Drug Detail:Varivax (Varicella virus (chickenpox) vaccine [ var-i-sel-a-vye-rus-vak-seen ])
Drug Class: Viral vaccines
Highlights of Prescribing Information
VARIVAX®
Varicella Virus Vaccine Live
Suspension for intramuscular or subcutaneous injection
Initial U.S. Approval: 1995
Recent Major Changes
| Dosage and Administration | |
| Dose and Schedule (2.1) | 02/2023 |
| Administration (2.3) | 02/2023 |
Indications and Usage for Varivax
VARIVAX is a vaccine indicated for active immunization for the prevention of varicella in individuals 12 months of age and older. (1)
Varivax Dosage and Administration
For intramuscular or subcutaneous injection only. (2.1, 2.3)
A single dose is approximately 0.5 mL.
Children (12 months to 12 years of age)
- The first dose is administered at 12 to 15 months of age. (2.1)
- The second dose is administered at 4 to 6 years of age. (2.1)
- There should be a minimum interval of 3 months between doses. (2.1)
Adolescents (≥13 years of age) and Adults
- Two doses are administered at a minimum interval of 4 weeks. (2.1)
Dosage Forms and Strengths
Suspension for injection (approximately 0.5 mL dose) supplied as a lyophilized vaccine to be reconstituted using the accompanying sterile diluent. (2.2, 3, 16)
Contraindications
- History of severe allergic reaction to any component of the vaccine (including neomycin and gelatin) or to a previous dose of varicella vaccine. (4.1)
- Immunosuppression. (4.2)
- Moderate or severe febrile illness. (4.3)
- Active untreated tuberculosis. (4.4)
- Pregnancy. (4.5, 8.1, 17)
Warnings and Precautions
- Evaluate individuals for immune competence prior to administration of VARIVAX if there is a family history of congenital or hereditary immunodeficiency. (5.1)
- Avoid close contact with high-risk individuals susceptible to varicella because of possible transmission of varicella vaccine virus. (5.3)
- Immune Globulins (IG) and other blood products should not be given concomitantly with VARIVAX. (5.4, 7.2)
- Avoid use of salicylates for 6 weeks following administration of VARIVAX to children and adolescents. (5.5, 7.1)
Adverse Reactions/Side Effects
- Frequently reported (≥10%) adverse reactions in children ages 1 to 12 years include:
- fever ≥102.0°F (38.9°C) oral: 14.7%
- injection-site complaints: 19.3% (6.1)
- Frequently reported (≥10%) adverse reactions in adolescents and adults ages 13 years and older include:
- fever ≥100.0°F (37.8°C) oral: 10.2%
- injection-site complaints: 24.4% (6.1)
- Other reported adverse reactions in all age groups include:
- varicella-like rash (injection site)
- varicella-like rash (generalized) (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Merck Sharp & Dohme LLC at 1-877-888-4231 or VAERS at 1-800-822-7967 or www.vaers.hhs.gov .
Drug Interactions
- Reye syndrome has been reported in children and adolescents following the use of salicylates during wild-type varicella infection. (5.5, 7.1)
- Administration of immune globulins and other blood products concurrently with VARIVAX vaccine may interfere with the expected immune response. (5.4, 7.2)
- VARIVAX vaccination may result in a temporary depression of purified protein derivative (PPD) tuberculin skin sensitivity. (7.3)
Use In Specific Populations
Pregnancy: Do not administer VARIVAX to females who are pregnant. Pregnancy should be avoided for 3 months following vaccination with VARIVAX. (4.5, 8.1, 17)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 3/2023
Full Prescribing Information
1. Indications and Usage for Varivax
VARIVAX is a vaccine indicated for active immunization for the prevention of varicella in individuals 12 months of age and older.
2. Varivax Dosage and Administration
Intramuscular or subcutaneous administration only
2.2 Reconstitution Instructions
Use a sterile syringe free of preservatives, antiseptics, and detergents for each reconstitution and injection of VARIVAX because these substances may inactivate the vaccine virus. When reconstituting the vaccine, use only the sterile diluent supplied with VARIVAX. The sterile diluent does not contain preservatives or other anti-viral substances which might inactivate the vaccine virus.
