Drug Detail:Vemlidy (Tenofovir alafenamide)
Drug Class: Nucleoside reverse transcriptase inhibitors (NRTIs)
Highlights of Prescribing Information
VEMLIDY® (tenofovir alafenamide) tablets, for oral use
Initial U.S. Approval: 2015
WARNING: POSTTREATMENT SEVERE ACUTE EXACERBATION OF HEPATITIS B
See full prescribing information for complete boxed warning.
Discontinuation of anti-hepatitis B therapy may result in severe acute exacerbations of hepatitis B. Hepatic function should be monitored closely in patients who discontinue VEMLIDY. If appropriate, resumption of anti-hepatitis B therapy may be warranted. (5.1)
Recent Major Changes
| Indications and Usage (1) | 10/2022 |
| Dosage and Administration: Recommended Dosage in Adults and Pediatric Patients 12 Years of Age and Older (2.2) | 10/2022 |
Indications and Usage for Vemlidy
VEMLIDY is a hepatitis B virus (HBV) nucleoside analog reverse transcriptase inhibitor and is indicated for the treatment of chronic hepatitis B virus infection in adults and pediatric patients 12 years of age and older with compensated liver disease. (1)
Vemlidy Dosage and Administration
- Testing: Prior to initiation of VEMLIDY, test patients for HIV infection. VEMLIDY alone should not be used in patients with HIV infection. Prior to or when initiating VEMLIDY, and during treatment on a clinically appropriate schedule, assess serum creatinine, estimated creatinine clearance, urine glucose, and urine protein in all patients. Also assess serum phosphorus in patients with chronic kidney disease. (2.1)
- Recommended dosage: 25 mg (one tablet) taken orally once daily with food. (2.2)
- Renal Impairment: VEMLIDY is not recommended in patients with estimated creatinine clearance below 15 mL per minute who are not receiving chronic hemodialysis. In patients on chronic hemodialysis, on hemodialysis days, administer VEMLIDY after hemodialysis. (2.3)
- Hepatic Impairment: VEMLIDY is not recommended in patients with decompensated (Child-Pugh B or C) hepatic impairment. (2.4)
Dosage Forms and Strengths
Tablets: 25 mg of tenofovir alafenamide. (3)
Contraindications
None. (4)
Warnings and Precautions
- HBV and HIV-1 coinfection: VEMLIDY alone is not recommended for the treatment of HIV-1 infection. HIV-1 resistance may develop in these patients. (5.2)
- New onset or worsening renal impairment: Prior to or when initiating VEMLIDY, and during treatment on a clinically appropriate schedule, assess serum creatinine, estimated creatinine clearance, urine glucose, and urine protein in all patients. Also assess serum phosphorus in patients with chronic kidney disease. (5.3)
- Lactic acidosis/severe hepatomegaly with steatosis: Discontinue treatment in patients who develop symptoms or laboratory findings suggestive of lactic acidosis or pronounced hepatotoxicity. (5.4)
Adverse Reactions/Side Effects
Most common adverse reaction (incidence greater than or equal to 10%, all grades) is headache. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Gilead Sciences, Inc. at 1-800-GILEAD-5 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
VEMLIDY is a substrate of P-glycoprotein (P-gp) and BCRP. Drugs that strongly affect P-gp and BCRP activity may lead to changes in VEMLIDY absorption. Consult the full prescribing information prior to and during treatment for potential drug-drug interactions. (7)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 10/2022
Related/similar drugs
tenofovir, entecavir, lamivudine, Viread, PegasysFull Prescribing Information
WARNING: POSTTREATMENT SEVERE ACUTE EXACERBATION OF HEPATITIS B
Discontinuation of anti-hepatitis B therapy, including VEMLIDY, may result in severe acute exacerbations of hepatitis B. Hepatic function should be monitored closely with both clinical and laboratory follow-up for at least several months in patients who discontinue anti-hepatitis B therapy, including VEMLIDY. If appropriate, resumption of anti-hepatitis B therapy may be warranted [see Warnings and Precautions (5.1)].
1. Indications and Usage for Vemlidy
VEMLIDY is indicated for the treatment of chronic hepatitis B virus (HBV) infection in adults and pediatric patients 12 years of age and older with compensated liver disease [see Clinical Studies (14)].
2. Vemlidy Dosage and Administration
2.1 Testing Prior to Initiation of VEMLIDY
Prior to initiation of VEMLIDY, patients should be tested for HIV-1 infection. VEMLIDY alone should not be used in patients with HIV-1 infection [see Warnings and Precautions (5.2)].
Prior to or when initiating VEMLIDY, and during treatment with VEMLIDY on a clinically appropriate schedule, assess serum creatinine, estimated creatinine clearance, urine glucose, and urine protein in all patients. In patients with chronic kidney disease, also assess serum phosphorus [see Warnings and Precautions (5.3)].
2.2 Recommended Dosage in Adults and Pediatric Patients 12 Years of Age and Older
The recommended dosage of VEMLIDY in adults and pediatric patients 12 years of age and older is one 25 mg tablet taken orally once daily with food [see Clinical Pharmacology (12.3)].
