Drug Class: Vaccine combinations
Highlights of Prescribing Information
VAXELIS® (Diphtheria and Tetanus Toxoids and Acellular Pertussis, Inactivated Poliovirus, Haemophilus b Conjugate and Hepatitis B Vaccine)
Suspension for Intramuscular Injection
Initial U.S. Approval: 2018
Indications and Usage for Vaxelis
VAXELIS is a vaccine indicated for active immunization to prevent diphtheria, tetanus, pertussis, poliomyelitis, hepatitis B, and invasive disease due to Haemophilus influenzae type b. VAXELIS is approved for use as a 3-dose series in children from 6 weeks through 4 years of age (prior to the 5th birthday). (1)
Vaxelis Dosage and Administration
The 3-dose immunization series consists of a 0.5 mL intramuscular injection, administered at 2, 4, and 6 months of age. (2.1)
Dosage Forms and Strengths
Suspension for injection (0.5 mL dose) available in single-dose vials and prefilled syringes. (3)
Contraindications
- Severe allergic reaction (e.g., anaphylaxis) to a previous dose of VAXELIS, any ingredient of VAXELIS, or any other diphtheria toxoid, tetanus toxoid, pertussis-containing vaccine, inactivated poliovirus vaccine, hepatitis B vaccine, or Haemophilus influenzae type b vaccine. (4.1)
- Encephalopathy within 7 days of a previous pertussis-containing vaccine with no other identifiable cause. (4.2)
- Progressive neurologic disorder until a treatment regimen has been established and the condition has stabilized. (4.3)
Warnings and Precautions
- Carefully consider benefits and risks before administering VAXELIS to persons with a history of:
- -
- fever ≥40.5°C (≥105°F), hypotonic-hyporesponsive episode (HHE) or persistent, inconsolable crying lasting ≥3 hours within 48 hours after a previous pertussis-containing vaccine. (5.2)
- -
- seizures within 3 days after a previous pertussis-containing vaccine. (5.2)
- If Guillain-Barré syndrome occurred within 6 weeks of receipt of a prior vaccine containing tetanus toxoid, the risk for Guillain-Barré syndrome may be increased following VAXELIS. (5.3)
- Apnea following intramuscular vaccination has been observed in some infants born prematurely. The decision about when to administer an intramuscular vaccine, including VAXELIS, to an infant born prematurely should be based on consideration of the individual infant's medical status and the potential benefits and possible risks of vaccination. (5.5)
- Urine antigen detection may not have definitive diagnostic value in suspected H. influenzae type b disease following vaccination with VAXELIS. (5.7) (7.1)
Adverse Reactions/Side Effects
The solicited adverse reactions following any dose were irritability (≥55%), crying (≥45%), injection site pain (≥44%), somnolence (≥40%), injection site erythema (≥25%), decreased appetite (≥23%), fever ≥38.0°C (≥19%), injection site swelling (≥18%), and vomiting (≥9%). (6)
To report SUSPECTED ADVERSE REACTIONS, contact Sanofi Pasteur Inc., at 1-800-822-2463 (1-800-VACCINE) or VAERS at 1-800-822-7967 and http://vaers.hhs.gov.
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 10/2022
Related/similar drugs
azithromycin, Zithromax, clarithromycin, rifampin, Biaxin, Daptacel (DTaP), hepatitis b adult vaccineFull Prescribing Information
1. Indications and Usage for Vaxelis
VAXELIS® is a vaccine indicated for active immunization to prevent diphtheria, tetanus, pertussis, poliomyelitis, hepatitis B, and invasive disease due to Haemophilus influenzae (H. influenzae) type b. VAXELIS is approved for use as a 3-dose series in children 6 weeks through 4 years of age (prior to the 5th birthday).
2. Vaxelis Dosage and Administration
For intramuscular use only.
2.1 Vaccination Schedule
VAXELIS is to be administered as a 3-dose series at 2, 4, and 6 months of age. The first dose may be given as early as 6 weeks of age. Three doses of VAXELIS constitute a primary immunization course against diphtheria, tetanus, H. influenzae type b invasive disease and poliomyelitis.
