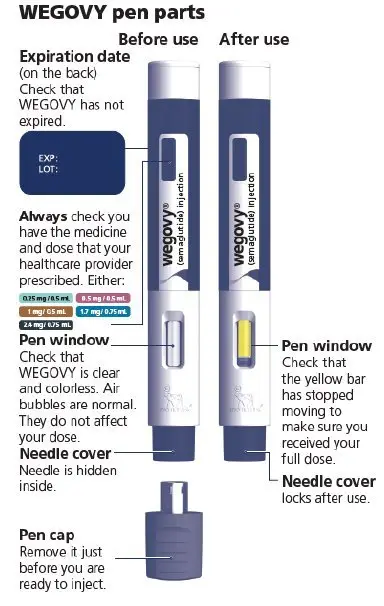
Drug Detail:Victoza (Liraglutide [ lir-a-gloo-tide ])
Drug Class: Incretin mimetics
Highlights of Prescribing Information
VICTOZA® (liraglutide) injection, for subcutaneous use
Initial U.S. Approval: 2010
WARNING: RISK OF THYROID C-CELL TUMORS
See full prescribing information for complete boxed warning.
- •
- Liraglutide causes thyroid C-cell tumors at clinically relevant exposures in both genders of rats and mice. It is unknown whether VICTOZA causes thyroid C-cell tumors, including medullary thyroid carcinoma (MTC), in humans, as the human relevance of liraglutide-induced rodent thyroid C-cell tumors has not been determined (5.1, 13.1).
- •
- VICTOZA is contraindicated in patients with a personal or family history of MTC or in patients with Multiple Endocrine Neoplasia syndrome type 2 (MEN 2). Counsel patients regarding the potential risk of MTC and the symptoms of thyroid tumors (4, 5.1).
Indications and Usage for Victoza
VICTOZA is a glucagon-like peptide-1 (GLP-1) receptor agonist indicated:
- •
- as an adjunct to diet and exercise to improve glycemic control in adults and pediatric patients aged 10 years and older with type 2 diabetes mellitus (1).
- •
- to reduce the risk of major adverse cardiovascular events in adults with type 2 diabetes mellitus and established cardiovascular disease (1).
Limitations of Use:
- •
- Not for treatment of type 1 diabetes mellitus.
- •
- Should not be coadministered with other liraglutide-containing products.
Victoza Dosage and Administration
-
- •
- Adult Patients: Initiate at 0.6 mg injected subcutaneously once daily for one week then increase to 1.2 mg daily. If additional glycemic control is required, increase the dose to 1.8 mg daily after one week of treatment with the 1.2 mg daily dose (2.1).
- •
- Pediatric Patients: Initiate at 0.6 mg injected subcutaneously once daily for at least one week. If additional glycemic control is required increase the dose to 1.2 mg daily and if additional glycemic control is still required, increase the dose to 1.8 mg daily after at least one week of treatment with the 1.2 mg daily dose (2.1).
- •
- Inspect visually prior to each injection. Only use if solution is clear, colorless, and contains no particles (2.3).
- •
- Inject VICTOZA subcutaneously once-daily at any time of day, independently of meals, in the abdomen, thigh or upper arm (2.3).
- •
- When using VICTOZA with insulin, administer as separate injections. Never mix. (2.3).
Dosage Forms and Strengths
Injection: 6 mg/mL solution in a pre-filled, single-patient-use pen that delivers doses of 0.6 mg, 1.2 mg, or 1.8 mg (3).
Contraindications
- •
- Patients with a personal or family history of medullary thyroid carcinoma or in patients with Multiple Endocrine Neoplasia syndrome type 2 (4).
- •
- Patients with a serious hypersensitivity reaction to liraglutide or any of the excipients in VICTOZA (4).
Warnings and Precautions
- •
- Pancreatitis: Postmarketing reports, including fatal and non-fatal hemorrhagic or necrotizing pancreatitis. Discontinue promptly if pancreatitis is suspected. Do not restart if pancreatitis is confirmed (5.2).
- •
- Never share a VICTOZA pen between patients, even if the needle is changed (5.3).
- •
- Hypoglycemia: Adult patients taking an insulin secretagogue or insulin may have an increased risk of hypoglycemia, including severe hypoglycemia. In pediatric patients 10 years of age and older, the risk of hypoglycemia was higher with VICTOZA regardless of insulin and/or metformin use. Reduction in the dose of insulin secretagogues or insulin may be necessary (5.4).
- •
- Acute Kidney Injury: Postmarketing, usually in association with nausea, vomiting, diarrhea, or dehydration which may sometimes require hemodialysis. Use caution when initiating or escalating doses of VICTOZA in patients with renal impairment (5.5).