2.3 Dosage in Patients with Renal Impairment
No dosage adjustment of VEMLIDY is required in patients with estimated creatinine clearance greater than or equal to 15 mL per minute, or in patients with end stage renal disease (ESRD; estimated creatinine clearance below 15 mL per minute) who are receiving chronic hemodialysis. On days of hemodialysis, administer VEMLIDY after completion of hemodialysis treatment.
VEMLIDY is not recommended in patients with ESRD who are not receiving chronic hemodialysis [see Use in Specific Populations (8.6) and Clinical Pharmacology (12.3)].
No data are available to make dose recommendations in pediatric patients with renal impairment.
2.4 Dosage in Patients with Hepatic Impairment
No dosage adjustment of VEMLIDY is required in patients with mild hepatic impairment (Child-Pugh A). VEMLIDY is not recommended in patients with decompensated (Child-Pugh B or C) hepatic impairment [see Use in Specific Populations (8.7) and Clinical Pharmacology (12.3)].
3. Dosage Forms and Strengths
Tablets: 25 mg of tenofovir alafenamide (equivalent to 28 mg of tenofovir alafenamide fumarate) — yellow, round, film-coated tablets, debossed with "GSI" on one side of the tablet and "25" on the other side.
5. Warnings and Precautions
5.1 Severe Acute Exacerbation of Hepatitis B after Discontinuation of Treatment
Discontinuation of anti-hepatitis B therapy, including VEMLIDY, may result in severe acute exacerbations of hepatitis B. Patients who discontinue VEMLIDY should be closely monitored with both clinical and laboratory follow-up for at least several months after stopping treatment. If appropriate, resumption of anti-hepatitis B therapy may be warranted.
5.2 Risk of Development of HIV-1 Resistance in Patients Coinfected with HBV and HIV-1
Due to the risk of development of HIV-1 resistance, VEMLIDY alone is not recommended for the treatment of HIV-1 infection. The safety and efficacy of VEMLIDY have not been established in patients coinfected with HBV and HIV-1. HIV antibody testing should be offered to all HBV-infected patients before initiating therapy with VEMLIDY, and, if positive, an appropriate antiretroviral combination regimen that is recommended for patients coinfected with HIV-1 should be used.
5.3 New Onset or Worsening Renal Impairment
Postmarketing cases of renal impairment, including acute renal failure, proximal renal tubulopathy (PRT), and Fanconi syndrome have been reported with TAF-containing products; while most of these cases were characterized by potential confounders that may have contributed to the reported renal events, it is also possible these factors may have predisposed patients to tenofovir-related adverse events [see Adverse Reactions (6.2)].
Patients taking tenofovir prodrugs who have impaired renal function and those taking nephrotoxic agents, including non-steroidal anti-inflammatory drugs, are at increased risk of developing renal-related adverse reactions [see Drug Interactions (7.2)].
Prior to or when initiating VEMLIDY, and during treatment with VEMLIDY on a clinically appropriate schedule, assess serum creatinine, estimated creatinine clearance, urine glucose, and urine protein in all patients. In patients with chronic kidney disease, also assess serum phosphorus. Discontinue VEMLIDY in patients who develop clinically significant decreases in renal function or evidence of Fanconi syndrome [see Adverse Reactions (6.1) and Use in Specific Populations (8.6)].
5.4 Lactic Acidosis/Severe Hepatomegaly with Steatosis
Lactic acidosis and severe hepatomegaly with steatosis, including fatal cases, have been reported with the use of nucleoside analogs, including tenofovir disoproxil fumarate (TDF), another prodrug of tenofovir, alone or in combination with other antiretrovirals. Treatment with VEMLIDY should be suspended in any patient who develops clinical or laboratory findings suggestive of lactic acidosis or pronounced hepatotoxicity (which may include hepatomegaly and steatosis even in the absence of marked transaminase elevations).
6. Adverse Reactions/Side Effects
The following adverse reactions are discussed in other sections of the labeling:
- Severe Acute Exacerbation of Hepatitis B [see Warnings and Precautions (5.1)]
- New Onset or Worsening of Renal Impairment [see Warnings and Precautions (5.3)]
- Lactic Acidosis/Severe Hepatomegaly with Steatosis [see Warnings and Precautions (5.4)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Adverse Reactions in Adult Subjects with Chronic Hepatitis B and Compensated Liver Disease
The safety assessment of VEMLIDY was based on pooled data through the Week 96 data analysis from 1298 subjects in two randomized, double-blind, active-controlled trials, Trial 108 and Trial 110, in adult subjects with chronic hepatitis B and compensated liver disease. A total of 866 subjects received VEMLIDY 25 mg once daily [see Clinical Studies (14.2)]. Further safety assessment was based on pooled data from Trials 108 and 110 from subjects who continued to receive their original blinded treatment through Week 120 and additionally from subjects who received open-label VEMLIDY from Week 96 through Week 120 (n = 361 remained on VEMLIDY; n = 180 switched from TDF to VEMLIDY at Week 96).
