Drug Detail:Tyvaso dpi maintenance kit (inhalation) (Treprostinil (inhalation) [ tre-pros-ti-nil ])
Drug Class: Agents for pulmonary hypertension
Highlights of Prescribing Information
TYVASO DPI® (treprostinil) inhalation powder, for oral inhalation use
Initial U.S. Approval: 2002
Indications and Usage for Tyvaso DPI
Tyvaso DPI is a prostacyclin mimetic indicated for the treatment of:
- Pulmonary arterial hypertension (PAH; WHO Group 1) to improve exercise ability. Studies with Tyvaso establishing effectiveness predominately included patients with NYHA Functional Class III symptoms and etiologies of idiopathic or heritable PAH (56%) or PAH associated with connective tissue diseases (33%). (1.1)
- Pulmonary hypertension associated with interstitial lung disease (PH-ILD; WHO Group 3) to improve exercise ability. The study with Tyvaso establishing effectiveness predominately included patients with etiologies of idiopathic interstitial pneumonia (IIP) (45%) inclusive of idiopathic pulmonary fibrosis (IPF), combined pulmonary fibrosis and emphysema (CPFE) (25%), and WHO Group 3 connective tissue disease (22%). (1.2)
Tyvaso DPI Dosage and Administration
- Use only with the Tyvaso DPI Inhaler. (2.1)
- Administer using a single inhalation per cartridge. (2.1)
- Administer in 4 separate treatment sessions each day approximately 4 hours apart, during waking hours. (2.1)
- Initial dosage: one 16 mcg cartridge per treatment session. (2.2)
- Dosage should be increased by an additional 16 mcg per treatment session at approximately 1- to 2-week intervals, if tolerated. (2.2)
- Titrate to target maintenance doses of 48 mcg to 64 mcg per treatment session, 4 times daily. (2.2)
Dosage Forms and Strengths
Inhalation powder: Single-dose plastic cartridges containing 16, 32, 48, or 64 mcg of treprostinil as a dry powder formulation. (3)
Contraindications
None. (4)
Warnings and Precautions
- Tyvaso DPI may cause symptomatic hypotension. (5.1)
- Tyvaso DPI inhibits platelet aggregation and increases the risk of bleeding. (5.2)
- Tyvaso DPI dosage adjustments may be necessary if inhibitors or inducers of CYP2C8 are added or withdrawn. (5.3, 7.3)
- May cause bronchospasm: Patients with a history of hyperreactive airway disease may be more sensitive. (5.4)
Adverse Reactions/Side Effects
Most common adverse reactions (≥4%) are cough, headache, throat irritation/pharyngolaryngeal pain, nausea, flushing, dyspnea, and syncope. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact United Therapeutics Corp. at 1-866-458-6479 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 6/2023
Full Prescribing Information
1. Indications and Usage for Tyvaso DPI
1.1 Pulmonary Arterial Hypertension
Tyvaso DPI is indicated for the treatment of pulmonary arterial hypertension (PAH; WHO Group 1) to improve exercise ability. Studies with Tyvaso establishing effectiveness predominately included patients with NYHA Functional Class III symptoms and etiologies of idiopathic or heritable PAH (56%) or PAH associated with connective tissue diseases (33%).
The effects diminish over the minimum recommended dosing interval of 4 hours; treatment timing can be adjusted for planned activities.
While there are long-term data on use of treprostinil by other routes of administration, nearly all clinical experience with inhaled treprostinil has been on a background of an endothelin receptor antagonist (ERA) and/or a phosphodiesterase type 5 (PDE-5) inhibitor. The controlled clinical experience with Tyvaso was limited to 12 weeks in duration [see Clinical Studies (14)].
1.2 Pulmonary Hypertension Associated with ILD
Tyvaso DPI is indicated for the treatment of pulmonary hypertension associated with interstitial lung disease (PH-ILD; WHO Group 3) to improve exercise ability. The study with Tyvaso establishing effectiveness predominately included patients with etiologies of idiopathic interstitial pneumonia (IIP) (45%) inclusive of idiopathic pulmonary fibrosis (IPF), combined pulmonary fibrosis and emphysema (CPFE) (25%), and WHO Group 3 connective tissue disease (22%) [see Clinical Studies (14.3)].
2. Tyvaso DPI Dosage and Administration
2.1 Administration
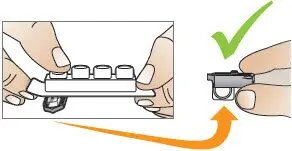
Use Tyvaso DPI only with the Tyvaso DPI Inhaler. Tyvaso DPI is administered using a single inhalation per cartridge. Administer Tyvaso DPI in 4 separate, equally spaced treatment sessions per day, during waking hours. The treatment sessions should be approximately 4 hours apart.
If the prescribed dose is higher than 64 mcg per treatment session, more than 1 cartridge will be needed per session. Patients should follow the instructions for use for operation and care of the Tyvaso DPI Inhaler.
Do not use the Tyvaso DPI Inhaler with other medications.
Between each of the 4 daily treatment sessions, store the Tyvaso DPI Inhaler with the mouthpiece attached and empty. Wipe the outside of the inhaler with a clean, dry cloth only, if needed. Do not rinse or wash the Tyvaso DPI Inhaler; always keep the inhaler dry. After 7 days of use, throw away the used Tyvaso DPI Inhaler into regular household trash.
3. Dosage Forms and Strengths
Inhalation powder: Single-dose plastic cartridges containing 16, 32, 48, or 64 mcg of treprostinil as a dry powder formulation.
5. Warnings and Precautions
5.1 Risk of Symptomatic Hypotension
Treprostinil is a pulmonary and systemic vasodilator. In patients with low systemic arterial pressure, treatment with Tyvaso DPI may produce symptomatic hypotension.