- •
- Hypersensitivity Reactions: Postmarketing reports of serious hypersensitivity reactions (e.g., anaphylactic reactions and angioedema). Discontinue VICTOZA and promptly seek medical advice (5.6).
- •
- Acute Gallbladder Disease: If cholelithiasis or cholecystitis are suspected, gallbladder studies are indicated (5.7).
Adverse Reactions/Side Effects
- •
- Most common adverse reactions (incidence ≥5%) in clinical trials are nausea, diarrhea, vomiting, decreased appetite, dyspepsia, constipation (6.1).
- •
- Immunogenicity-related events, including urticaria, were more common among VICTOZA-treated patients (0.8%) than among comparator-treated patients (0.4%) in clinical trials (12.6).
To report SUSPECTED ADVERSE REACTIONS, contact Novo Nordisk Inc. at 1-877-484-2869 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
- •
- Effects of delayed gastric emptying on oral medications:VICTOZA delays gastric emptying and may impact absorption of concomitantly administered oral medications (7).
Use In Specific Populations
- •
- Pregnancy: VICTOZA should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus (8.1).
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 7/2023
Related/similar drugs
metformin, Ozempic, Xarelto, Jardiance, simvastatin, Trulicity, LantusFull Prescribing Information
WARNING: RISK OF THYROID C-CELL TUMORS
- •
- Liraglutide causes dose-dependent and treatment-duration-dependent thyroid C-cell tumors at clinically relevant exposures in both genders of rats and mice. It is unknown whether VICTOZA causes thyroid C-cell tumors, including medullary thyroid carcinoma (MTC), in humans, as the human relevance of liraglutide-induced rodent thyroid C-cell tumors has not been determined [see Warnings and Precautions (5.1) and Nonclinical Toxicology (13.1)].
- •
- VICTOZA is contraindicated in patients with a personal or family history of MTC and in patients with Multiple Endocrine Neoplasia syndrome type 2 (MEN 2). Counsel patients regarding the potential risk for MTC with the use of VICTOZA and inform them of symptoms of thyroid tumors (e.g., a mass in the neck, dysphagia, dyspnea, persistent hoarseness). Routine monitoring of serum calcitonin or using thyroid ultrasound is of uncertain value for early detection of MTC in patients treated with VICTOZA [see Contraindications (4) and Warnings and Precautions (5.1)].
1. Indications and Usage for Victoza
VICTOZA is indicated:
- •
- as an adjunct to diet and exercise to improve glycemic control in adults and pediatric patients aged 10 years and older with type 2 diabetes mellitus,
- •
- to reduce the risk of major adverse cardiovascular events (cardiovascular death, non-fatal myocardial
infarction, or non-fatal stroke) in adults with type 2 diabetes mellitus and established cardiovascular disease.
Limitations of Use:
VICTOZA should not be used in patients with type 1 diabetes mellitus.
VICTOZA contains liraglutide and should not be coadministered with other liraglutide-containing products.
2. Victoza Dosage and Administration
2.1 Recommended Dosage
Adult Patients
- •
- The recommended starting dosage of VICTOZA is 0.6 mg injected subcutaneously once daily for one week. The 0.6 mg once daily dosage is intended to reduce gastrointestinal symptoms [see Adverse Reactions (6.1)] during initial titration and is not effective for glycemic control in adults.
- •
- After one week at the 0.6 mg once daily dosage, increase the dosage to 1.2 mg injected subcutaneously once daily.
- •
- If additional glycemic control is required, increase the dosage to the maximum recommended dosage of 1.8 mg injected subcutaneously once daily after at least one week of treatment with the 1.2 mg once daily dosage.
Pediatric Patients Aged 10 Years and Older
- •
- The recommended starting dosage of VICTOZA is 0.6 mg injected subcutaneously once daily.
- •
- If additional glycemic control is required, increase the dosage in 0.6 mg increments after at least one week on the current dosage.
- •
- The maximum recommended dosage is 1.8 mg injected subcutaneously once daily.
2.2 Recommendations Regarding Missed Dose
- •
- Instruct patients who miss a dose of VICTOZA to resume the once -daily dosage regimen as prescribed with the next scheduled dose. Do not administer an extra dose or increase the dose to make up for the missed dose.
- •
- If more than 3 days have elapsed since the last VICTOZA dose, reinitiate VICTOZA at 0.6 mg once daily to mitigate any gastrointestinal symptoms associated with reinitiation of treatment. Upon reinitiation, VICTOZA should be titrated at the discretion of the healthcare provider.