Based on the Week 96 analysis, the most common adverse reaction (all Grades) reported in at least 10% of subjects in the VEMLIDY group was headache. The proportion of subjects who discontinued treatment with VEMLIDY or TDF due to adverse reactions of any severity was 1.5% and 0.9%, respectively. Table 1 displays the frequency of the adverse reactions (all Grades) greater than or equal to 5% in the VEMLIDY group.
| VEMLIDY (N=866) | TDF (N=432) |
|
|---|---|---|
|
||
| Headache | 12% | 10% |
| Abdominal pain‡ | 9% | 6% |
| Cough | 8% | 8% |
| Back pain | 6% | 6% |
| Fatigue | 6% | 5% |
| Nausea | 6% | 6% |
| Arthralgia | 5% | 6% |
| Diarrhea | 5% | 5% |
| Dyspepsia | 5% | 5% |
Additional adverse reactions occurring in less than 5% of subjects in Trials 108 and 110 included vomiting, rash, and flatulence.
The safety profile of VEMLIDY in subjects who continued to receive blinded treatment through Week 120 was similar to that at Week 96. The safety profile of VEMLIDY in subjects who remained on VEMLIDY in the open-label phase through Week 120 was similar to that in subjects who switched from TDF to VEMLIDY at Week 96.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post approval use of VEMLIDY or other products containing tenofovir alafenamide. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Skin and Subcutaneous Tissue Disorders
Angioedema, urticaria
Renal and Urinary Disorders
Acute renal failure, acute tubular necrosis, proximal renal tubulopathy, and Fanconi syndrome
7. Drug Interactions
7.1 Potential for Other Drugs to Affect VEMLIDY
VEMLIDY is a substrate of P-glycoprotein (P-gp) and BCRP. Drugs that strongly affect P-gp and BCRP activity may lead to changes in tenofovir alafenamide absorption (see Table 4). Drugs that induce P-gp activity are expected to decrease the absorption of tenofovir alafenamide, resulting in decreased plasma concentrations of tenofovir alafenamide, which may lead to loss of therapeutic effect of VEMLIDY. Coadministration of VEMLIDY with other drugs that inhibit P-gp and BCRP may increase the absorption and plasma concentration of tenofovir alafenamide.
7.2 Drugs Affecting Renal Function
Because tenofovir is primarily excreted by the kidneys by a combination of glomerular filtration and active tubular secretion, coadministration of VEMLIDY with drugs that reduce renal function or compete for active tubular secretion may increase concentrations of tenofovir and other renally eliminated drugs and this may increase the risk of adverse reactions. Some examples of drugs that are eliminated by active tubular secretion include, but are not limited to, acyclovir, cidofovir, ganciclovir, valacyclovir, valganciclovir, aminoglycosides (e.g., gentamicin), and high-dose or multiple NSAIDs [see Warnings and Precautions (5.3)].
7.3 Established and Other Potentially Significant Interactions
Table 4 provides a listing of established or potentially clinically significant drug interactions. The drug interactions described are based on studies conducted with tenofovir alafenamide or are predicted drug interactions that may occur with VEMLIDY [For magnitude of interaction, see Clinical Pharmacology (12.3)]. Information regarding potential drug-drug interactions with HIV antiretrovirals is not provided (see the prescribing information for emtricitabine/tenofovir alafenamide for interactions with HIV antiretrovirals). The table includes potentially significant interactions but is not all inclusive.
| Concomitant Drug Class: Drug Name | Effect on Concentration† | Clinical Comment |
|---|---|---|
|
||
| Anticonvulsants:
Carbamazepine‡§ Oxcarbazepine§ Phenobarbital§ Phenytoin§ | ↓ tenofovir alafenamide | When coadministered with carbamazepine, the tenofovir alafenamide dose should be increased to two tablets once daily. Coadministration of VEMLIDY with oxcarbazepine, phenobarbital, or phenytoin is not recommended. |
| Antimycobacterials:
Rifabutin§ Rifampin§ Rifapentine§ | ↓ tenofovir alafenamide | Coadministration of VEMLIDY with rifabutin, rifampin or rifapentine is not recommended. |
| Herbal Products:
St. John's wort§ (Hypericum perforatum) | ↓ tenofovir alafenamide | Coadministration of VEMLIDY with St. John's wort is not recommended. |
7.4 Drugs without Clinically Significant Interactions with VEMLIDY
Based on drug interaction studies conducted with VEMLIDY, no clinically significant drug interactions have been observed with: ethinyl estradiol, ledipasvir/sofosbuvir, midazolam, norgestimate, sertraline, sofosbuvir, sofosbuvir/velpatasvir, and sofosbuvir/velpatasvir/voxilaprevir.
8. Use In Specific Populations
8.2 Lactation
Data
Studies in rats and monkeys have demonstrated that tenofovir is secreted in milk. Tenofovir was excreted into the milk of lactating rats following oral administration of TDF (up to 600 mg/kg/day) at up to approximately 24% of the median plasma concentration in the highest dosed animals at lactation day 11 (see Data 8.1). Tenofovir was excreted into the milk of lactating monkeys following a single subcutaneous (30 mg/kg) dose of tenofovir at concentrations up to approximately 4% of plasma concentration, resulting in exposure (AUC) of approximately 20% of plasma exposure.