5.3 Effect of Other Drugs on Treprostinil
Co-administration of a cytochrome P450 (CYP) 2C8 enzyme inhibitor (e.g., gemfibrozil) may increase exposure (both Cmax and AUC) to treprostinil. Co-administration of a CYP2C8 enzyme inducer (e.g., rifampin) may decrease exposure to treprostinil. Increased exposure is likely to increase adverse events associated with treprostinil administration, whereas decreased exposure is likely to reduce clinical effectiveness [see Drug Interactions (7.3) and Clinical Pharmacology (12.3)].
5.4 Bronchospasm
Like other inhaled prostaglandins, Tyvaso DPI may cause acute bronchospasm. Patients with asthma or chronic obstructive pulmonary disease (COPD), or other bronchial hyperreactivity, are at increased risk for bronchospasm. Ensure that such patients are treated optimally for reactive airway disease prior to and during treatment with Tyvaso DPI.
6. Adverse Reactions/Side Effects
The following potential adverse reactions are described in Warnings and Precautions (5):
- -
- Decrease in systemic blood pressure [see Warnings and Precautions (5.1)].
- -
- Bleeding [see Warnings and Precautions (5.2)].
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
7. Drug Interactions
7.1 Bosentan
In a human pharmacokinetic study conducted with bosentan (250 mg/day) and an oral formulation of treprostinil (treprostinil diolamine), no pharmacokinetic interactions between treprostinil and bosentan were observed.
7.2 Sildenafil
In a human pharmacokinetic study conducted with sildenafil (60 mg/day) and an oral formulation of treprostinil (treprostinil diolamine), no pharmacokinetic interactions between treprostinil and sildenafil were observed.
7.3 Effect of Cytochrome P450 Inhibitors and Inducers
In vitro studies of human hepatic microsomes showed that treprostinil does not inhibit cytochrome P450 (CYP) isoenzymes CYP1A2, CYP2A6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, CYP2E1, and CYP3A. Additionally, treprostinil does not induce cytochrome P450 isoenzymes CYP1A2, CYP2B6, CYP2C9, CYP2C19, and CYP3A.
Human pharmacokinetic studies with an oral formulation of treprostinil (treprostinil diolamine) indicated that co-administration of the cytochrome P450 (CYP) 2C8 enzyme inhibitor, gemfibrozil, increases exposure (both Cmax and AUC) to treprostinil. Co-administration of the CYP2C8 enzyme inducer, rifampin, decreases exposure to treprostinil. It is unclear if the safety and efficacy of treprostinil by the inhalation route are altered by inhibitors or inducers of CYP2C8 [see Warnings and Precautions (5.3)].
7.4 Effect of Other Drugs on Treprostinil
Drug interaction studies have been carried out with treprostinil (oral or subcutaneous) co-administered with acetaminophen (4 g/day), warfarin (25 mg/day), and fluconazole (200 mg/day), respectively, in healthy volunteers. These studies did not show a clinically significant effect on the pharmacokinetics of treprostinil. Treprostinil does not affect the pharmacokinetics or pharmacodynamics of warfarin. The pharmacokinetics of R- and S- warfarin and the international normalized ratio (INR) in healthy subjects given a single 25 mg dose of warfarin were unaffected by continuous subcutaneous infusion of treprostinil at an infusion rate of 10 ng/kg/min.
8. Use In Specific Populations
8.4 Pediatric Use
Safety and effectiveness in pediatric patients have not been established. Clinical studies of inhaled treprostinil did not include patients younger than 18 years to determine whether they respond differently from older patients.
8.5 Geriatric Use
Across clinical studies used to establish the effectiveness of Tyvaso Inhalation Solution in patients with PAH and PH-ILD, 268 (47.8%) patients aged 65 years and over were enrolled. The treatment effects and safety profile observed in geriatric patients were similar to younger patients. In general, dose selection for an elderly patient should be cautious, reflecting the greater frequency of hepatic, renal, or cardiac dysfunction, and of concomitant diseases or other drug therapy.
8.6 Patients with Hepatic Insufficiency
Plasma clearance of treprostinil, delivered subcutaneously, was reduced up to 80% in subjects with mild-to-moderate hepatic insufficiency. Uptitrate slowly when treating patients with hepatic insufficiency because of the risk of an increase in systemic exposure which may lead to an increase in dose-dependent adverse effects. Treprostinil has not been studied in patients with severe hepatic insufficiency [see Clinical Pharmacology (12.3)].
10. Overdosage
In general, symptoms of overdose with inhaled treprostinil include flushing, headache, hypotension, nausea, vomiting, and diarrhea. Provide general supportive care until the symptoms of overdose have resolved.
11. Tyvaso DPI Description
11.1 Tyvaso DPI Cartridges
Tyvaso DPI consists of single-dose plastic cartridges filled with a white powder containing 1% of treprostinil, a prostacyclin mimetic, which is intended for administration by oral inhalation using the Tyvaso DPI Inhaler only. Treprostinil is adsorbed onto carrier particles consisting of fumaryl diketopiperazine (FDKP). Each cartridge contains 16, 32, 48, or 64 mcg of treprostinil with approximate fill weights of 1.6, 3.2, 4.8, or 6.4 mg of Tyvaso DPI, respectively.
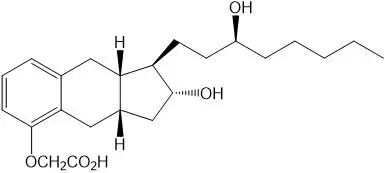
Treprostinil is (1R,2R,3aS,9aS)-[[2,3,3a,4,9,9a-hexahydro-2-hydroxy-1-[(3S)-3-hydroxyoctyl]-1H-benz[f]inden-5-yl]oxy]acetic acid. Treprostinil has a molecular weight of 390.52 and a molecular formula of C23H34O5.