2.3 Important Administration Instructions
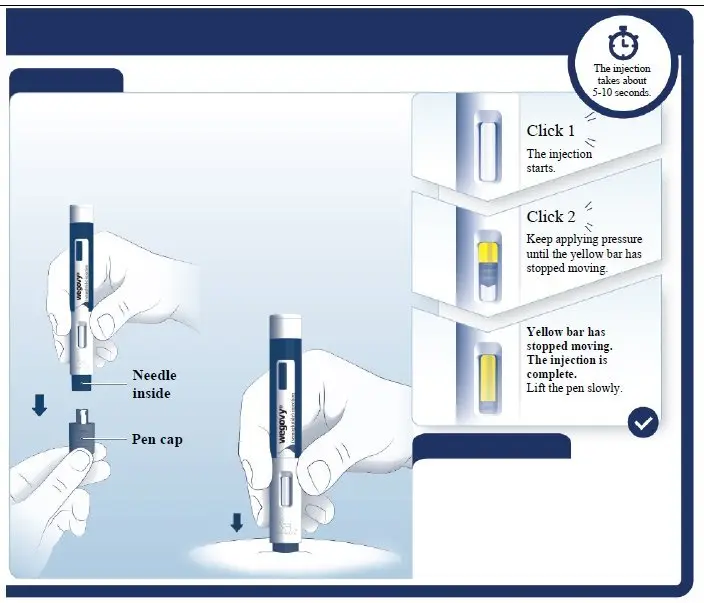
- •
- Inspect visually prior to each injection. Only use if solution is clear, colorless, and contains no particles.
- •
- Inject VICTOZA subcutaneously once daily at any time of day, independently of meals.
- •
- Inject VICTOZA subcutaneously in the abdomen, thigh or upper arm. No dosage adjustment is needed if changing the injection site and/or timing.
- •
- Rotate injection sites within the same region in order to reduce the risk of cutaneous amyloidosis [see Adverse Reactions (6.2)].
- •
- When using VICTOZA with insulin, administer as separate injections. Never mix. It is acceptable to inject VICTOZA and insulin in the same body region but the injections should not be adjacent to each other.
3. Dosage Forms and Strengths
Injection: 18 mg/3 mL (6 mg/mL) clear, colorless solution in a pre-filled, single-patient-use pen that delivers doses of 0.6 mg, 1.2 mg, or 1.8 mg.
4. Contraindications
VICTOZA is contraindicated in patients with a:
- •
- personal or family history of medullary thyroid carcinoma (MTC) or in patients with Multiple Endocrine Neoplasia syndrome type 2 (MEN 2) [see Warnings and Precautions (5.1)].
- •
- serious hypersensitivity reaction to liraglutide or to any of the excipients in VICTOZA. Serious hypersensitivity reactions including anaphylactic reactions and angioedema have been reported with VICTOZA [see Warnings and Precautions (5.6)].
5. Warnings and Precautions
5.1 Risk of Thyroid C-cell Tumors
Liraglutide causes dose-dependent and treatment-duration-dependent thyroid C-cell tumors (adenomas and/or carcinomas) at clinically relevant exposures in both genders of rats and mice [see Nonclinical Toxicology (13.1)]. Malignant thyroid C-cell carcinomas were detected in rats and mice. It is unknown whether VICTOZA will cause thyroid C-cell tumors, including medullary thyroid carcinoma (MTC), in humans, as the human relevance of liraglutide-induced rodent thyroid C-cell tumors has not been determined.
Cases of MTC in patients treated with VICTOZA have been reported in the postmarketing period; the data in these reports are insufficient to establish or exclude a causal relationship between MTC and VICTOZA use in humans.
VICTOZA is contraindicated in patients with a personal or family history of MTC or in patients with MEN 2. Counsel patients regarding the potential risk for MTC with the use of VICTOZA and inform them of symptoms of thyroid tumors (e.g., a mass in the neck, dysphagia, dyspnea, persistent hoarseness).
Routine monitoring of serum calcitonin or using thyroid ultrasound is of uncertain value for early detection of MTC in patients treated with VICTOZA. Such monitoring may increase the risk of unnecessary procedures, due to low test specificity for serum calcitonin and a high background incidence of thyroid disease. Significantly elevated serum calcitonin may indicate MTC and patients with MTC usually have calcitonin values >50 ng/L. If serum calcitonin is measured and found to be elevated, the patient should be further evaluated. Patients with thyroid nodules noted on physical examination or neck imaging should also be further evaluated.