8.4 Pediatric Use
The pharmacokinetics, safety, and effectiveness of VEMLIDY for the treatment of chronic HBV infection have been established in pediatric patients between the ages of 12 to less than 18 years (N=47) in Trial 1092 for 24 weeks. No clinically meaningful differences in pharmacokinetics or safety were observed in comparison to those observed in adults [see Adverse Reactions (6.1), Clinical Pharmacology (12.3), and Clinical Studies (14.5)].
Safety and effectiveness of VEMLIDY has not been established in pediatric patients with chronic HBV infection who are less than 12 years of age.
8.5 Geriatric Use
In clinical trials, VEMLIDY was administered to 89 subjects aged 65 and over. No clinically significant differences in safety or efficacy have been observed between elderly subjects and subjects between 18 and less than 65 years of age.
8.6 Renal Impairment
No dosage adjustment of VEMLIDY is required in patients with mild, moderate, or severe renal impairment, or in patients with ESRD (estimated creatinine clearance below 15 mL per minute) who are receiving chronic hemodialysis. On days of hemodialysis, administer VEMLIDY after completion of hemodialysis treatment [see Dosage and Administration (2.3)].
The safety and efficacy of VEMLIDY in HBV-infected adult subjects with moderate to severe renal impairment (estimated creatinine clearance between 15 and 59 mL per minute by Cockcroft-Gault method) and ESRD (estimated creatinine clearance of less than 15 mL per minute by Cockcroft-Gault method) receiving chronic hemodialysis were evaluated in 78 and 15 subjects, respectively, in an open-label trial (Trial 4035, Part A). Overall, 98% of subjects achieved HBV DNA <20 IU/mL at Week 24 (Cohort 1, 97%; Cohort 2, 100%) and the safety of VEMLIDY was similar to that observed in clinical trials of VEMLIDY in subjects with compensated liver disease but without renal impairment [see Adverse Reactions (6.1) and Clinical Studies (14.4)].
The safety and efficacy of elvitegravir/cobicistat/emtricitabine/tenofovir alafenamide 150/150/200/10 mg was previously evaluated in 55 virologically suppressed HIV-1 infected subjects with ESRD receiving chronic hemodialysis in an open-label trial (Trial 1825). Tenofovir alafenamide exposures are similar when comparing tenofovir alafenamide 25 mg and tenofovir alafenamide 10 mg as part of elvitegravir/cobicistat/emtricitabine/tenofovir alafenamide.
Among subjects with ESRD receiving chronic hemodialysis and administered tenofovir alafenamide, higher exposures of tenofovir were observed in HBV-infected subjects (Trial 4035 Part A) compared to HIV-infected subjects (Trial 1825). The clinical significance of these higher exposures is not established [see Clinical Pharmacology (12.3)].
VEMLIDY is not recommended in patients with ESRD (estimated creatinine clearance below 15 mL per minute by Cockcroft-Gault method) who are not receiving chronic hemodialysis as the safety of VEMLIDY has not been established in this population [see Dosage and Administration (2.3) and Clinical Pharmacology (12.3)].
8.7 Hepatic Impairment
No dosage adjustment of VEMLIDY is required in patients with mild hepatic impairment (Child-Pugh A). The safety and efficacy of VEMLIDY in patients with decompensated cirrhosis (Child-Pugh B or C) have not been established; therefore, VEMLIDY is not recommended in patients with decompensated (Child-Pugh B or C) hepatic impairment [see Dosage and Administration (2.4) and Clinical Pharmacology (12.3)].
10. Overdosage
If overdose occurs, monitor patient for evidence of toxicity. Treatment of overdosage with VEMLIDY consists of general supportive measures including monitoring of vital signs as well as observation of the clinical status of the patient. Tenofovir is efficiently removed by hemodialysis with an extraction coefficient of approximately 54%.
11. Vemlidy Description
VEMLIDY is a tablet containing tenofovir alafenamide for oral administration. Tenofovir alafenamide, a hepatitis B virus (HBV) nucleoside analog reverse transcriptase inhibitor, is converted in vivo to tenofovir, an acyclic nucleoside phosphonate (nucleotide) analog of adenosine 5′-monophosphate.
Each tablet contains 25 mg of tenofovir alafenamide (equivalent to 28 mg of tenofovir alafenamide fumarate). The tablets include the following inactive ingredients: croscarmellose sodium, lactose monohydrate, magnesium stearate, and microcrystalline cellulose. The tablets are film coated with a coating material containing: iron oxide yellow, polyethylene glycol, polyvinyl alcohol, talc, and titanium dioxide.
The chemical name of tenofovir alafenamide fumarate drug substance is L-alanine, N-[(S)-[[(1R)-2-(6-amino-9H-purin-9-yl)-1-methylethoxy]methyl]phenoxyphosphinyl]-, 1-methylethyl ester, (2E)-2-butenedioate (2:1).