VAXELIS may be used to complete the hepatitis B immunization series.
A 3-dose series of VAXELIS does not constitute a primary immunization series against pertussis; an additional dose of pertussis-containing vaccine is needed to complete the primary series. [See Pertussis Vaccination Following VAXELIS.]
Pertussis Vaccination following VAXELIS
VAXELIS, Pentacel® [(Diphtheria and Tetanus Toxoids and Acellular Pertussis Adsorbed, Inactivated Poliovirus and Haemophilus b Conjugate (Tetanus Toxoid Conjugate) Vaccine): DTaP-IPV/Hib], Quadracel® [(Diphtheria and Tetanus Toxoids and Acellular Pertussis Adsorbed and Inactivated Poliovirus Vaccine): DTaP-IPV] and DAPTACEL® [(Diphtheria and Tetanus Toxoids and Acellular Pertussis Vaccine Adsorbed): DTaP] contain the same pertussis antigens manufactured by the same process. Children who have received a 3-dose series of VAXELIS should complete the primary and pertussis vaccination series with Pentacel, Quadracel or DAPTACEL according to the respective prescribing information in the approved package inserts. [See ADVERSE REACTIONS (6.1) AND CLINICAL STUDIES (14).]
2.2 Administration
Just before use, shake the vial or syringe until a uniform, white, cloudy suspension results.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. If either of these conditions exist, the product should not be administered.
Administer a single 0.5 mL dose of VAXELIS intramuscularly.
In infants younger than 1 year, the anterolateral aspect of the thigh is the preferred site of injection. The vaccine should not be injected into the gluteal area.
VAXELIS should not be combined through reconstitution or mixed with any other vaccine. Discard unused portion.
3. Dosage Forms and Strengths
VAXELIS is a suspension for injection available in 0.5 mL single-dose vials and prefilled syringes. [See HOW SUPPLIED/STORAGE AND HANDLING (16).]
4. Contraindications
4.1 Hypersensitivity
Do not administer VAXELIS to anyone with a history of a severe allergic reaction (e.g., anaphylaxis) to a previous dose of VAXELIS, any ingredient of VAXELIS, or any other diphtheria toxoid, tetanus toxoid, pertussis-containing vaccine, inactivated poliovirus vaccine, hepatitis B vaccine, or H. influenzae type b vaccine [See DESCRIPTION (11).]
5. Warnings and Precautions
5.1 Management of Acute Allergic Reactions
Epinephrine hydrochloride solution (1:1,000) and other appropriate agents and equipment must be available for immediate use in case an anaphylactic or acute hypersensitivity reaction occurs.
5.2 Adverse Reactions Following Prior Pertussis Vaccination
If any of the following events occur after administration of a pertussis vaccine, the decision to administer VAXELIS should be based on careful consideration of potential benefits and possible risks.
- Temperature of ≥40.5°C (≥105°F) within 48 hours, not attributable to another identifiable cause.
- Collapse or shock-like state (hypotonic-hyporesponsive episode [HHE]) within 48 hours.
- Persistent, inconsolable crying lasting ≥3 hours within 48 hours.
- Seizures with or without fever within 3 days.
5.3 Guillain-Barré Syndrome and Brachial Neuritis
A review by the Institute of Medicine (IOM) found evidence for a causal relation between tetanus toxoid and both brachial neuritis and Guillain-Barré syndrome. If Guillain-Barré syndrome occurred within 6 weeks of receipt of a prior vaccine containing tetanus toxoid, the risk for Guillain-Barré syndrome may be increased following VAXELIS. (1)
5.4 Altered Immunocompetence
If VAXELIS is administered to immunocompromised persons, including persons receiving immunosuppressive therapy, the expected immune response may not be obtained.
5.5 Apnea in Premature Infants
Apnea following intramuscular vaccination has been observed in some infants born prematurely. The decision about when to administer an intramuscular vaccine, including VAXELIS, to an infant born prematurely should be based on consideration of the infant's medical status and the potential benefits and possible risks of vaccination.