The structural formula of treprostinil is:
12. Tyvaso DPI - Clinical Pharmacology
12.1 Mechanism of Action
Treprostinil is a prostacyclin analogue. The major pharmacologic actions of treprostinil are direct vasodilation of pulmonary and systemic arterial vascular beds and inhibition of platelet aggregation.
12.2 Pharmacodynamics
In a clinical trial of 240 healthy volunteers, single doses of Tyvaso Inhalation Solution 54 mcg (the target maintenance dose per session) and 84 mcg (supratherapeutic inhalation dose) prolonged the corrected QTc interval by approximately 10 ms. The QTc effect dissipated as the concentration of treprostinil decreased.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
A 2-year rat carcinogenicity study was performed with treprostinil inhalation at target doses of 5.26, 10.6, and 34.1 mcg/kg/day. There was no evidence for carcinogenic potential associated with treprostinil inhalation in rats at systemic exposure levels up to 36 times the clinical exposure at the 64 mcg dose of treprostinil inhalation powder. In vitro and in vivo genetic toxicology studies did not demonstrate any mutagenic or clastogenic effects of treprostinil. Treprostinil sodium did not affect fertility or mating performance of male or female rats given continuous subcutaneous infusions at rates of up to 450 ng treprostinil/kg/min. In this study, males were dosed from 10 weeks prior to mating and through the 2-week mating period. Females were dosed from 2 weeks prior to mating until gestational day 6.
Oral administration of treprostinil diolamine to Tg.rasH2 mice at 0, 5, 10, and 20 mg/kg/day in males and 0, 3, 7.5, and 15 mg/kg/day in females daily for 26 weeks did not significantly increase the incidence of tumors.
Treprostinil diolamine was tested in vivo in a rat micronucleus assay and did not induce an increased incidence of micronucleated polychromatic erythrocytes.
13.2 Animal Toxicology and/or Pharmacology
In a 2-year rat study with treprostinil inhalation solution at target doses of 5.26, 10.6, and 34.1 mcg/kg/day, there were more deaths (11) in the mid- and high-dose treprostinil groups during the first 9 weeks of the study, compared to 1 in control groups. At the high-dose level, males showed a higher incidence of inflammation in teeth and preputial gland, and females showed higher incidences of inflammation and urothelial hyperplasia in the urinary bladder. The exposures in rats at mid- and high-dose levels were about 14 and 36 times, respectively, the clinical exposure at the 64 mcg dose of treprostinil inhalation powder.
14. Clinical Studies
14.1 Pulmonary Arterial Hypertension (WHO Group 1) (TRIUMPH I)
TRIUMPH I, was a 12-week, randomized, double-blind, placebo-controlled, multicenter study of patients with PAH (NCT00147199). The study population included 235 clinically stable subjects with PAH (WHO Group 1), nearly all with NYHA Class III (98%) symptoms who were receiving either bosentan (an endothelin receptor antagonist) or sildenafil (a phosphodiesterase-5 inhibitor) for at least 3 months prior to study initiation. Concomitant therapy also could have included anticoagulants, other vasodilators (e.g., calcium channel blockers), diuretics, oxygen, and digitalis, but not a prostacyclin. These patients were administered either placebo or Tyvaso Inhalation Solution in 4 daily treatment sessions with a target dose of 9 breaths (54 mcg) per session over the course of the 12-week study. Patients were predominately female (82%), had the origin of PAH as idiopathic/heritable (56%), secondary to connective tissue diseases (33%) or secondary to HIV or previous use of anorexigens (12%); bosentan was the concomitant oral medication in 70% of those enrolled, sildenafil in 30%.
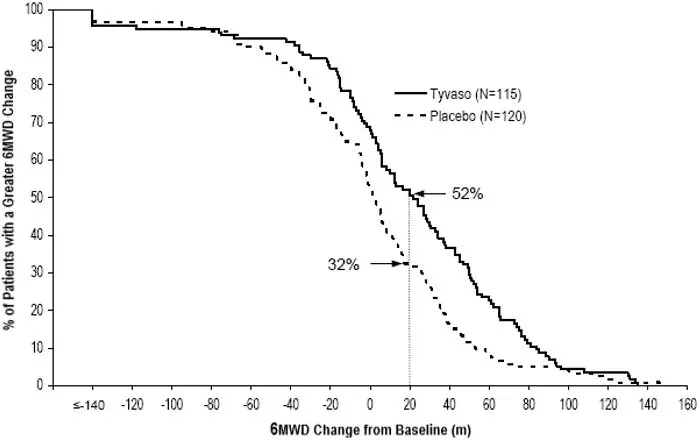
The primary efficacy endpoint of the trial was the change in 6MWD relative to baseline at 12 weeks. 6MWD was measured at peak exposure (between 10 and 60 minutes after dosing), and 3 to 5 hours after bosentan or 0.5 to 2 hours after sildenafil. Patients receiving Tyvaso Inhalation Solution had a placebo-corrected median change from baseline in peak 6MWD of 20 meters at Week 12 (p<0.001). The distribution of these 6MWD changes from baseline at Week 12 were plotted across the range of observed values (Figure 1). 6MWD measured at trough exposure (defined as measurement of 6MWD at least 4 hours after dosing) improved by 14 meters. There were no placebo-controlled 6MWD assessments made after 12 weeks.