To reconstitute the vaccine, withdraw the total volume of supplied sterile diluent and inject into the lyophilized vaccine vial. Agitate to dissolve completely. Discard if the lyophilized vaccine cannot be dissolved.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Do not use the product if particulates are present or if it appears discolored. Visually inspect the vaccine before and after reconstitution prior to administration. Before reconstitution, the lyophilized vaccine is a white compact crystalline plug. VARIVAX, when reconstituted, is a clear, colorless to pale yellow liquid.
Withdraw the entire amount of reconstituted vaccine, inject the total volume and discard vial.
To minimize loss of potency, administer VARIVAX immediately after reconstitution. Discard if reconstituted vaccine is not used within 30 minutes.
Do not freeze reconstituted vaccine.
Do not combine VARIVAX with any other vaccine through reconstitution or mixing.
3. Dosage Forms and Strengths
VARIVAX is a suspension for injection supplied as a single-dose vial of lyophilized vaccine to be reconstituted using the accompanying sterile diluent [see Dosage and Administration (2.2) and How Supplied/Storage and Handling (16)]. A single dose after reconstitution is approximately 0.5 mL.
4. Contraindications
4.1 Severe Allergic Reaction
Do not administer VARIVAX to individuals with a history of anaphylactic or severe allergic reaction to any component of the vaccine (including neomycin and gelatin) or to a previous dose of a varicella-containing vaccine.
4.2 Immunosuppression
Do not administer VARIVAX to individuals who are immunodeficient or immunosuppressed due to disease or medical therapy.
Disseminated varicella disease and extensive vaccine-associated rash have been reported in individuals who are immunosuppressed or immunodeficient who were inadvertently vaccinated with a varicella-containing vaccine.
4.3 Moderate or Severe Febrile Illness
Do not administer VARIVAX to individuals with an active febrile illness with fever >101.3°F (>38.5°C).
5. Warnings and Precautions
5.1 Family History of Immunodeficiency
Vaccination should be deferred in individuals with a family history of congenital or hereditary immunodeficiency until the individual's immune status has been evaluated and the individual has been found to be immunocompetent.
5.2 Use in HIV-Infected Individuals
The Advisory Committee on Immunization Practices (ACIP) has recommendations on the use of varicella vaccine in HIV-infected individuals.
5.3 Risk of Vaccine Virus Transmission
Post-marketing experience suggests that transmission of varicella vaccine virus (Oka/Merck) resulting in varicella infection including disseminated disease may occur between vaccine recipients (who develop or do not develop a varicella-like rash) and contacts susceptible to varicella including healthy as well as high-risk individuals.
Due to the concern for transmission of vaccine virus, vaccine recipients should attempt to avoid whenever possible close association with susceptible high-risk individuals for up to six weeks following vaccination with VARIVAX. Susceptible high-risk individuals include:
- Immunocompromised individuals;
- Pregnant women without documented history of varicella or laboratory evidence of prior infection;
- Newborn infants of mothers without documented history of varicella or laboratory evidence of prior infection and all newborn infants born at <28 weeks gestation regardless of maternal varicella immunity.
5.4 Immune Globulins and Transfusions
Immune Globulins (IG) and other blood products should not be given concomitantly with VARIVAX [see Drug Interactions (7.2)]. These products may contain antibodies that interfere with vaccine virus replication and decrease the expected immune response.
The ACIP has specific recommendations for intervals between administration of antibody-containing products and live virus vaccines.
5.5 Salicylate Therapy
Avoid use of salicylates (aspirin) or salicylate-containing products in children and adolescents 12 months through 17 years of age for six weeks following vaccination with VARIVAX because of the association of Reye syndrome with salicylate therapy and wild-type varicella infection [see Drug Interactions (7.1)].
6. Adverse Reactions/Side Effects
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a vaccine cannot be directly compared to rates in the clinical trials of another vaccine and may not reflect the rates observed in clinical practice. Vaccine-related adverse reactions reported during clinical trials were assessed by the study investigators to be possibly, probably, or definitely vaccine-related and are summarized below.
In clinical trials {1-8}, VARIVAX was administered subcutaneously to over 11,000 healthy children, adolescents, and adults.
In a double-blind, placebo-controlled study among 914 healthy children and adolescents who were serologically confirmed to be susceptible to varicella, the only adverse reactions that occurred at a significantly (p<0.05) greater rate in vaccine recipients than in placebo recipients were pain and redness at the injection site {1}.