It has an empirical formula of C21H29O5N6P∙½(C4H4O4) and a formula weight of 534.50. It has the following structural formula:
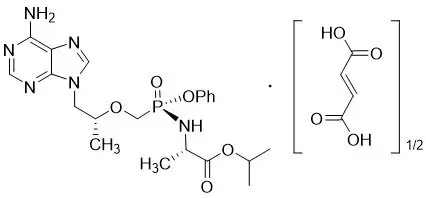
Tenofovir alafenamide fumarate is a white to off-white or tan powder with a solubility of 4.7 mg per mL in water at 20 °C.
12. Vemlidy - Clinical Pharmacology
12.1 Mechanism of Action
Tenofovir alafenamide is an antiviral drug against the hepatitis B virus [see Microbiology (12.4)].
12.3 Pharmacokinetics
The pharmacokinetic properties of VEMLIDY are provided in Table 5. The multiple dose PK parameters of tenofovir alafenamide and its metabolite tenofovir are provided in Table 6.
| Tenofovir Alafenamide | |
|---|---|
| CES1 = carboxylesterase 1; PBMCs = peripheral blood mononuclear cells. | |
|
|
| Absorption | |
| Tmax (h) | 0.48 |
| Effect of high fat meal (relative to fasting): AUClast Ratio* | 1.65 (1.51, 1.81) |
| Distribution | |
| % Bound to human plasma proteins | 80% |
| Source of protein binding data | Ex vivo |
| Blood-to-plasma ratio | 1.0 |
| Metabolism | |
| Metabolism† | CES1 (hepatocytes) Cathepsin A (PBMCs) CYP3A (minimal) |
| Elimination | |
| Major route of elimination | Metabolism (>80% of oral dose) |
| t1/2 (h)‡ | 0.51 |
| % Of dose excreted in urine§ | <1 |
| % Of dose excreted in feces§ | 31.7 |
| Parameter Mean (CV%) | Tenofovir Alafenamide* | Tenofovir* |
|---|---|---|
| CV = coefficient of variation; NA = not applicable | ||
|
||
| Cmax
(mcg per mL) | 0.27 (63.3) | 0.03 (24.6) |
| AUCtau
(mcg∙hour per mL) | 0.27 (47.8) | 0.40 (35.2) |
| Ctrough (mcg per mL) | NA | 0.01 (39.6) |
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Since tenofovir alafenamide is rapidly converted to tenofovir and a lower tenofovir exposure in rats and mice was observed after tenofovir alafenamide administration compared to TDF administration, carcinogenicity studies were conducted only with TDF. Long-term oral carcinogenicity studies of TDF in mice and rats were carried out at exposures up to approximately 10 times (mice) and 4 times (rats) those observed in humans at the 300 mg therapeutic dose of TDF for chronic hepatitis B. The tenofovir exposure in these studies was approximately 151 times (mice) and 50 times (rat) those observed in humans after administration of VEMLIDY treatment. At the high dose in female mice, liver adenomas were increased at tenofovir exposures approximately 151 times those observed after VEMLIDY administration in humans. In rats, the study was negative for carcinogenic findings.
Tenofovir alafenamide was not genotoxic in the reverse mutation bacterial test (Ames test), mouse lymphoma or rat micronucleus assays.
There were no effects on fertility, mating performance or early embryonic development when tenofovir alafenamide was administered to male rats at a dose equivalent to 155 times the human dose based on body surface area comparisons for 28 days prior to mating and to female rats for 14 days prior to mating through Day 7 of gestation.
13.2 Animal Toxicology and/or Pharmacology
Minimal to slight infiltration of mononuclear cells in the posterior uvea was observed in dogs with similar severity after three- and nine-month administration of tenofovir alafenamide; reversibility was seen after a three-month recovery period. At the NOAEL for eye toxicity, the systemic exposure in dogs was 5 (tenofovir alafenamide) and 14 (tenofovir) times the exposure seen in humans at the recommended daily VEMLIDY dosage.
14. Clinical Studies
14.1 Description of Clinical Trials
The efficacy and safety of VEMLIDY were evaluated in the trials summarized in Table 11.
| Trial | Population | Trial Arms (N) | Primary Endpoint (Week) |
|---|---|---|---|
| TE = treatment-experienced, TN = treatment-naive | |||
|
|||
| Trial 108*
(NCT01940341) | HBeAg-negative TN and TE adults with compensated liver disease | VEMLIDY (285) TDF (140) | 48 |
| Trial 110*
(NCT01940471) | HBeAg-positive TN and TE adults with compensated liver disease | VEMLIDY (581) TDF (292) | 48 |
| Trial 4018*
(NCT02979613) | HBeAg-negative and HBeAg-positive virologically suppressed† adults with compensated liver disease | VEMLIDY (243) TDF (245) | 48 |
| Trial 4035 (Part A, Cohort 1)‡
(NCT03180619) | Virologically suppressed† adults with moderate to severe renal impairment§ | VEMLIDY (78) | 24 |
| Trial 4035 (Part A, Cohort 2)‡
(NCT03180619) | Virologically suppressed† adults with ESRD¶ receiving chronic hemodialysis | VEMLIDY (15) | 24 |
| Trial 1092#
(NCT02932150) | Pediatric subjects between the ages of 12 to less than 18 years (at least 35 kg) with compensated liver disease | VEMLIDY (47) Placebo (23) | 24 |
14.2 Clinical Trials in Adults with Chronic Hepatitis B Virus Infection and Compensated Liver Disease
The efficacy and safety of VEMLIDY in the treatment of adults with chronic hepatitis B virus infection with compensated liver disease are based on 48-week data from two randomized, double-blind, active-controlled trials, Trial 108 (N=425) and Trial 110 (N=873). In both trials, besides trial treatment, subjects were not allowed to receive other nucleosides, nucleotides, or interferon.