Figure 1: Distributions of 6MWD Changes from Baseline at Week 12 During Peak Plasma Concentration of Tyvaso Inhalation Solution
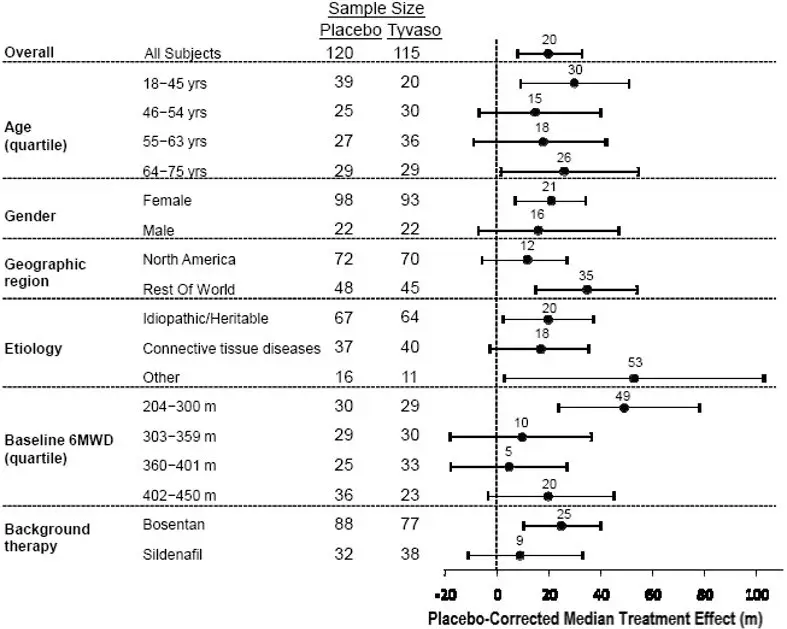
The placebo-corrected median treatment effect on 6MWD was estimated (using the Hodges-Lehmann estimator) within various subpopulations defined by age quartile, gender, geographic region of the study site, disease etiology, baseline 6MWD quartile, and type of background therapy (Figure 2).
Figure 2: Placebo-Corrected Median Treatment Effect (Hodges-Lehmann Estimate with 95% CI) on 6MWD Change from Baseline at Week 12 During Peak Plasma Concentration of Tyvaso Inhalation Solution for Various Subgroups
14.2 Long-term Treatment of PAH
In long-term follow-up of patients who were treated with Tyvaso Inhalation Solution in the pivotal study and the open-label extension (N=206) (NCT00147199), Kaplan-Meier estimates of survival at 1, 2, and 3 years were 97%, 91%, and 82%, respectively. These uncontrolled observations do not allow comparison with a control group not given Tyvaso Inhalation Solution and cannot be used to determine the long-term effect of Tyvaso Inhalation Solution on mortality.
14.3 Pulmonary Hypertension Associated with ILD (WHO Group 3)
INCREASE was a 16-week, randomized, double-blind, placebo-controlled, multicenter study that enrolled 326 patients with PH-ILD (NCT02630316). Enrolled study patients predominately had etiologies of idiopathic interstitial pneumonia (45%) inclusive of idiopathic pulmonary fibrosis, combined pulmonary fibrosis and emphysema (25%), and WHO Group 3 connective tissue disease (22%). The mean baseline 6MWD was 260 meters.
Patients in the INCREASE study were randomized (1:1) to either placebo or Tyvaso Inhalation Solution in 4 daily treatment sessions with a target dose of 9 breaths (54 mcg) per session and a maximum dose of 12 breaths (72 mcg) per session over the course of the 16-week study. Approximately 75% of patients randomized to Tyvaso Inhalation Solution titrated up to a dose of 9 breaths, 4 times daily or greater, with 48% of patients randomized to Tyvaso Inhalation Solution reaching a dose of 12 breaths, 4 times daily during the study.
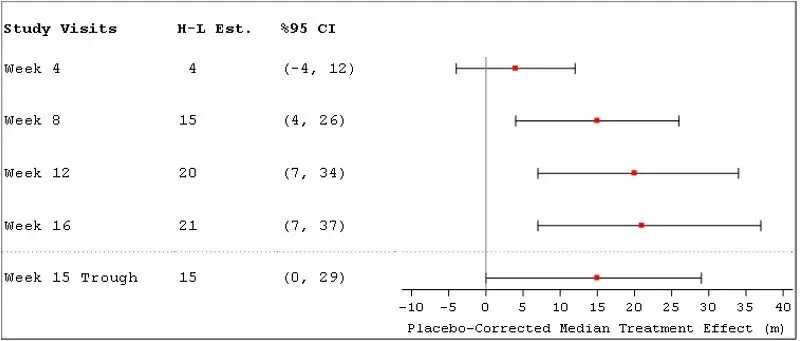
The primary efficacy endpoint was the change in 6MWD measured at peak exposure (between 10 and 60 minutes after dosing) from baseline to Week 16. Patients receiving Tyvaso Inhalation Solution had a placebo-corrected median change from baseline in peak 6MWD of 21 meters at Week 16 (p=0.004) using Hodges-Lehmann estimate (Figure 3).
Figure 3: Hodges-Lehmann Estimate of Treatment Effect by Visit for 6MWD at Peak Exposure of Tyvaso Inhalation Solution (PH-ILD)
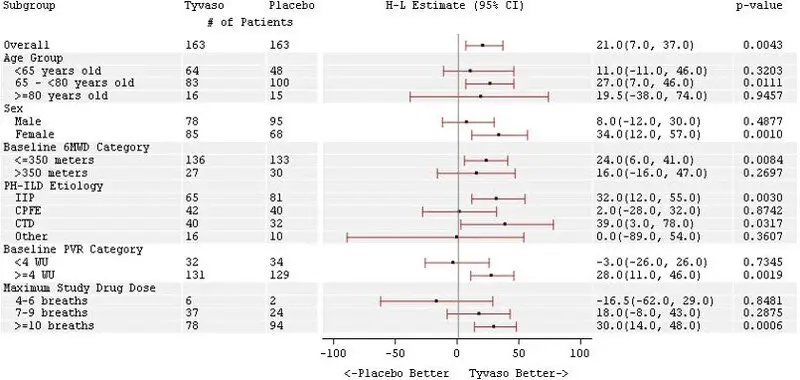
The treatment effect on 6MWD at Week 16 was consistent for various subgroups, including etiology of PH-ILD, disease severity, age, sex, baseline hemodynamics, and dose (Figure 4).