6. Adverse Reactions/Side Effects
Rates of adverse reactions varied by number of doses of VAXELIS received. The solicited adverse reactions 0-5 days following any dose were irritability (≥55%), crying (≥45%), injection site pain (≥44%), somnolence (≥40%), injection site erythema (≥25%), decreased appetite (≥23%), fever ≥38.0°C (≥19%), injection site swelling (≥18%), and vomiting (≥9%).
6.1 Clinical Trials Experience
Because clinical trials are conducted under varying conditions, adverse reaction rates observed in the clinical trials of a vaccine cannot be directly compared to rates in the clinical trials of another vaccine and may not reflect the rates observed in practice. The adverse reaction information from clinical trials does, however, provide a basis for identifying the adverse events that appear to be related to vaccine use and for approximating rates of those events.
The safety of VAXELIS was evaluated in 6 clinical studies, in which a total of 5,251 infants 43 to 99 days of age at enrollment received at least 1 dose of VAXELIS. Two of these (study 005 and 006) were controlled clinical studies conducted in the US, in which a total of 3,380 infants 46 to 89 days of age at enrollment received at least 1 dose of VAXELIS. The vaccination schedules of VAXELIS, Control vaccines, and concomitantly administered vaccines used in these studies are provided in Table 1. At 15 months of age, participants in Study 005 received a dose of DAPTACEL and a H. influenzae type b conjugate vaccine, whereas participants in Study 006 received a dose of Pentacel. In a non-US study, 294 children received a dose of VAXELIS at 15 months of age.
Across the 2 studies conducted in the US, among all randomized participants (3,392 in the VAXELIS group and 889 in the Control group), 52.6% were male and 47.4% were female. The race distribution was as follows: 71.7% were White, 11.0% were Black, 4.5% were American Indian or Alaska Native, 3.5% were Asian, and 9.3% were of other racial groups. Most participants (81.8%) were of non-Hispanic or Latino ethnicity. The racial/ethnic distribution of participants who received VAXELIS and Control vaccines was similar.
| Study | Vaccine | Concomitantly Administered Vaccines |
|---|---|---|
| Prevnar 13 (Pneumococcal 13-valent Conjugate Vaccine [Diphtheria CRM197 Protein]) | ||
| RotaTeq (Rotavirus Vaccine, Live, Oral, Pentavalent) | ||
| PedvaxHIB [Haemophilus b Conjugate Vaccine (Meningococcal Protein Conjugate)] | ||
| RECOMBIVAX HB (Hepatitis B Vaccine [Recombinant]) | ||
|
||
| 005* | VAXELIS at 2, 4, 6 months and DAPTACEL + PedvaxHIB® at 15 months | RotaTeq® at 2, 4, and 6 months Prevnar 13® at 2, 4, 6, and 15 months |
| Control group vaccines: Pentacel at 2, 4, 6 months and RECOMBIVAX HB® at 2 and 6 months DAPTACEL+ ActHIB® at 15 months | RotaTeq at 2, 4, and 6 months Prevnar 13 at 2, 4, 6, and 15 months |
|
| 006* | VAXELIS at 2, 4, 6 months and Pentacel at 15 months | RotaTeq at 2, 4, and 6 months Prevnar 13 at 2, 4, 6, and 15 months |
| Control group vaccines: Pentacel at 2, 4, 6, and 15 months RECOMBIVAX HB at 2 and 6 months | RotaTeq at 2, 4, and 6 months Prevnar 13 at 2, 4, 6, and 15 months |
|
6.2 Postmarketing Experience
The following adverse events have been reported during post-marketing use of VAXELIS or other vaccines containing the antigens of VAXELIS. These adverse events are included based on a suspected causal connection to VAXELIS or the components of DAPTACEL® (Diphtheria and Tetanus Toxoids and Acellular Pertussis Vaccine Adsorbed), IPOL® (Poliovirus Vaccine Inactivated), COMVAX® [Haemophilus b Conjugate (Meningococcal Protein Conjugate) and Hepatitis B (Recombinant) Vaccine]. (COMVAX is no longer licensed in the US.)