Children 1 to 12 Years of Age
One-Dose Regimen in Children
In clinical trials involving healthy children monitored for up to 42 days after a single dose of VARIVAX, the frequency of fever, injection-site complaints, or rashes were reported as shown in Table 1:
| Reaction | N | % Experiencing Reaction | Peak Occurrence During Postvaccination Days | |
|---|---|---|---|---|
| Fever ≥102.0°F (38.9°C) Oral | 8827 | 14.7% | 0 to 42 | |
| Injection-site complaints | 8916 | 19.3% | 0 to 2 | |
| (pain/soreness, swelling and/or erythema, rash, pruritus, hematoma, induration, stiffness) | ||||
| Varicella-like rash (injection site) | 8916 | 3.4% | 8 to 19 | |
| Median number of lesions | 2 | |||
| Varicella-like rash (generalized) | 8916 | 3.8% | 5 to 26 | |
| Median number of lesions | 5 | |||
In addition, adverse events occurring at a rate of ≥1% are listed in decreasing order of frequency: upper respiratory illness, cough, irritability, fatigue, disturbed sleep, diarrhea, loss of appetite, vomiting, otitis, headache, malaise, abdominal pain, other rash, nausea, chills, lymphadenopathy, myalgia, lower respiratory illness, allergic reactions (including allergic rash, hives), stiff neck, arthralgia, itching.
Pneumonitis has been reported rarely (<1%) in children vaccinated with VARIVAX.
Febrile seizures have occurred at a rate of <0.1% in children vaccinated with VARIVAX.
Adolescents (13 Years of Age and Older) and Adults
In clinical trials involving healthy adolescents and adults, the majority of whom received two doses of VARIVAX and were monitored for up to 42 days after any dose, the frequencies of fever, injection-site complaints, or rashes are shown in Table 2.
| Reaction | N | % Post Dose 1 | Peak Occurrence in Postvaccination Days | N | % Post Dose 2 | Peak Occurrence in Postvaccination Days |
|
|---|---|---|---|---|---|---|---|
| Fever ≥100.0°F (37.8°C) Oral | 1584 | 10.2% | 14 to 27 | 956 | 9.5% | 0 to 42 | |
| Injection-site complaints | 1606 | 24.4% | 0 to 2 | 955 | 32.5% | 0 to 2 | |
| (soreness, erythema, swelling, rash, pruritus, pyrexia, hematoma, induration, numbness) | |||||||
| Varicella-like rash (injection site) | 1606 | 3% | 6 to 20 | 955 | 1% | 0 to 6 | |
| Median number of lesions | 2 | 2 | |||||
| Varicella-like rash (generalized) | 1606 | 5.5% | 7 to 21 | 955 | 0.9% | 0 to 23 | |
| Median number of lesions | 5 | 5.5 | |||||
In addition, adverse events reported at a rate of ≥1% are listed in decreasing order of frequency: upper respiratory illness, headache, fatigue, cough, myalgia, disturbed sleep, nausea, malaise, diarrhea, stiff neck, irritability, lymphadenopathy, chills, abdominal pain, loss of appetite, arthralgia, otitis, itching, vomiting, other rashes, lower respiratory illness, allergic reactions (including allergic rash, hives).
In a randomized open-label clinical trial (NCT00432523), conducted in France and Germany, 752 children 12 months through 18 months of age received M-M-R II concomitantly administered with VARIVAX at a separate site, by either the intramuscular (n=374) or subcutaneous (n=378) route. In the overall population, 55.3% were male and the median age was 13.2 months. Local and systemic solicited adverse reactions were recorded by parents or guardians using standardized diary cards. Local solicited reactions were recorded for 4 days after vaccination, and systemic solicited adverse reactions were recorded for 42 days after vaccination. In the event that a participant experienced a rash or a mumps-like illness, parents and/or guardians were instructed to contact the investigator for an examination as soon as possible and no later than 72 hours following onset of symptoms. The nature of any rash was characterized by principal investigator either as a measles-like, rubella-like, varicella-like or “other”. Study investigators reviewed the diary card with the participant or participant’s legal guardian 42 days vaccination to ensure consistency with protocol definitions. Table 3 below presents the frequency of solicited adverse reactions based on the final assessment by the study investigators.