Figure 4: Forest Plot on Subgroup Analyses of Peak 6MWD (Meter) at Week 16 (PH-ILD)
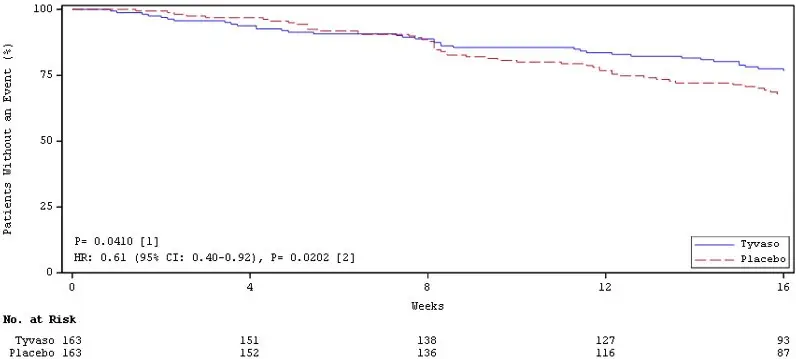
Time to clinical worsening in the INCREASE study was defined as the time of randomization until 1 of the following criteria were met: hospitalization due to a cardiopulmonary indication, decrease in 6MWD >15% from baseline directly related to PH-ILD at 2 consecutive visits and at least 24 hours apart, death (all causes), or lung transplantation. Treatment with Tyvaso Inhalation Solution in patients with PH-ILD resulted in numerically fewer hospitalizations. The numbers of reported deaths were the same for both treatment groups (Table 3). Overall, treatment with Tyvaso Inhalation Solution demonstrated a statistically significant increase in the time to first clinical worsening event (log-rank test p=0.041; Figure 5), and a 39% overall reduction in the risk of a clinical worsening event (HR=0.61 [95% CI; 0.40, 0.92]; Figure 5).
| Tyvaso Inhalation Solution n=163 n (%) | Placebo n=163 n (%) | HR (95% CI) | ||
|---|---|---|---|---|
| Clinical worsening | 37 (22.7%) | 54 (33.1%) | 0.61 (0.40, 0.92) | |
| First contributing event | Hospitalization due to a cardiopulmonary indication | 18 (11.0%) | 24 (14.7%) | |
| Decrease in 6MWD >15% from baseline directly related to PH-ILD | 13 (8.0%) | 26 (16.0%) | ||
| Death (all causes) | 4 (2.5%) | 4 (2.5%) | ||
| Lung transplantation | 2 (1.2%) | 0 | ||
| First of each event | Hospitalization due to a cardiopulmonary indication | 21 (12.9%) | 30 (18.4%) | |
| Decrease in 6MWD >15% from baseline directly related to PH-ILD | 16 (9.8%) | 31 (19.0%) | ||
| Death (all causes) | 8 (4.9%) | 10 (6.1%) | ||
| Lung transplantation | 2 (1.2%) | 1 (0.6%) | ||
Figure 5: Kaplan-Meier Plot of Time to Clinical Worsening Events (PH-ILD)
16. How is Tyvaso DPI supplied
Tyvaso DPI (treprostinil) inhalation powder is available as 16 mcg, 32 mcg, 48 mcg, or 64 mcg of treprostinil in single-dose plastic cartridges with approximate fill weights of 1.6 mg, 3.2 mg, 4.8 mg, or 6.4 mg of Tyvaso DPI, respectively. Four cartridges are contained in a single cavity of a blister strip. A card contains 7 blister strips separated by perforations for a total of 28 cartridges of each labeled strength in Titration and Maintenance Kits. For convenience, the perforation allows users to remove a single blister strip containing 4 cartridges. The Institutional Kits contain 4 blister strips for a total of 16 cartridges of each labeled strength.
The cartridges are color-coded, purple for 16 mcg, dark blue for 32 mcg, light blue for 48 mcg, and light green for 64 mcg. Each cartridge is marked with "Tyvaso DPI" and the corresponding dosage strength of "16 mcg", "32 mcg", "48 mcg", or "64 mcg".
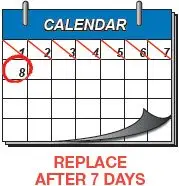
The Tyvaso DPI Inhaler is individually packaged in a clear overwrap. The inhaler is fully assembled with a removable mouthpiece cover. The Tyvaso DPI Inhaler can be used for up to 7 days from the date of first use. After 7 days of use, the inhaler must be discarded and replaced with a new inhaler.
Tyvaso DPI is available in the following configurations:
| Kit Contents | |||
|---|---|---|---|
| Description | NDC | Number of Cartridges and Strength | Number of Inhalers |
| Tyvaso DPI (treprostinil) Inhalation Powder Titration Kit | 66302-600-02 | 112 cartridges, each containing 16 mcg per cartridge | 5 |
| 84 cartridges, each containing 32 mcg per cartridge | |||
| 66302-610-02 | 112 cartridges, each containing 16 mcg per cartridge | 5 | |
| 112 cartridges, each containing 32 mcg per cartridge | |||
| 28 cartridges, each containing 48 mcg per cartridge | |||
| Tyvaso DPI (treprostinil) Inhalation Powder Maintenance Kit | 66302-616-03 | 112 cartridges, each containing 16 mcg per cartridge | 5 |
| 66302-632-03 | 112 cartridges, each containing 32 mcg per cartridge | 5 | |
| 66302-648-03 | 112 cartridges, each containing 48 mcg per cartridge | 5 | |
| 66302-664-03 | 112 cartridges, each containing 64 mcg per cartridge | 5 | |
| 66302-620-03 | 112 cartridges, each containing 32 mcg per cartridge | 5 | |
| 112 cartridges, each containing 48 mcg per cartridge | |||
| 66302-630-03 | 112 cartridges, each containing 32 mcg per cartridge | 5 | |
| 112 cartridges, each containing 64 mcg per cartridge | |||
| 66302-640-03 | 112 cartridges, each containing 48 mcg per cartridge | 5 | |
| 112 cartridges, each containing 64 mcg per cartridge | |||
| 66302-650-03 | 112 cartridges, each containing 16 mcg per cartridge | 5 | |
| 112 cartridges, each containing 48 mcg per cartridge | |||
| 112 cartridges, each containing 64 mcg per cartridge | |||
| Tyvaso DPI (treprostinil) Inhalation Powder Institutional Kit | 66302-716-04 | 16 cartridges, each containing 16 mcg per cartridge | 2 |
| 66302-732-04 | 16 cartridges, each containing 32 mcg per cartridge | 2 | |
| 66302-748-04 | 16 cartridges, each containing 48 mcg per cartridge | 2 | |
| 66302-764-04 | 16 cartridges, each containing 64 mcg per cartridge | 2 | |
| 66302-720-04 | 16 cartridges, each containing 32 mcg per cartridge | 2 | |
| 16 cartridges, each containing 48 mcg per cartridge | |||
17. Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Instructions for Use).