Because these events are reported voluntarily from a population of uncertain size, it is not possible to reliably estimate their frequency or establish a causal relationship to vaccination.
-
Immune System Disorders
Hypersensitivity (such as rash, urticaria, dyspnea, erythema multiforme), anaphylactic reaction (such as urticaria, angioedema, edema, face edema, shock). -
General Disorders and Administration Site Conditions
Extensive swelling of injected limb (including swelling that involves adjacent joints). -
Nervous System
Seizure, febrile seizure, hypotonic-hyporesponsive episode (HHE).
7. Drug Interactions
7.1 Interference with Laboratory Tests
Sensitive tests (e.g., Latex Agglutination kits) have detected vaccine-derived polyribosylribitol phosphate (PRP) in the urine of vaccinees for at least 30 days following vaccination with PedvaxHIB [Haemophilus b Conjugate Vaccine (Meningococcal Protein Conjugate)]. (2) Therefore, urine antigen detection may not have definite diagnostic value in suspected H. influenzae type b disease following vaccination with VAXELIS. [See WARNINGS AND PRECAUTIONS (5.7).]
8. Use In Specific Populations
8.1 Pregnancy
VAXELIS is not approved for use in individuals 5 years of age and older. No human or animal data are available to assess vaccine-associated risks in pregnancy.
8.2 Lactation
VAXELIS is not approved for use in individuals 5 years of age and older. No human or animal data are available to assess the impact of VAXELIS on milk production, its presence in breast milk, or its effects on the breastfed infant.
8.4 Pediatric Use
The safety of VAXELIS has been established in the age group 6 weeks through 15 months, and the effectiveness of VAXELIS was established in the age group 6 weeks through 6 months on the basis of clinical studies. [See ADVERSE REACTIONS (6.1) AND CLINICAL STUDIES (14).]
The safety and effectiveness of VAXELIS in older children through 4 years of age are supported by evidence in younger children. The safety and effectiveness of VAXELIS in infants less than 6 weeks of age and in children and adolescents 5 through 17 years of age have not been established.
11. Vaxelis Description
VAXELIS (Diphtheria and Tetanus Toxoids and Acellular Pertussis, Inactivated Poliovirus, Haemophilus b Conjugate and Hepatitis B Vaccine) is a sterile suspension for intramuscular injection.
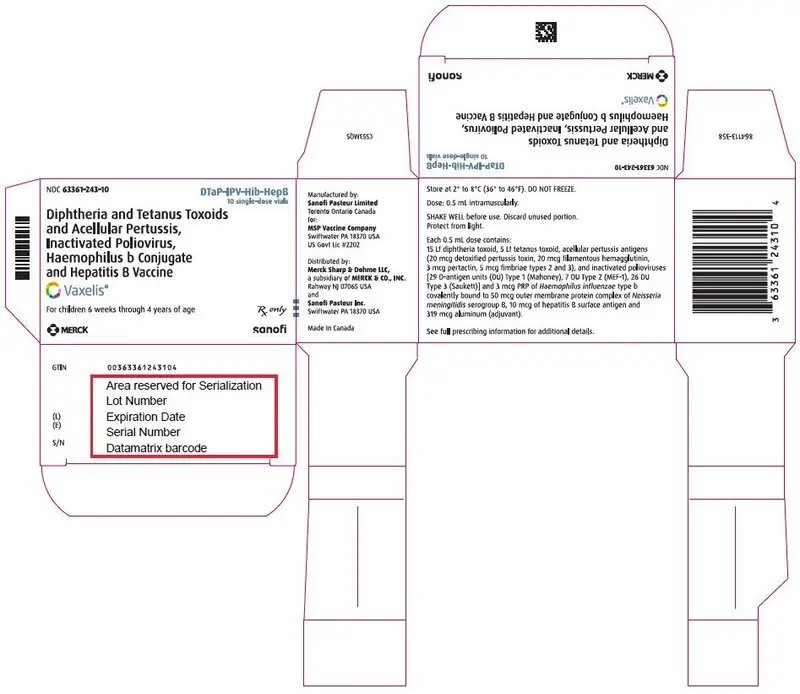
Each 0.5 mL dose is formulated to contain 15 Lf diphtheria toxoid, 5 Lf tetanus toxoid, acellular pertussis antigens [20 mcg detoxified pertussis toxin (PT), 20 mcg filamentous hemagglutinin (FHA), 3 mcg pertactin (PRN), 5 mcg fimbriae types 2 and 3 (FIM)], inactivated polioviruses [29 D-antigen units (DU) Type 1 (Mahoney), 7 DU Type 2 (MEF-1), 26 DU Type 3 (Saukett)], 3 mcg polyribosylribitol phosphate (PRP) of H. influenzae type b covalently bound to 50 mcg of the outer membrane protein complex (OMPC) of Neisseria meningitidis serogroup B, and 10 mcg hepatitis B surface antigen (HBsAg). Each 0.5 mL dose contains 319 mcg aluminum from aluminum salts used as adjuvants.
