Drug Detail:Vicodin (Acetaminophen and hydrocodone [ a-seet-a-min-oh-fen-and-hye-droe-koe-done ])
Drug Class: Narcotic analgesic combinations
Vicodin Description
Hydrocodone bitartrate and acetaminophen is supplied in tablet form for oral administration.
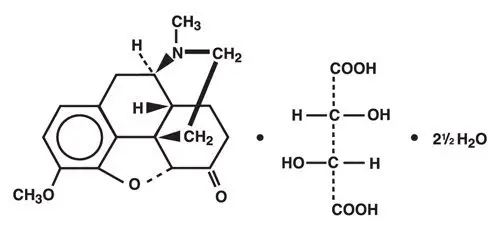 C18H21NO3·C4H6O6·2½H2O M.W. = 494.490
C18H21NO3·C4H6O6·2½H2O M.W. = 494.490
Hydrocodone Bitartrate and Acetaminophen Tablets, USP is available in the following strengths:
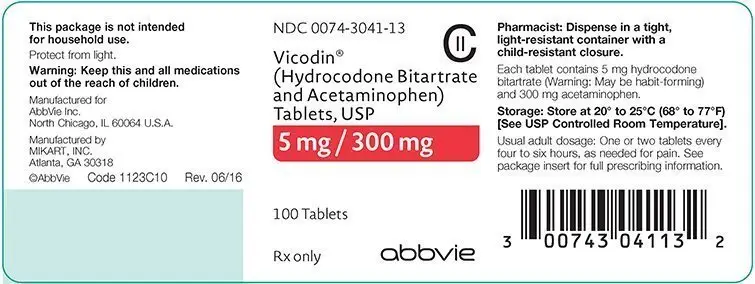
VICODIN®: Hydrocodone Bitartrate..... 5 mg
WARNING: May be habit-forming.
VICODIN ES®: Hydrocodone Bitartrate..... 7.5 mg
WARNING: May be habit-forming.
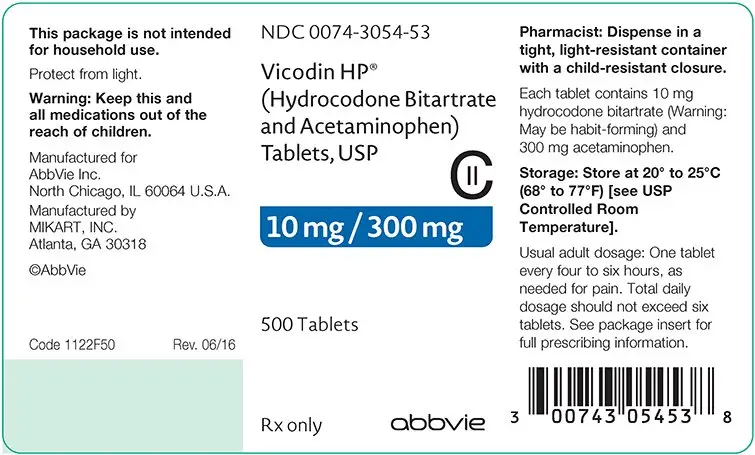
VICODIN HP®: Hydrocodone Bitartrate..... 10 mg
Related/similar drugs
aspirin, acetaminophen, tramadol, ibuprofen, duloxetine, naproxen, oxycodoneVicodin - Clinical Pharmacology
Contraindications
Patients known to be hypersensitive to other opioids may exhibit cross sensitivity to hydrocodone.
Warnings
Precautions
Information for Patients/Caregivers
- Do not take hydrocodone bitartrate and acetaminophen tablets if you are allergic to any of its ingredients.
- If you develop signs of allergy such as a rash or difficulty breathing stop taking hydrocodone bitartrate and acetaminophen tablets and contact your healthcare provider immediately.
- Do not take more than 4000 milligrams of acetaminophen per day. Call your doctor if you took more than the recommended dose.
Drug/Laboratory Test Interactions
Acetaminophen may produce false-positive test results for urinary 5-hydroxyindoleacetic acid.
Adverse Reactions/Side Effects
Other adverse reactions include:
Genitourinary System
Ureteral spasm, spasm of vesical sphincters and urinary retention have been reported with opiates.
Drug Abuse and Dependence
Controlled Substance
Hydrocone bitartrate and acetaminophen tablets is classified as a Schedule II controlled substance.
Overdosage
Following an acute overdosage, toxicity may result from hydrocodone or acetaminophen.
How is Vicodin supplied
Bottles of 100 - NDC 0074-3041-13
Bottles of 500 - NDC 0074-3041-53
Bottles of 100 - NDC 0074-3043-13
Bottles of 500 - NDC 0074-3043-53
How is Vicodin supplied
Store at 20° to 25°C (68° to 77°F). [See USP Controlled Room Temperature].
PHARMACIST: Dispense in a tight, light-resistant container with a child-resistant closure.
| VICODIN HP
hydrocodone bitartrate and acetaminophen tablet |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| VICODIN ES
hydrocodone bitartrate and acetaminophen tablet |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| VICODIN
hydrocodone bitartrate and acetaminophen tablet |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - AbbVie Inc. (078458370) |




 C
C