Train patients in the administration process for Tyvaso DPI, including dosing, Tyvaso DPI Inhaler setup, operation, cleaning, and maintenance, according to the instructions for use [see Dosage and Administration (2.1, 2.2)].
Advise patients that after 7 days of use, the inhaler must be discarded and replaced with a new inhaler [see Dosage and Administration (2.1)].
Instruct patients to use Tyvaso DPI only with the Tyvaso DPI Inhaler [see Dosage and Administration (2.1)].
If a scheduled treatment session is missed, resume therapy as soon as possible [see Dosage and Administration (2.2)].
Instructions
for Use
TYVASO [ tī-vā'-sō ] DPI™
(treprostinil) Inhalation Powder
For oral inhalation only
| Table of Contents | |
| page(s) | |
| Read Before Starting | 1 |
| Important Information | 2 |
| Storing TYVASO DPI Inhalers and Cartridges | 3 |
| Preparing to Inhale TYVASO DPI | 4-7 |
| Inhaling TYVASO DPI | 8-9 |
| Removing the Used Cartridge | 10 |
| Disposing of TYVASO DPI Cartridge | 11 |
| Inhaling Multiple Cartridges of TYVASO DPI | 12 |
| Caring for Your TYVASO DPI Inhaler | 13 |
| Disposing of Your TYVASO DPI Inhaler | 13 |
Read Before Starting
| Parts of the TYVASO DPI Inhaler
(see Figure A) | Your Starter Kit includes a Carrying Case
(see Figure B) | TYVASO DPI Blister Cards
(see Figure C) |
|
| This Instructions for Use contains information on how to inhale TYVASO DPI (treprostinil) Inhalation Powder. Read this Instructions for Use carefully before you start using your inhaler and each time you get a new inhaler. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or your treatment. Your healthcare provider should show you how to use your inhaler the right way before you use it for the first time. | 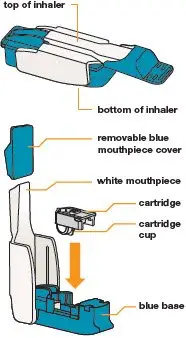 Figure A | The TYVASO DPI inhaler and blister strips can be stored in the carrying case when using outside of your home or traveling. | 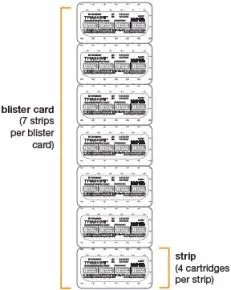 Figure C |
 Figure B |
Important Information
| Important information you need to know before inhaling TYVASO DPI Inhalation Powder using the TYVASO DPI Inhaler |
|
| TYVASO DPI cartridges come in 4 strengths (see Figure D). Important: Always make sure you have the right number of TYVASO DPI cartridges for your dose before you start. Only use TYVASO DPI cartridges with the TYVASO DPI Inhaler. |
|
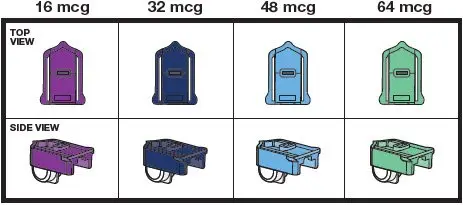 Figure D |
|
| If you are having problems with your TYVASO DPI Inhaler, have any side effects, or if your TYVASO DPI Inhaler breaks and you need a new one, please call 1-877-UNITHER (1-877-864-8437). |
Storing TYVASO DPI Inhalers and Cartridges
| Storing TYVASO DPI Inhalers | Storing Unopened Blister Cards and Strips | Storing Opened Blister Strips | |||
|---|---|---|---|---|---|
| Store TYVASO DPI Inhalers, with the mouthpiece on, in a clean, dry place at room temperature between 68°F to 77°F (20°C to 25°C), such as a drawer or medicine cabinet (see Figure E). Inhalers may be stored in the refrigerator between 36°F to 46°F (2°C to 8°C), but should be left out at room temperature for 10 minutes before use. | Store unopened blister cards and strips in a clean, dry place at room temperature, such as a drawer or medicine cabinet. You can store in the carrying case when using outside your home or traveling (see Figure F).
|  Figure F | Store opened blister strips in a clean, dry place at room temperature (see Figure H), such as a drawer or medicine cabinet. Opened blister strips must be used within 3 days.
|
||
|
|  Figure H Figure H |
|||
 Figure E | Important: If refrigerated, cartridges and inhaler should be left out at room temperature for 10 minutes before use. |  Figure G Figure G |
|||
 ROOM TEMPERATURE |  10 mins. |
||||
Preparing to Inhale TYVASO DPI
| Step 1 : Select the TYVASO DPI cartridges for your dose (see Figure I) | Step 2 : Tear off 1 strip | Step 3 : Check the expiration date on the strip | ||||
Select the TYVASO DPI cartridges for your dose (see Figure I).