Other ingredients per 0.5 mL dose include <0.0056% polysorbate 80 and the following residuals from the manufacturing process: ≤14 mcg formaldehyde, ≤50 ng glutaraldehyde, ≤50 ng bovine serum albumin, <5 ng of neomycin, <200 ng streptomycin sulfate, <25 ng polymyxin B sulfate, ≤0.125 μg ammonium thiocyanate and ≤0.1 mcg yeast protein (maximum 1% relative to HBsAg protein).
Corynebacterium diphtheriae is grown in modified Mueller's growth medium. (3) After purification by ammonium sulfate fractionation, the diphtheria toxin is detoxified with formaldehyde and diafiltered.
Clostridium tetani is grown in modified Mueller-Miller casamino acid medium without beef heart infusion. (4) Tetanus toxin is detoxified with formaldehyde and purified by ammonium sulfate fractionation and diafiltration. Diphtheria and tetanus toxoids are individually adsorbed onto aluminum phosphate.
The acellular pertussis vaccine antigens are produced from Bordetella pertussis cultures grown in Stainer-Scholte medium (5) modified by the addition of casamino acids and dimethyl-beta-cyclodextrin. PT, FHA and PRN are isolated separately from the supernatant culture medium. FIM are extracted and copurified from the bacterial cells. The pertussis antigens are purified by sequential filtration, salt-precipitation, ultrafiltration and chromatography. PT is detoxified with glutaraldehyde. FHA is treated with formaldehyde and the residual aldehydes are removed by ultrafiltration. The individual antigens are adsorbed separately onto aluminum phosphate.
The Type 1, Type 2, and Type 3 polioviruses are individually grown in Vero cells. The viral harvests are concentrated and purified, then inactivated with formaldehyde to produce monovalent suspensions of each serotype. Specified quantities of monovalent suspensions of each serotype are mixed to produce the trivalent poliovirus concentrate.
The HBsAg antigen is harvested and purified from fermentation cultures of a recombinant strain of the yeast Saccharomyces cerevisiae containing the gene for the adw subtype of HBsAg. The recombinant Saccharomyces cerevisiae is grown in a fermentation medium which consists of an extract of yeast, soy peptone, dextrose, amino acids, and mineral salts. The HBsAg protein is released from the yeast cells by cell disruption and purified by a series of physical and chemical methods which includes ion and hydrophobic chromatography, and diafiltration. The purified protein is treated in phosphate buffer with formaldehyde and then co-precipitated with alum (potassium aluminum sulfate) to form bulk vaccine adjuvanted with amorphous aluminum hydroxyphosphate sulfate.
