate / hydrochlorothiazide tablet, film coated |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Labeler - Daiichi Sankyo, Inc. (068605067) |




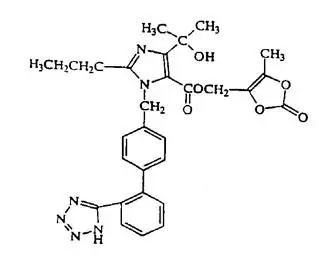
![The structural formula for amlodipine besylate is chemically described as 3 ethyl 5-methyl (±)-2-[(2-aminoethoxy)methyl]-4-(2-chlorophenyl)-1,4-dihydro-6-methyl-3,5-pyridinedicarboxylate, monobenzenesulphonate. Its empirical formula is C20H25CIN2O5•C6H6O3S.](https://cdn.themeditary.com/images/2023/09/05/tribenzor-02.webp)