| Tear along the perforation to remove 1 strip from the blister card (see Figure J). | 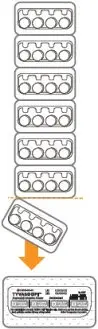 Figure J | Check the expiration date on the foil strip label (see Figure K).
|
|||
If your prescribed TYVASO DPI dose is 16 mcg, use... | If your prescribed TYVASO DPI dose is 32 mcg, use... | If your prescribed TYVASO DPI dose is 48 mcg, use... | If your prescribed TYVASO DPI dose is 64 mcg, use... |  Figure K |
||
| If your prescribed TYVASO DPI dose is more than 64 mcg per treatment session, you will need to use more than 1 cartridge to get the right dose. Example: If your prescribed TYVASO DPI dose is 80 mcg per treatment session, you can use... |
||||||
 Figure I |
||||||
| Step 4 : Remove cartridge(s) from strip | Step 5 : Check supplies before continuing | |||||
|---|---|---|---|---|---|---|
|  | Check that you have the right cartridge(s) for your dose. | ||||
 | Only use one inhaler for multiple cartridges. Your inhaler lasts for 7 days. | |||||
|
Figure L | ||||||
| Important: If refrigerated, cartridges and inhaler should be left out at room temperature for 10 minutes before use. |  ROOM TEMPERATURE |  10 mins. | ||||
| Step 6: Load a cartridge | |||
|---|---|---|---|
| Place Inhaler on Flat Surface
Place the inhaler on a flat surface (see Figure M). | Open Inhaler
Open the inhaler by lifting the mouthpiece to an upright (vertical) position (see Figure N). Important: If the cartridge came from a strip stored in the refrigerator (or if you stored the inhaler in the refrigerator), leave the cartridge and inhaler at room temperature for 10 minutes to remove condensation. | Place Cartridge in Inhaler
|
|
 Figure M |
|||
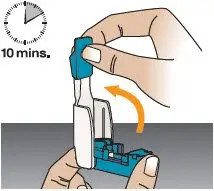 Figure N | 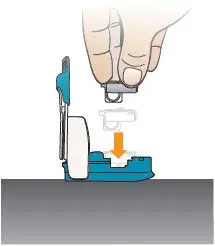 Figure O | 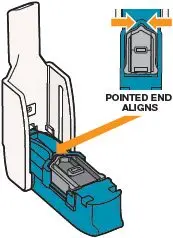 Figure P |
|
| Close Inhaler
Close the inhaler (this will open the cartridge). You should feel a snap when the inhaler is closed (see Figure Q). Important: Now that the cartridge is loaded, keep the inhaler level to avoid loss of the TYVASO DPI powder, until it is in your mouth (see Figure R). | Not keeping the inhaler level could cause a loss of TYVASO DPI powder (see Figure S) | |||
If any powder from the cartridge spills:
|
||||
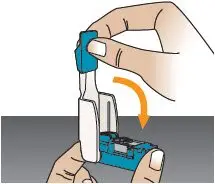 Figure Q | 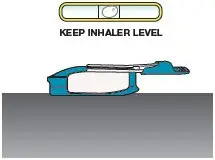 Figure R | 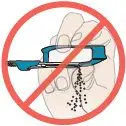 DO NOT turn inhaler upside down. | 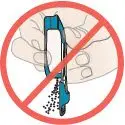 DO NOT point the mouthpiece down. | 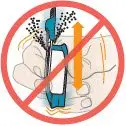 DO NOT shake or drop the inhaler. |
| This can cause a loss of TYVASO DPI powder. | ||||
| Figure S | ||||
Inhaling TYVASO DPI
Before inhaling TYVASO DPI, fully review all parts of Step 7 before you take your dose.
| Step 7: Inhale Your Dose | ||
|---|---|---|
| Remove the Mouthpiece Cover (see Figure T). Important: Keep the inhaler level during and after removal of the blue mouthpiece cover to prevent loss of TYVASO DPI powder. | Hold Inhaler Near Cheek
While keeping the inhaler level, carefully pick up the inhaler and bring it near your cheek, but not in front of your mouth (see Figure U). | Exhale
Holding the inhaler away from your mouth, fully blow out (exhale) (see Figure V). |
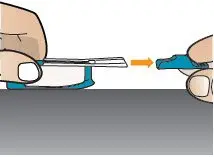 Figure T | 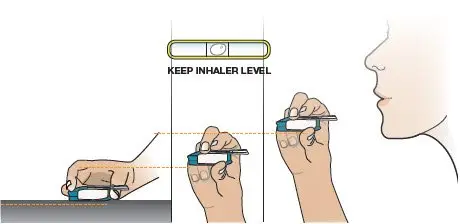 Figure U | 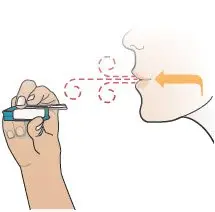 Figure V |
Position Inhaler in Mouth
| Inhale Deeply, Hold Breath, then Exhale
|
||
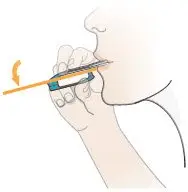 Figure W | 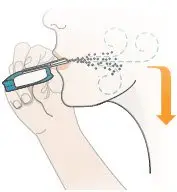 Figure X | 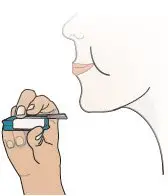 Figure Y | 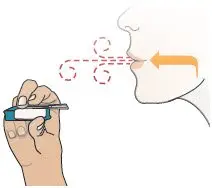 Figure Z |
Removing the Used Cartridge
| Step 8 : Remove the used cartridge | |||
|---|---|---|---|
| Replace Mouthpiece Cover
Place the mouthpiece cover back onto the inhaler (see Figure AA).