The purified PRP of H. influenzae type b (Haemophilus b, Ross strain) is conjugated to an OMPC of the B11 strain of N. meningitidis serogroup B. H. influenzae type b is grown in a fermentation medium which includes an extract of yeast, nicotinamide adenine dinucleotide, hemin chloride, soy peptone, dextrose, and mineral salts. The PRP is purified from the culture broth by purification procedures which include ethanol fractionation, enzyme digestion, phenol extraction and diafiltration. N. meningitidis serogroup B is grown in a fermentation medium which includes an extract of yeast, amino acids and mineral salts. The OMPC is purified by detergent extraction, ultracentrifugation, diafiltration and sterile filtration. PRP is conjugated to OMPC by chemical coupling and the PRP-OMPC is then adsorbed onto an amorphous aluminum hydroxyphosphate sulfate adjuvant.
The adsorbed diphtheria, tetanus, and acellular pertussis antigens are combined with aluminum phosphate (as adjuvant) and water for injection into an intermediate concentrate. The individual HBsAg and PRP-OMPC adjuvanted bulks are added followed by the trivalent poliovirus concentrate, to produce VAXELIS.
Both diphtheria and tetanus toxoids induce at least 2 neutralizing units per mL of serum in the guinea pig potency test. The potency of the acellular pertussis antigens is evaluated by the antibody response of immunized mice to detoxified PT, FHA, PRN and FIM as measured by enzyme-linked immunosorbent assay (ELISA). The immunogenicity of the inactivated polioviruses is evaluated by the antibody response in rats measured by virus neutralization. The potency of the HBsAg component is measured relative to a standard by an in vitro immunoassay. The potency of the PRP-OMPC component is measured by quantitating the polysaccharide concentration using an HPLC method.
VAXELIS does not contain a preservative. The vial stopper, syringe plunger stopper, and syringe tip cap are not made with natural rubber latex.
14. Clinical Studies
14.2 Immunogenicity
In the US Study 005 (Table 1), infants were randomized to receive 3 doses of VAXELIS at 2, 4, and 6 months of age and DAPTACEL and PedvaxHIB at 15 months of age, or Control group vaccines (3 doses of Pentacel vaccine at 2, 4, and 6 months of age + RECOMBIVAX HB at 2 and 6 months of age and DAPTACEL and ActHIB at 15 months of age). All subjects received concomitant vaccines: RotaTeq at 2, 4 and 6 months and Prevnar 13 at 2, 4, 6, and 15 months of age. [See ADVERSE REACTIONS (6.1).] All infants had received a dose of hepatitis B vaccine prior to study initiation, prior to or at one month of age. Among all randomized participants, 53.0% were male and 47.0% were female. Most (79.2%) participants were White, 14.1% were Black and 5.2% were multi-racial. Most (91.4%) participants were of non-Hispanic or non-Latin ethnicity.
Antibody responses to diphtheria, tetanus, pertussis (PT, FHA, PRN and FIM), poliovirus types 1, 2 and 3, hepatitis B and H. influenzae type b antigens were measured in sera obtained one month following the third dose of VAXELIS or Pentacel + RECOMBIVAX HB vaccines. VAXELIS was non-inferior to Pentacel + RECOMBIVAX HB administered concomitantly at separate sites, as demonstrated by the proportions of participants achieving seroprotective levels of antibodies to diphtheria, tetanus, poliovirus, hepatitis B and PRP antigens, and pertussis vaccine response rates and GMCs (except FHA), following 3 doses of the vaccine. See Table 3.
To complete the 4-dose pertussis primary vaccination series, participants in both groups received DAPTACEL at 15 months of age and were evaluated for immune responses to pertussis antigens one month later. The non-inferiority criteria for vaccine response rates and GMCs for all pertussis antigens were met following the fourth dose.