In Trial 108, HBeAg-negative treatment-naïve and treatment-experienced subjects with compensated liver disease (no evidence of ascites, hepatic encephalopathy, variceal bleeding, INR <1.5× ULN, total bilirubin <2.5× ULN, and albumin >3.0 mg/dL) were randomized in a 2:1 ratio to receive VEMLIDY 25 mg (N=285) once daily or TDF 300 mg (N=140) once daily for 48 weeks. The mean age was 46 years, 61% were male, 72% were Asian, 25% were White, 2% were Black, and 1% were other races. 24%, 38%, and 31% had HBV genotype B, C, and D, respectively. 21% were treatment experienced [previous treatment with oral antivirals, including entecavir (N=41), lamivudine (N=42), TDF (N=21), or other (N=18)]. At baseline, mean plasma HBV DNA was 5.8 log10 IU/mL, mean serum ALT was 94 U/L, and 9% of subjects had a history of cirrhosis.
In Trial 110, HBeAg-positive treatment-naïve and treatment-experienced subjects with compensated liver disease were randomized in a 2:1 ratio to receive VEMLIDY 25 mg (N=581) once daily or TDF 300 mg (N=292) once daily for 48 weeks. The mean age was 38 years, 64% were male, 82% were Asian, 17% were White, and 1% were Black or other races. 17%, 52%, and 23% had HBV genotype B, C, and D, respectively. 26% were treatment experienced [previous treatment with oral antivirals, including adefovir (N=42), entecavir (N=117), lamivudine (N=84), telbivudine (N=25), TDF (N=70), or other (n=17)]. At baseline, mean plasma HBV DNA was 7.6 log10 IU/mL, mean serum ALT was 120 U/L, and 7% of subjects had a history of cirrhosis.
In both trials, randomization was stratified on prior treatment history (nucleoside naïve or experienced) and baseline HBV DNA (<7, ≥7 to <8, and ≥8 log10 IU/mL in Trial 108; and <8 and ≥8 log10 IU/mL in Trial 110). The efficacy endpoint in both trials was the proportion of subjects with plasma HBV DNA levels below 29 IU/mL at Week 48. Additional efficacy endpoints include the proportion of subjects with ALT normalization, HBsAg loss and seroconversion, and HBeAg loss and seroconversion in Trial 110.
Treatment outcomes of Trials 108 and 110 at Week 48 are presented in Table 12 and Table 13.
| Trial 108 (HBeAg-Negative) | Trial 110 (HBeAg-Positive) | |||
|---|---|---|---|---|
| VEMLIDY (N=285) | TDF (N=140) | VEMLIDY (N=581) | TDF (N=292) |
|
|
||||
| HBV DNA <29 IU/mL | 94% | 93% | 64% | 67% |
| Treatment Difference† | 1.8% (95% CI = -3.6% to 7.2%) | -3.6% (95% CI = -9.8% to 2.6%) | ||
| HBV DNA ≥29 IU/mL | 2% | 3% | 31% | 30% |
| Baseline HBV DNA | ||||
| <7 log10 IU/mL | 96% (221/230) | 92% (107/116) | N/A | N/A |
| ≥7 log10 IU/mL | 85% (47/55) | 96% (23/24) | ||
| Baseline HBV DNA | ||||
| <8 log10 IU/mL | N/A | N/A | 82% (254/309) | 82% (123/150) |
| ≥8 log10 IU/mL | 43% (117/272) | 51% (72/142) | ||
| Nucleoside Naïve‡ | 94% (212/225) | 93% (102/110) | 68% (302/444) | 70% (156/223) |
| Nucleoside Experienced | 93% (56/60) | 93% (28/30) | 50% (69/137) | 57% (39/69) |
| No Virologic Data at Week 48§ | 4% | 4% | 5% | 3% |
In Trial 108, the proportion of subjects with cirrhosis who achieved HBV DNA <29 IU/mL at Week 48 was 92% (22/24) in the VEMLIDY group and 93% (13/14) in the TDF group. The corresponding proportions in Trial 110 were 63% (26/41) and 67% (16/24) in the VEMLIDY and TDF groups, respectively.