| Intramuscular N=374 % | Subcutaneous N=376 % |
|
|---|---|---|
| N=total number of participants in the group | ||
|
||
| Solicited local reactions at Varivax injection site (Days 0 to 4)* | ||
| Erythema† | 8.8 | 16.8 |
| Mild | 8.0 | 12.8 |
| Moderate | 0.5 | 3.7 |
| Severe | 0 | 0 |
| Missing | 0.3 | 0.3 |
| Pain‡ | 7.0 | 8.5 |
| Mild | 4.8 | 7.2 |
| Moderate | 2.1 | 1.3 |
| Severe | 0 | 0 |
| Swelling† | 3.2 | 4.8 |
| Mild | 1.6 | 3.5 |
| Moderate | 1.1 | 0.5 |
| Severe | 0 | 0 |
| Missing | 0.5 | 0.8 |
| Solicited systemic adverse reactions (Days 0 to 42) | ||
| Measles-like rash (Days 0 to 42)§ | 2.9 | 2.7 |
| Rubella-like rash (Days 0 to 42)§ | 2.7 | 2.7 |
| Varicella-like rash (Days 0 to 42)§ | 0.5 | 3.2 |
| Mumps-like illness (Days 0 to 42) | 0 | 0.3 |
| Fever (temperature ≥38.0°C) (Days 0 to 42)¶,# | 66.5 | 66.8 |
| 38.0-38.5°C | 20.4 | 22.2 |
| >38.5-39.0°C | 17.4 | 16.6 |
| >39.0-39.5°C | 14.2 | 13.4 |
| >39.5-40.0°C | 11.8 | 11.0 |
| >40.0°C | 2.7 | 3.7 |
Unsolicited adverse events that occurred within 42 days following vaccination were recorded using diary cards supplemented by medical review. Data on unsolicited adverse events were transcribed into the study database during an on-site visit at day 42. The rates and types of reported adverse events (AEs) across groups were similar and included common clinical events that are often reported in the evaluated populations. Serious adverse events occurred at rates of 0.3% and 1% in the intramuscular and subcutaneous groups, respectively. One moderate intensity case of otitis media occurred in a participant in the subcutaneous group was considered related to the vaccination.
Herpes Zoster
Overall, 9454 healthy children (12 months to 12 years of age) and 1648 adolescents and adults (13 years of age and older) have been vaccinated with VARIVAX in clinical trials. Eight cases of herpes zoster have been reported in children during 42,556 person-years of follow-up in clinical trials, resulting in a calculated incidence of at least 18.8 cases per 100,000 person-years. The completeness of this reporting has not been determined. One case of herpes zoster has been reported in the adolescent and adult age group during 5410 person-years of follow-up in clinical trials, resulting in a calculated incidence of 18.5 cases per 100,000 person-years. All 9 cases were mild and without sequelae. Two cultures (one child and one adult) obtained from vesicles were positive for wild-type VZV as confirmed by restriction endonuclease analysis {11}. The long-term effect of VARIVAX on the incidence of herpes zoster, particularly in those vaccinees exposed to wild-type varicella, is unknown at present.
In children, the reported rate of herpes zoster in vaccine recipients appears not to exceed that previously determined in a population-based study of healthy children who had experienced wild-type varicella {12}. The incidence of herpes zoster in adults who have had wild-type varicella infection is higher than that in children.
6.2 Post-Marketing Experience
The following adverse events have been identified during post approval use of VARIVAX. Because the events are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to vaccine exposure.
Body as a Whole
Anaphylaxis (including anaphylactic shock) and related phenomena such as angioneurotic edema, facial edema, and peripheral edema.
Eye Disorders
Necrotizing retinitis (in immunocompromised individuals).
Hemic and Lymphatic System
Aplastic anemia; thrombocytopenia (including idiopathic thrombocytopenic purpura (ITP)).
Infections and Infestations
Varicella (vaccine strain).
Nervous/Psychiatric
Encephalitis; cerebrovascular accident; transverse myelitis; Guillain-Barré syndrome; Bell's palsy; ataxia; non-febrile seizures; aseptic meningitis; meningitis; dizziness; paresthesia; syncope.
Cases of encephalitis or meningitis caused by vaccine strain varicella virus have been reported in immunocompromised and immunocompetent individuals previously vaccinated with VARIVAX months to years after vaccination. Reported cases were commonly associated with preceding or concurrent herpes zoster rash.
Respiratory
Pharyngitis; pneumonia/pneumonitis.
Skin
Stevens-Johnson syndrome; erythema multiforme; Henoch-Schönlein purpura; secondary bacterial infections of skin and soft tissue, including impetigo and cellulitis; wild-type or vaccine strain herpes zoster.
Herpes Zoster
The vaccine virus (Oka/Merck strain) contained in VARIVAX may establish latency of varicella zoster virus in immunocompetent individuals, with the potential for later development of herpes zoster.