| VAXELIS + Prevnar 13 + RotaTeq (N=688 – 810) | Pentacel + RECOMBIVAX HB + Prevnar 13 + RotaTeq (N=353 – 400) |
|
|---|---|---|
| N = The number of participants with available data. | ||
|
||
| Anti-Diphtheria Toxoid | ||
| % ≥0.1 IU/mL | 82.4* | 86.3 |
| Anti-Tetanus Toxoid | ||
| % ≥0.1 IU/mL | 99.9† | 99.5 |
| Anti-PT | ||
| % vaccine response‡ | 98.1* | 98.5 |
| GMC | 109.6§ | 85.4 |
| Anti-FHA | ||
| % vaccine response‡ | 87.3* | 92.0 |
| GMC | 46.6¶ | 72.3 |
| Anti-PRN | ||
| % vaccine response‡ | 79.3* | 82.0 |
| GMC | 55.8§ | 66.8 |
| Anti-FIM | ||
| % vaccine response‡ | 90.2* | 86.2 |
| GMC | 235.9§ | 184.4 |
| Anti-Poliovirus Type 1 | ||
| % ≥1:8 dilution | 100.0† | 98.2 |
| Anti-Poliovirus Type 2 | ||
| % ≥1:8 dilution | 100.0† | 99.7 |
| Anti-Poliovirus Type 3 | ||
| % ≥1:8 dilution | 100.0† | 99.8 |
| Anti-PRP | ||
| % ≥0.15 μg/mL | 97.3† | 92.4 |
| % ≥1.0 μg/mL | 85.0* | 75.3 |
| Anti-HBsAg | ||
| % ≥10 mIU/mL | 99.4* | 98.6 |
Study 006 (Table 1) was a lot consistency study conducted in the US, where infants were randomized to receive 3 doses of VAXELIS at 2, 4, and 6 months of age and Pentacel at 15 months of age (N=2,406), or control group vaccines (4 doses of Pentacel at 2, 4, 6, and 15 months of age + RECOMBIVAX HB at 2 and 6 months of age; N=402). All subjects received concomitant vaccines: RotaTeq at 2, 4 and 6 months and Prevnar 13 at 2, 4, 6, and 15 months of age. All infants had received a dose of hepatitis B vaccine prior to study initiation, from birth up to one month of age.
Antibody responses to diphtheria, tetanus, pertussis (PT, FHA, PRN and FIM), poliovirus types 1, 2 and 3, hepatitis B and H. influenzae type b antigens were measured in sera obtained one month following the third dose of VAXELIS or Pentacel + RECOMBIVAX HB. VAXELIS was non-inferior to Pentacel + RECOMBIVAX HB administered concomitantly at separate sites, as demonstrated by the proportions of participants achieving seroprotective levels of antibodies to diphtheria, tetanus, poliovirus, hepatitis B and PRP antigens, and pertussis vaccine response rates and GMCs, except for GMCs for FHA (lower bound of 2-sided 95% CI for GMC ratio [VAXELIS group/Control group vaccines] was 0.62, which was below the non-inferiority criterion >0.67).
To complete the 4-dose pertussis primary vaccination series, participants in both groups received Pentacel at 15 months of age and were evaluated for immune responses to pertussis antigens one month later. The non-inferiority criteria for antibody vaccine response rates and GMCs for all pertussis antigens were met following the fourth dose except for GMCs for PRN (lower bound of 2-sided 95% CI for GMC ratio [VAXELIS group/Control group vaccines] was 0.66, which was below the non-inferiority criterion >0.67).
14.3 Concomitantly Administered Vaccines
In Study 006 conducted in the US (Table 1), the immune responses to Prevnar 13 were measured one month after the third dose. Non-inferiority criteria were met for GMCs to 12 of the 13 serotype antigens in Prevnar 13 for participants who received VAXELIS relative to Control vaccines. For serotype 6B, the non-inferiority criterion was not met (lower bound of 2-sided 95% CI for GMC ratio [VAXELIS group/Control vaccines group] is 0.64, which is below the non-inferiority criterion >0.67).
15. References
- 1
- Stratton K, Ford A, Rusch E, Clayton EW, eds. Institute of Medicine (IOM). Adverse Effects of Vaccines: Evidence and Causality. Washington, DC: The National Academies Press. 2011.
- 2
- Goepp JG, Hohenboken M, Almeido-Hill J, Santosham M. Persistent urinary antigen excretion in infants vaccinated with Haemophilus influenzae type b capsular polysaccharide conjugated with outer membrane protein from Neisseria meningitidis. Pediatr Infect Dis J 1992;11(1):2–5.