5.2 Pancreatitis
Based on spontaneous postmarketing reports, acute pancreatitis, including fatal and non-fatal hemorrhagic or necrotizing pancreatitis, has been observed in patients treated with VICTOZA. After initiation of VICTOZA, observe patients carefully for signs and symptoms of pancreatitis (including persistent severe abdominal pain, sometimes radiating to the back and which may or may not be accompanied by vomiting). If pancreatitis is suspected, VICTOZA should promptly be discontinued and appropriate management should be initiated. If pancreatitis is confirmed, VICTOZA should not be restarted.
In glycemic control trials of VICTOZA, there have been 13 cases of pancreatitis among VICTOZA-treated patients and 1 case in a comparator (glimepiride) treated patient (2.7 vs. 0.5 cases per 1000 patient-years). Nine of the 13 cases with VICTOZA were reported as acute pancreatitis and four were reported as chronic pancreatitis. In one case in a VICTOZA-treated patient, pancreatitis, with necrosis, was observed and led to death; however clinical causality could not be established. Some patients had other risk factors for pancreatitis, such as a history of cholelithiasis or alcohol abuse.
VICTOZA has been studied in a limited number of patients with a history of pancreatitis. It is unknown if patients with a history of pancreatitis are at higher risk for development of pancreatitis on VICTOZA.
5.3 Never Share a VICTOZA Pen Between Patients
VICTOZA pens must never be shared between patients, even if the needle is changed. Pen-sharing poses a risk for transmission of blood-borne pathogens.
5.4 Hypoglycemia
Adult patients receiving VICTOZA in combination with an insulin secretagogue (e.g., sulfonylurea) or insulin may have an increased risk of hypoglycemia, including severe hypoglycemia. In pediatric patients 10 years of age and older, the risk of hypoglycemia was higher with VICTOZA regardless of insulin and/or metformin use. [see Adverse Reactions (6.1), Drug Interactions (7.2)].
The risk of hypoglycemia may be lowered by a reduction in the dose of sulfonylurea (or other concomitantly administered insulin secretagogues) or insulin. Inform patients using these concomitant medications and pediatric patients of the risk of hypoglycemia and educate them on the signs and symptoms of hypoglycemia.
5.5 Acute Kidney Injury
VICTOZA has not been found to be directly nephrotoxic in animal studies or clinical trials.
There have been postmarketing reports of acute renal failure and worsening of chronic renal failure, which may sometimes require hemodialysis in VICTOZA-treated patients [see Adverse Reactions (6.2)]. Some of these events were reported in patients without known underlying renal disease. A majority of the reported events occurred in patients who had experienced nausea, vomiting, diarrhea, or dehydration [see Adverse Reactions (6.1)]. Some of the reported events occurred in patients receiving one or more medications known to affect renal function or hydration status. Altered renal function has been reversed in many of the reported cases with supportive treatment and discontinuation of potentially causative agents, including VICTOZA. Use caution when initiating or escalating doses of VICTOZA in patients with renal impairment [see Use in Specific Populations (8.6)].
5.6 Hypersensitivity Reactions
There have been postmarketing reports of serious hypersensitivity reactions (e.g., anaphylactic reactions and angioedema) in patients treated with VICTOZA [see Adverse Reactions (6.2)]. If a hypersensitivity reaction occurs, discontinue VICTOZA; treat promptly per standard of care, and monitor until signs and symptoms resolve.
Anaphylaxis and angioedema have been reported with other GLP-1 receptor agonists. Use caution in a patient with a history of anaphylaxis or angioedema with another GLP-receptor agonist because it is unknown whether such patients will be predisposed to these reactions with VICTOZA. VICTOZA is contraindicated in patients who have had a serious hypersensitivity reaction to liraglutide or any of the excipients in VICTOZA [see Contraindications (4)].
5.7 Acute Gallbladder Disease
Acute events of gallbladder disease such as cholelithiasis or cholecystitis have been reported in GLP-1 receptor agonist trials and postmarketing. In the LEADER trial [see Clinical Studies (14.3)], 3.1% of VICTOZA-treated patients versus 1.9% of placebo-treated patients reported an acute event of gallbladder disease, such as cholelithiasis or cholecystitis
[seeAdverse Reactions (6.1)]. If cholelithiasis is suspected, gallbladder studies and appropriate clinical follow-up are indicated.