7. Drug Interactions
7.1 Salicylates
No cases of Reye syndrome have been observed following vaccination with VARIVAX. Vaccine recipients should avoid use of salicylates for 6 weeks after vaccination with VARIVAX, as Reye syndrome has been reported following the use of salicylates during wild-type varicella infection [see Warnings and Precautions (5.5)].
7.2 Immune Globulins and Transfusions
Administration of immune globulins and other blood products concurrently with VARIVAX may interfere with the expected immune response [see Warnings and Precautions (5.4)] {9}. The ACIP has specific recommendations for intervals between administration of antibody-containing products and live virus vaccines.
7.3 Tuberculin Skin Testing
Tuberculin skin testing, with tuberculin purified protein derivative (PPD), may be performed before VARIVAX is administered or on the same day, or at least 4 weeks following vaccination with VARIVAX, as other live virus vaccines may cause a temporary depression of tuberculin skin test sensitivity leading to false negative results.
7.4 Use with Other Vaccines
VARIVAX can be administered concurrently with other live viral vaccines. If not given concurrently, at least 1 month should elapse between a dose of a live attenuated measles virus-containing vaccine and a dose of VARIVAX. In children through the age of 12 years at least 3 months should elapse between administration of 2 doses of a live attenuated varicella virus-containing vaccine. For adolescents and adults, 2 doses of VARIVAX may be separated by 1 month [see Dosage and Administration (2.1)].
VARIVAX may be administered concomitantly with M-M-R II (Measles, Mumps, and Rubella Virus Vaccine Live), Haemophilus influenzae type b conjugate (meningococcal protein conjugate) and hepatitis B (recombinant). Additionally, VARIVAX may be administered concomitantly with inactivated diphtheria-tetanus and acellular pertussis vaccines [see Clinical Studies (14.4)].
8. Use In Specific Populations
11. Varivax Description
VARIVAX [Varicella Virus Vaccine Live] is a preparation of the Oka/Merck strain of live, attenuated varicella virus. The virus was initially obtained from a child with wild-type varicella, then introduced into human embryonic lung cell cultures, adapted to and propagated in embryonic guinea pig cell cultures and finally propagated in human diploid cell cultures (WI-38). Further passage of the virus for varicella vaccine was performed at Research Laboratories of Merck Sharp & Dohme LLC, Rahway, NJ, USA in human diploid cell cultures (MRC-5) that were free of adventitious agents. This live, attenuated varicella vaccine is a lyophilized preparation containing sucrose, phosphate, glutamate, and processed gelatin as stabilizers.
VARIVAX, when reconstituted as directed, is a sterile preparation for intramuscular or subcutaneous injection. Each approximately 0.5 mL dose contains a minimum of 1350 plaque-forming units (PFU) of Oka/Merck varicella virus when reconstituted and stored at room temperature for a maximum of 30 minutes. Each approximately 0.5 mL dose also contains approximately 24 mg of sucrose, 12.0 mg hydrolyzed gelatin, 3.1 mg of sodium chloride, 0.5 mg of monosodium L-glutamate, 0.44 mg of sodium phosphate dibasic, 0.08 mg of potassium phosphate monobasic, and 0.08 mg of potassium chloride. The product also contains residual components of MRC-5 cells including DNA and protein and trace quantities of sodium phosphate monobasic, EDTA, neomycin and fetal bovine serum. The product contains no preservative.
12. Varivax - Clinical Pharmacology
12.1 Mechanism of Action
VARIVAX induces both cell-mediated and humoral immune responses to varicella-zoster virus. The relative contributions of humoral immunity and cell-mediated immunity to protection from varicella are unknown.
12.4 Duration of Protection
The duration of protection of VARIVAX is unknown; however, long-term efficacy studies have demonstrated continued protection up to 10 years after vaccination [see Clinical Studies (14.1)] {13}. A boost in antibody levels has been observed in vaccinees following exposure to wild-type varicella which could account for the apparent long-term protection after vaccination in these studies.
14. Clinical Studies
14.1 Clinical Efficacy
The protective efficacy of VARIVAX administered subcutaneously was established by: (1) a placebo-controlled, double-blind clinical trial, (2) comparing varicella rates in vaccinees versus historical controls, and (3) assessing protection from disease following household exposure.