- 3
- Stainer DW. Production of diphtheria toxin. In: Manclark CR, editor. Proceedings of an informal consultation on the World Health Organization requirements for diphtheria, tetanus, pertussis and combined vaccines. United States Public Health Service, Bethesda, MD. DHHS 91-1174; 1991. p. 7–11.
- 4
- Mueller JH, Miller PA. Variable factors influencing the production of tetanus toxin. J Bacteriol 1954;67(3):271–7.
- 5
- Stainer DW, Scholte MJ. A simple chemically defined medium for the production of phase I Bordetella pertussis. J Gen Microbiol 1971;63:211–20.
- 6
- Department of Health and Human Services, Food and Drug Administration. Biological products; bacterial vaccines and toxoids; implementation of efficacy review; proposed rule. Federal Register 1985;50(240):51002–117.
- 7
- Tiwari TSP, Wharton M. Diphtheria toxoid. In: Plotkin SA, Orenstein WA, and Offit PA, editors. Vaccines. 6th ed. Philadelphia, PA: WB Saunders; 2013:153–66.
- 8
- Roper M, Wassilak SGF, Tiwari TSP, Orenstein WA. Tetanus toxoid. In: Plotkin SA, Orenstein WA, Offit PA, editors. Vaccines. 6th ed. Philadelphia, PA: WB Saunders; 2013. p. 746–72.
- 9
- Sutter RW, et al. Defining surrogate serologic tests with respect to predicting protective vaccine efficacy: Poliovirus vaccination. In: Williams JC, et al. eds. Combined vaccines and simultaneous administration. Current issues and perspectives. New York, NY: The New York Academy of Sciences. 1995:289–99.
- 10
- Robbins, J. B., et al: Quantitative measurement of 'natural' and immunization-induced Haemophilus influenzae type b capsular polysaccharide antibodies. Pediatr Res 1973;7(3):103–10.
- 11
- Kayhty H, et al. The protective level of serum antibodies to the capsular polysaccharide of Haemophilus influenzae type b. J Infect Dis 1983;147:1100.
- 12
- Anderson P. The protective level of serum antibodies to the capsular polysaccharide of Haemophilus influenzae type b. J Infect Dis 1984;149:1034.
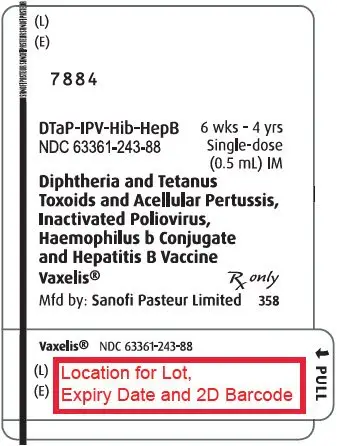
16. How is Vaxelis supplied
16.1 How Supplied
Single-dose vial (NDC 63361-243-58) in packages of 10 vials (NDC 63361-243-10).
Single-dose, prefilled syringe with Luer lock connection and a tip cap, without needle, 0.5 mL (NDC 63361-243-88). Supplied as package of 10 (NDC 63361-243-15).
The vial stopper, syringe plunger stopper, and syringe tip cap are not made with natural rubber latex.
17. Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Inform the parent or guardian of the following:
- The potential benefits and risks of immunization with VAXELIS.
- The common adverse reactions that have occurred following administration of VAXELIS or other vaccines containing similar ingredients.
- Other adverse reactions can occur. Call healthcare provider with any adverse reactions of concern.
Provide the Vaccine Information Statements (VIS), which are required by the National Childhood Vaccine Injury Act of 1986.
| VAXELIS
diphtheria and tetanus toxoids and acellular pertussis, inactivated poliovirus, haemophilus b conjugate and hepatitis b vaccine injection, suspension |
|||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||
| Labeler - MSP Vaccine Company (079352454) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Sanofi Pasteur Limited | 208206623 | MANUFACTURE(63361-243) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Sanofi Pasteur SA | 578763542 | MANUFACTURE(63361-243) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Merck Sharp & Dohme LLC | 002387926 | MANUFACTURE(63361-243) | |