| Trial 108 (HBeAg-Negative) | Trial 110 (HBeAg-Positive) | |||
|---|---|---|---|---|
| VEMLIDY (N=285) | TDF (N=140) | VEMLIDY (N=581) | TDF (N=292) |
|
| N/A = not applicable | ||||
|
||||
| ALT | ||||
| Normalized ALT (Central Lab)† | 83% | 75% | 72% | 67% |
| Normalized ALT (AASLD)‡ | 50% | 32% | 45% | 36% |
| Serology | ||||
| HBeAg Loss / Seroconversion§ | N/A | N/A | 14% / 10% | 12% / 8% |
| HBsAg Loss / Seroconversion | 0 / 0 | 0 / 0 | 1% / 1% | <1% / 0 |
14.3 Clinical Trials in Virologically Suppressed Adults with Chronic Hepatitis B Virus Infection Who Switched to VEMLIDY
The efficacy and safety of switching from TDF to VEMLIDY in virologically suppressed adults with chronic hepatitis B virus infection is based on 48-week data from a randomized, double-blind, active-controlled trial, Trial 4018 (N=488). Subjects must have been taking TDF 300 mg once daily for at least 12 months, with HBV DNA less than the Lower Limit of Quantitation by local laboratory assessment for at least 12 weeks prior to screening and HBV DNA <20 IU/mL at screening. Subjects were stratified by HBeAg status (HBeAg-positive or HBeAg-negative) and age (≥50 or <50 years) and randomized in a 1:1 ratio to either switch to VEMLIDY 25 mg once daily (N=243) or stay on TDF 300 mg once daily (N=245). The mean age was 51 years (22% were ≥60 years), 71% were male, 82% were Asian, 14% were White, and 68% were HBeAg-negative. At baseline, median duration of prior TDF treatment was 220 and 224 weeks in the VEMLIDY and TDF groups, respectively. At baseline, mean serum ALT was 27 U/L, and 16% of subjects had a history of cirrhosis.
The primary efficacy endpoint was the proportion of subjects with plasma HBV DNA levels ≥20 IU/mL at Week 48. Additional efficacy endpoints in Trial 4018 included the proportion of subjects with HBV DNA <20 IU/mL, ALT normal and normalization, HBsAg loss and seroconversion, and HBeAg loss and seroconversion.
Treatment outcomes of Trial 4018 at Week 48 are presented in Table 14 and Table 15.
| VEMLIDY (N=243) | TDF (N=245) |
|
|---|---|---|
|
||
| HBV DNA ≥20 IU/mL† | <1% | <1% |
| Treatment Difference‡ | 0.0% (95% CI = -1.9% to 2.0%) | |
| HBV DNA <20 IU/mL | 96% | 96% |
| Treatment Difference‡ | 0.0% (95% CI = -3.7% to 3.7%) | |
| No Virologic Data at Week 48 | 3% | 3% |
| Discontinued Study Drug Due to AE or Death and Last Available HBV DNA <20 IU/mL | 1% | 0 |
| Discontinued Study Drug Due to Other Reasons§ and Last Available HBV DNA <20 IU/mL | 2% | 3% |
| VEMLIDY (N=243) | TDF (N=245) |
|
|---|---|---|
|
||
| ALT | ||
| Normal ALT (Central Lab) | 89% | 85% |
| Normal ALT (AASLD) | 79% | 75% |
| Normalized ALT (Central Lab)†,‡ | 50% | 37% |
| Normalized ALT (AASLD)§,¶ | 50% | 26% |
| Serology | ||
| HBeAg Loss / Seroconversion# | 8% / 3% | 6% / 0 |
| HBsAg Loss / Seroconversion | 0 / 0 | 2% / 0 |
14.4 Clinical Trial in Adults with Chronic Hepatitis B Virus Infection and Renal Impairment
In Trial 4035, Part A, the efficacy and safety of switching from TDF and/or other antivirals to VEMLIDY were evaluated in an open-label clinical trial of virologically suppressed chronic hepatitis B infected adults with moderate to severe renal impairment (estimated creatinine clearance between 15 and 59 mL per minute by Cockcroft-Gault method) (Cohort 1, N=78) or ESRD (estimated creatinine clearance below 15 mL per minute by Cockcroft-Gault method) on hemodialysis (Cohort 2, N=15). At baseline, 98% of subjects in Part A had baseline HBV DNA <20 IU/mL. Median age was 65 years, 74% were male, 77% were Asian, 16% were White, and 83% were HBeAg-negative. Previous treatment with oral antivirals included TDF (Cohort 1, N=57; Cohort 2, N=1), lamivudine (N=46), adefovir dipivoxil (N=46), and entecavir (N=43). At baseline, 97% and 95% of subjects had ALT ≤ULN based on central laboratory criteria and 2018 AASLD criteria, respectively; median estimated creatinine clearance by Cockcroft-Gault was 43 mL per minute (45 mL per minute in Cohort 1 and 7 mL per minute in Cohort 2); and 34% of subjects had a history of cirrhosis.
Overall, 98% of subjects achieved HBV DNA <20 IU/mL at Week 24 (Cohort 1, 97%; Cohort 2, 100%). Two subjects in Cohort 1 discontinued treatment early (due to subject decision); last available HBV DNA for both of these subjects was <20 IU/mL. The overall mean (SD) change from baseline in ALT values was +1 (11.3) U/L (Cohort 1, +1 [11.9] U/L; Cohort 2, +3 [7.9] U/L) at Week 24. No subject had HBeAg or HBsAg loss at Week 24. The mean (SD) changes in HBsAg level from baseline were -0.05 (0.122) log10 IU/mL (-0.05 [0.124] log10 IU/mL for Cohort 1 and -0.07 [0.115] log10 IU/mL for Cohort 2) at Week 24.