14.2 Immunogenicity
In clinical trials, varicella antibodies have been evaluated following vaccination with formulations of VARIVAX containing attenuated virus ranging from 1000 to 50,000 PFU per dose in healthy individuals ranging from 12 months to 55 years of age {1,8}.
15. References
- Weibel, R.E.; et al.: Live Attenuated Varicella Virus Vaccine. Efficacy Trial in Healthy Children. N Engl J Med. 310(22): 1409-1415, 1984.
- Arbeter, A.M.; et al.: Varicella Vaccine Trials in Healthy Children. A Summary of Comparative and Follow-up Studies. Am J Dis Child. 138: 434-438, 1984.
- Weibel, R.E.; et al.: Live Oka/Merck Varicella Vaccine in Healthy Children. Further Clinical and Laboratory Assessment. JAMA. 254(17): 2435-2439, 1985.
- Chartrand, D.M.; et al.: New Varicella Vaccine Production Lots in Healthy Children and Adolescents. Abstracts of the 1988 Inter-Science Conference Antimicrobial Agents and Chemotherapy: 237(Abstract #731).
- Johnson, C.E.; et al.: Live Attenuated Varicella Vaccine in Healthy 12- to 24-Month-Old Children. Pediatrics. 81(4): 512-518, 1988.
- Gershon, A.A.; et al.: Immunization of Healthy Adults with Live Attenuated Varicella Vaccine. J Infect Dis. 158(1): 132-137, 1988.
- Gershon, A.A.; et al.: Live Attenuated Varicella Vaccine: Protection in Healthy Adults Compared with Leukemic Children. J Infect Dis. 161: 661-666, 1990.
- White, C.J.; et al.: Varicella Vaccine (VARIVAX) in Healthy Children and Adolescents: Results From Clinical Trials, 1987 to 1989. Pediatrics. 87(5): 604-610, 1991.
- Peter, G.; et al (eds): Report of the Committee on Infectious Diseases, Twenty-fourth Edition, American Academy of Pediatrics, 344-357, 1997.
- Galea, S.; et al.: The Safety Profile of Varicella Vaccine: A 10-Year Review. J Infect Dis. 197(S2): 165-169, 2008.
- Hammerschlag, M.R.; et al.: Herpes Zoster in an Adult Recipient of Live Attenuated Varicella Vaccine. J Infect Dis. 160(3): 535-537, 1989.
- Guess, H.A.; et al.: Population-Based Studies of Varicella Complications. Pediatrics. 78(suppl): 723-727, 1986.
- Kuter, B.J.; et al.: Ten Year Follow-up of Healthy Children who Received One or Two Injections of Varicella Vaccine. Pediatr Infect Dis J. 23: 132-37, 2004.
- Kuter, B.J.; et al.: Oka/Merck Varicella Vaccine in Healthy Children: Final Report of a 2-Year Efficacy Study and 7-Year Follow-up Studies. Vaccine. 9: 643-647, 1991.
- Bernstein, H.H.; et al.: Clinical Survey of Natural Varicella Compared with Breakthrough Varicella After Immunization with Live Attenuated Oka/Merck Varicella Vaccine. Pediatrics. 92(6): 833-837, 1993.
- Wharton, M.: The Epidemiology of Varicella-zoster Virus Infections. Infect Dis Clin North Am. 10(3):571-581, 1996.
- White, C.J. et al.: Measles, Mumps, Rubella, and Varicella Combination Vaccine: Safety and Immunogenicity Alone and in Combination with Other Vaccines Given to Children. Clin Infect Dis. 24(5): 925-931, 1997.
- Reuman, P.D.; et al.: Safety and Immunogenicity of Concurrent Administration of Measles-Mumps-Rubella-Varicella Vaccine and PedvaxHIB® Vaccines in Healthy Children Twelve to Eighteen Months Old. Pediatr Infect Dis J. 16(7): 662-667, 1997.