| Open Inhaler
Open the inhaler by lifting up the mouthpiece to an upright (vertical) position (see Figure AB). | Remove Cartridge
|
|
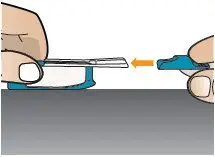 Figure AA | 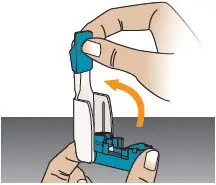 Figure AB | 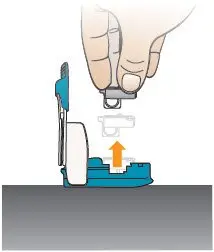 Figure AC | 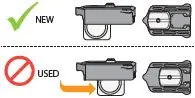 |
| The cup moves to the middle of the cartridge when it has been used. | |||
| Figure AD | |||
Disposing of TYVASO DPI Cartridges
| Step 9 : Throw away used cartridge | |
|---|---|
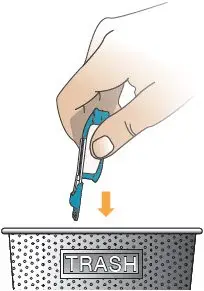
| Throw away the used cartridge in your regular household trash (see Figure AE). | |
 Figure AE | |
Inhaling Multiple Cartridges of TYVASO DPI
| Step 10 : Inhaling multiple cartridges (skip if not needed) | ||||
|---|---|---|---|---|
| If your dose requires you to inhale multiple cartridges, repeat steps 6 through 9 for each cartridge. | Warning: Be careful not to mix NEW cartridges with used cartridges (see Figure AG). | |||
| 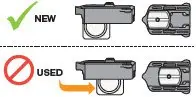 | |||
| The cup moves to the middle of the cartridge when it has been used. | ||||
 Figure AF | Figure AG | |||
| Caring for Your TYVASO DPI Inhaler | Disposing of Your TYVASO DPI Inhaler | ||
| Inhaler Care Instructions | Throw away used inhaler after 7 days of use | ||
| Cleaning
After taking your dose, powder residue in the mouthpiece is normal; this will not affect your dose. | Use Time
Only use 1 inhaler at a time. The same inhaler can be used to take 16 mcg, 32 mcg, 48 mcg, or 64 mcg cartridges. | After 7 days of use, throw away the used inhaler in your regular household trash (see Figure AH and Figure AI). | |
| The outside of the inhaler can be wiped with a clean, dry cloth only, if needed. Never wash the inhaler. Always keep the inhaler dry. | Replace the inhaler after 7 days (see Figure AH and Figure AI). Keep track of 7 days from when you start using the inhaler with a calendar. |
Figure AI | |
|
Figure AH |
|||
For further questions and information, or to report a problem with your device or any side effects with your TYVASO DPI, please call 1-877-UNITHER (1-877-864-8437).
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Issued: May 2022
TYVASO DPI™ is a trademark of United Therapeutics Corporation.
Patents: www.tyvasodpi.com/patent
Distributed by:
United Therapeutics Corporation
Research Triangle Park, NC 27709
USA
Manufactured by:
MannKind Corporation
Danbury, CT 06810
USA
05/2022
30-1311-006-01
| TYVASO DPI
treprostinil inhalant |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| TYVASO DPI
treprostinil inhalant |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| TYVASO DPI
treprostinil inhalant |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| TYVASO DPI
treprostinil inhalant |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| TYVASO DPI
treprostinil inhalant |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| TYVASO DPI
treprostinil inhalant |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| TYVASO DPI
treprostinil inhalant |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| TYVASO DPI
treprostinil inhalant |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| TYVASO DPI
treprostinil kit |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| TYVASO DPI
treprostinil kit |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| TYVASO DPI
treprostinil kit |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| TYVASO DPI
treprostinil kit |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| TYVASO DPI
treprostinil kit |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| TYVASO DPI
treprostinil kit |
|||||||||||||||||
|
|||||||||||||||||
|
|||||||||||||||||
|
|||||||||||||||||
|
|||||||||||||||||
|
|||||||||||||||||
|
|||||||||||||||||
|
|||||||||||||||||
|
|||||||||||||||||
|
|||||||||||||||||
|
|||||||||||||||||
|
|||||||||||||||||
|
|||||||||||||||||
|
|||||||||||||||||
|
|||||||||||||||||
|
|||||||||||||||||
|
|||||||||||||||||
|
|||||||||||||||||
|
|||||||||||||||||
|
|||||||||||||||||
|
|||||||||||||||||
|
|||||||||||||||||
|
|||||||||||||||||
| TYVASO DPI
treprostinil kit |
|||||||||||||||||
|
|||||||||||||||||
|
|||||||||||||||||
|
|||||||||||||||||
|
|||||||||||||||||
|
|||||||||||||||||
|
|||||||||||||||||
|
|||||||||||||||||
|
|||||||||||||||||
|
|||||||||||||||||
|
|||||||||||||||||
|
|||||||||||||||||
|
|||||||||||||||||
|
|||||||||||||||||
|
|||||||||||||||||
|
|||||||||||||||||
|
|||||||||||||||||
|
|||||||||||||||||
|
|||||||||||||||||
|
|||||||||||||||||
|
|||||||||||||||||
|
|||||||||||||||||
|
|||||||||||||||||
| Labeler - United Therapeutics Corporation (965460025) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| United Therapeutics Corporation | 965460025 | API MANUFACTURE(66302-616, 66302-632, 66302-648, 66302-664, 66302-600, 66302-610, 66302-620, 66302-630, 66302-640, 66302-650, 66302-716, 66302-732, 66302-748, 66302-764, 66302-720) , ANALYSIS(66302-616, 66302-632, 66302-648, 66302-664, 66302-600, 66302-610, 66302-620, 66302-630, 66302-640, 66302-650, 66302-716, 66302-732, 66302-748, 66302-764, 66302-720) | |