14.5 Clinical Trial in Pediatric Subjects 12 Years of Age and Older with Chronic Hepatitis B Virus Infection
In Trial 1092, the efficacy and safety of VEMLIDY in chronic HBV-infected subjects were evaluated in a randomized, double-blind, placebo-controlled clinical trial of treatment-naïve and treatment-experienced subjects between the ages of 12 to less than 18 years weighing at least 35 kg (Cohort 1; N=70). Subjects were randomized to receive VEMLIDY (N=47) or placebo (N=23) once daily. Baseline demographics and HBV disease characteristics were comparable between the VEMLIDY treatment arm and the placebo arm: in the VEMLIDY group, 57% were male, 70% were Asian, and 19% were White; 9%, 23%, 26%, and 40% had HBV genotype A, B, C, and D, respectively. Ninety-eight percent were HBeAg positive. At baseline, median HBV DNA was 8.1 log10 IU/mL, mean ALT was 112 U/L, median HBsAg was 4.5 log10 IU/mL. Previous treatment included oral antivirals (N=12 [26%]), including entecavir (N=7 [15%]), lamivudine (N=6 [13%]), and TDF (N=1 [2%]), and/or interferons (N=7 [15%]).
Overall, 21% (10/47) of subjects in Cohort 1 treated with VEMLIDY achieved HBV DNA <20 IU/mL at Week 24 versus 0% (0/23) of subjects in Cohort 1 treated with placebo. At Week 24, the overall mean (SD) change from baseline in HBV DNA for the Cohort 1 VEMLIDY and placebo groups, respectively, was -5.04 (1.544) log10 IU/mL and -0.13 (0.689) log10 IU/mL. The overall median (Q1, Q3) change from baseline in ALT values for the Cohort 1 VEMLIDY and placebo groups, respectively, was -32.0 U/L (-63.0, -13.0 U/L) and 1.0 U/L (-10.0, 25.0 U/mL) at Week 24. ALT normalization (AASLD criteria) was achieved for 44% of VEMLIDY-treated subjects and 0% of placebo-treated subjects in Cohort 1. At Week 24 in Cohort 1, 3/46 (7%) of VEMLIDY-treated subjects and 1/23 (4%) placebo-treated subject experienced HBeAg loss, all of whom experienced anti-HBe seroconversion. No subject had HBsAg loss or seroconversion at Week 24.
16. How is Vemlidy supplied
VEMLIDY tablets containing 25 mg of tenofovir alafenamide are yellow, round, film-coated, debossed with "GSI" on one side and "25" on the other side. Each bottle contains 30 tablets (NDC 61958-2301-1), a silica gel desiccant, polyester coil, and is closed with a child-resistant closure.
17. Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Patient Information).
| This Patient Information has been approved by the U.S. Food and Drug Administration. | Revised: 10/2022 |
| Patient Information
VEMLIDY® (VEM-lih-dee) (tenofovir alafenamide) tablets |
|
| What is the most important information I should know about VEMLIDY?
VEMLIDY can cause serious side effects, including:
|
|
| What is VEMLIDY?
VEMLIDY is a prescription medicine used to treat chronic (long-lasting) hepatitis B virus (HBV) in adults and children 12 years of age and older with stable (compensated) liver disease.
|
|
| What should I tell my healthcare provider before taking VEMLIDY? Before taking VEMLIDY, tell your healthcare provider about all of your medical conditions, including if you:
Some medicines may affect how VEMLIDY works.
|
|
How should I take VEMLIDY?
|
|
| What are the possible side effects of VEMLIDY? VEMLIDY may cause serious side effects, including:
These are not all the possible side effects of VEMLIDY. For more information, ask your healthcare provider or pharmacist. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
|
How should I store VEMLIDY?
|
|
| General information about the safe and effective use of VEMLIDY.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use VEMLIDY for a condition for which it was not prescribed. Do not give VEMLIDY to other people, even if they have the same symptoms you have. It may harm them. If you would like more information, talk with your healthcare provider. You can ask your healthcare provider or pharmacist for information about VEMLIDY that is written for health professionals. |
|
| What are the ingredients in VEMLIDY? Active ingredients: tenofovir alafenamide Inactive ingredients: croscarmellose sodium, lactose monohydrate, magnesium stearate, and microcrystalline cellulose. The tablets are film-coated with a coating material containing: iron oxide yellow, polyethylene glycol, polyvinyl alcohol, talc, and titanium dioxide. Manufactured and distributed by: Gilead Sciences, Inc. Foster City, CA 94404 VEMLIDY is a registered trademark of Gilead Sciences, Inc., or its related companies. © 2022 Gilead Sciences, Inc. All rights reserved. 208464-GS-008 For more information, call 1-800-445-3235 or go to www.VEMLIDY.com. |
|
| VEMLIDY
tenofovir alafenamide tablet |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - Gilead Sciences, Inc. (185049848) |