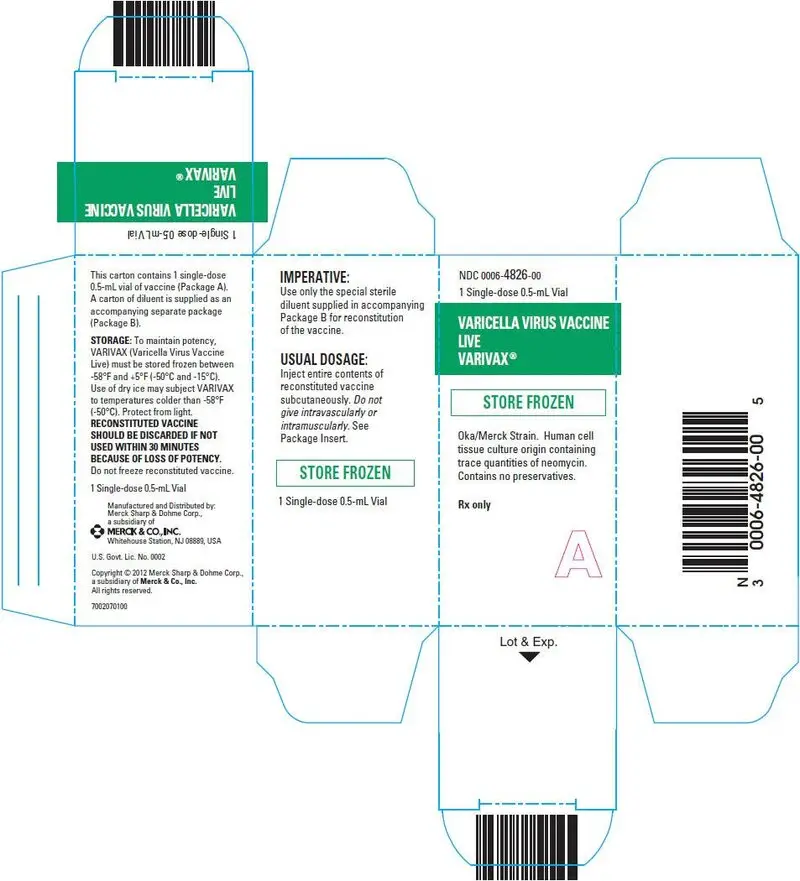
16. How is Varivax supplied
No. 4827/4309 —VARIVAX is supplied as follows:
(1) a box of 10 single-dose vials of lyophilized vaccine (package A), NDC 0006-4827-00
(2) a box of 10 vials of diluent (package B).
17. Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Discuss the following with the patient:
- Question the patient, parent, or guardian about reactions to previous vaccines.
- Provide a copy of the patient information (PPI) located at the end of this insert and discuss any questions or concerns.
- Inform patient, parent, or guardian that vaccination with VARIVAX may not result in protection of all healthy, susceptible children, adolescents, and adults.
- Inform female patients to avoid pregnancy for three months following vaccination.
- Inform patient, parent, or guardian of the benefits and risks of VARIVAX.
- Instruct patient, parent, or guardian to report any adverse reactions or any symptoms of concern to their healthcare professional.
The U.S. Department of Health and Human Services has established a Vaccine Adverse Event Reporting System (VAERS) to accept all reports of suspected adverse events after the administration of any vaccine. For information or a copy of the vaccine reporting form, call the VAERS toll-free number at 1-800-822-7967, or report online at www.vaers.hhs.gov .
Patient Information
VARIVAX® (pronounced "VAR ih vax")
Varicella Virus Vaccine Live
This is a summary of information about VARIVAX®. You should read it before you or your child get the vaccine. If you have any questions about the vaccine after reading this leaflet, you should ask your healthcare professional. This is a summary only. It does not take the place of talking about VARIVAX with your doctor, nurse, or other healthcare professional. Only your healthcare professional can decide if VARIVAX is right for you or your child.
What is VARIVAX and how does it work?
VARIVAX is also known as Varicella Virus Vaccine Live. It is a live virus vaccine that is given as a shot. It is meant to help prevent chickenpox. Chickenpox is sometimes called varicella (pronounced VAR ih sell a).
VARIVAX contains a weakened form of chickenpox virus.
VARIVAX works by helping the immune system protect you or your child from getting chickenpox.
VARIVAX may not protect everyone who gets it.
VARIVAX does not treat chickenpox once you or your child have it.
What do I need to know about chickenpox?
Chickenpox is an illness that occurs most often in children who are 5 to 9 years old. It can be passed to others. The illness can include headache, fever, and general discomfort. Then an itchy rash occurs, which can turn into blisters. The most common complication is that the blisters can get infected. Less common but very serious complications can occur. These include pneumonia, inflammation of the brain, Reye syndrome (which affects the liver and the brain), and death. Severe disease and serious complications are more likely to occur in adolescents and adults.
Who should not get VARIVAX?
Do not get VARIVAX if you or your child:
- are allergic to any of its ingredients. (This includes gelatin or neomycin. See the ingredient list at the end of this leaflet.)
- have a weakened immune system (which includes taking high doses of steroids by mouth or in a shot).
- have a fever.
- have active tuberculosis that is not treated.
- are pregnant or plan to get pregnant within the next three months.
What should I tell my healthcare professional before getting VARIVAX?
Tell your healthcare professional if you or your child:
- have or have had any medical problems.
- have received blood or plasma transfusions or human serum globulin.
- take any medicines. (This includes non-prescription medicines and dietary supplements.)
- have any allergies. (This includes allergies to neomycin or gelatin.)
- had an allergic reaction to any other vaccine.
How is VARIVAX given?
VARIVAX is given as a shot to people who are 12 months old or older. If your child is 12 months to 12 years old and your doctor gives a second dose, the second dose must be given at least 3 months after the first shot.
A second dose should be given to those who first get the vaccine when they are 13 years old or older. This second dose should be given 4 to 8 weeks after the first dose.
Your doctor or healthcare professional will use the official recommendations to decide the number of shots needed and when to get them.
If a dose is missed, your healthcare professional will let you know when you should have it.
What should you or your child avoid when getting VARIVAX?
Do not take aspirin or aspirin-containing products for 6 weeks after getting VARIVAX.
In rare circumstances, it is possible to catch chickenpox, including severe chickenpox, from a person who has been vaccinated with VARIVAX. This may occur in persons who have not previously been vaccinated or had chickenpox, as well as persons who fall into one of the following categories:
- people who have a weakened immune system.
- pregnant women who have never had chickenpox.
- newborn babies whose mothers have never had chickenpox.
- newborn babies born at less than 28 weeks of pregnancy.
Whenever possible, individuals who have been vaccinated with VARIVAX should attempt to avoid close contact for up to six weeks following the vaccination, with anyone who falls into one of the categories above. Tell your doctor or healthcare professional if you or your child expect to have close contact with someone who falls into one of these groups.
What are the possible side effects of VARIVAX?
The most common side effects reported after taking VARIVAX are:
- Fever
- Pain, swelling, itching, or redness at the site of the shot
- Chickenpox-like rash on the body or at the site of the shot
- Irritability
Other less common side effects have also been reported.
- Tingling of the skin
- Shingles (herpes zoster)
Tell your healthcare professional if you have any of the following problems within a short time after getting VARIVAX because they may be signs of an allergic reaction:
- Shortness of breath or wheezing
- Rash or hives
Other side effects have been reported. Some of them were serious. These include bruising more easily than normal; red or purple, flat, pinhead spots under the skin; severe paleness; difficulty walking; severe skin disorders; skin infection; and chickenpox. Rarely, swelling of the brain (encephalitis), stroke, inflammation of the coverings of the brain and spinal cord (meningitis), inflammation of the lungs (known as pneumonia or pneumonitis), and seizures with or without a fever have been reported. It is not known if these rare side effects are related to the vaccine.
Your doctor has a more complete list of side effects for VARIVAX.
Tell your doctor or healthcare professional if you or your child have any new or unusual symptoms after getting VARIVAX.
Report the following to your doctor or your child's doctor:
- any adverse reactions following vaccination
- exposure to VARIVAX during pregnancy
- exposure to VARIVAX during the 3 months before getting pregnant.
You may also report these events to Merck Sharp & Dohme LLC at 1-877-888-4231, or directly to the Vaccine Adverse Event Reporting System (VAERS). The VAERS toll-free number is 1-800-822-7967 or report online to www.vaers.hhs.gov .
What are the ingredients of VARIVAX?
Active Ingredient: a weakened form of chickenpox virus.
Inactive Ingredients: sucrose, hydrolyzed gelatin, sodium chloride, monosodium L-glutamate, sodium phosphate dibasic, potassium phosphate monobasic, potassium chloride, residual components of MRC-5 cells including DNA and protein, sodium phosphate monobasic, EDTA, neomycin, fetal bovine serum.
What else should I know about VARIVAX?
This leaflet summarizes important information about VARIVAX.
If you would like more information, talk to your healthcare professional, or call 1-800-637-2590.
| VARIVAX
varicella virus vaccine live injection, powder, lyophilized, for suspension |
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| VARIVAX
varicella virus vaccine live injection, powder, lyophilized, for suspension |
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| Labeler - Merck Sharp & Dohme LLC (118446553) |