6. Adverse Reactions/Side Effects
The following serious adverse reactions are described below or elsewhere in the prescribing information:
- •
- Risk of Thyroid C-cell Tumors [see Warnings and Precautions (5.1)]
- •
- Pancreatitis [see Warnings and Precautions (5.2)]
- •
- Hypoglycemia [see Warnings and Precautions (5.4)]
- •
- Acute Kidney Injury [see Warnings and Precautions (5.5)]
- •
- Hypersensitivity Reactions [see Warnings and Precautions (5.6)]
- •
- Acute Gallbladder Disease [see Warnings and Precautions (5.7)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Common Adverse Reactions
The safety of VICTOZA in patients with type 2 diabetes mellitus was evaluated in 5 glycemic control, placebo-controlled trials in adults and one trial of 52 weeks duration in pediatric patients 10 years of age and older [see Clinical Studies (14.1)]. The data in Table 1 reflect exposure of 1,673 adult patients to VICTOZA and a mean duration of exposure to VICTOZA of 37.3 weeks. The mean age of adult patients was 58 years, 4% were 75 years or older and 54% were male. The population was 79% White, 6% Black or African American, 13% Asian; 4% were of Hispanic or Latino ethnicity. At baseline the population had diabetes for an average of 9 years and a mean HbA1c of 8.4%. Baseline estimated renal function was normal or mildly impaired in 88% and moderately impaired in 12% of the pooled population.
Table 1 shows common adverse reactions in adults, excluding hypoglycemia, associated with the use of VICTOZA for the treatment of type 2 diabetes mellitus. These adverse reactions occurred more commonly on VICTOZA than on placebo and occurred in at least 5% of patients treated with VICTOZA. Overall, the type, and severity of adverse reactions in pediatric patients 10 years of age and older and above were comparable to that observed in the adult population.
Table 1 Adverse reactions reported in ≥ 5% of Adult Patients Treated with VICTOZA for Type 2 Diabetes Mellitus
|
Placebo N=661 |
Liraglutide 1.2 mg N= 645 |
Liraglutide 1.8 mg N= 1024 |
|
|
Adverse Reaction |
(%) |
(%) |
(%) |
|
Nausea |
5 |
18 |
20 |
|
Diarrhea |
4 |
10 |
12 |
|
Headache |
7 |
11 |
10 |
|
Nasopharyngitis |
8 |
9 |
10 |
|
Vomiting |
2 |
6 |
9 |
|
Decreased appetite |
1 |
10 |
9 |
|
Dyspepsia |
1 |
4 |
7 |
|
Upper Respiratory Tract Infection |
6 |
7 |
6 |
|
Constipation |
1 |
5 |
5 |
|
Back Pain |
3 |
4 |
5 |
Cumulative proportions were calculated combining studies using Cochran-Mantel-Haenszel weights.
In an analysis of placebo- and active-controlled trials, the types and frequency of common adverse reactions, excluding hypoglycemia, were similar to those listed in Table 1.
Other Adverse Reactions
Gastrointestinal Adverse Reactions
In the pool of 5 glycemic control, placebo-controlled adult clinical trials, withdrawals due to gastrointestinal adverse reactions, occurred in 4.3% of VICTOZA-treated patients and 0.5% of placebo-treated patients. Withdrawal due to gastrointestinal adverse events mainly occurred during the first 2-3 months of the trials.
Injection site reactions
Injection site reactions (e.g., injection site rash, erythema) were reported in approximately 2% of VICTOZA-treated adult patients in the five double-blind, glycemic control trials of at least 26 weeks duration. Less than 0.2% of VICTOZA-treated patients discontinued due to injection site reactions.
Hypoglycemia
In 5 adult glycemic control, placebo-controlled clinical trials of at least 26 weeks duration, hypoglycemia requiring the assistance of another person for treatment occurred in 8 VICTOZA-treated patients (7.5 events per 1000 patient-years). Of these 8 VICTOZA-treated patients, 7 patients were concomitantly using a sulfonylurea.
|
|
|
|
Add-on to Metformin |
Placebo + Metformin (N = 121) |
VICTOZA + Metformin
|
|
Patient not able to self-treat |
|
|
|
Patient able to self-treat |
|
|
|
Add-on to Glimepiride |
|
|
|
Patient not able to self-treat |
|
|
|
Patient able to self-treat |
|
|
|
Not classified |
|
|
|
Add-on to Metformin + Rosiglitazone |
(N = 175) |
VICTOZA + Metformin + Rosiglitazone
|
|
Patient not able to self-treat |
|
|
|
Patient able to self-treat |
|
|
|
Not classified |
|
|
|
Add-on to Metformin + Glimepiride |
(N = 114) |
VICTOZA + Metformin + Glimepiride
|
|
Patient not able to self-treat |
|
|
|
Patient able to self-treat |
|
|
|
Not classified |
|
|
“Patient not able to self-treat” is defined as an event requiring the assistance of another person for treatment.
In a 26-week placebo-controlled clinical trial in pediatric patients 10 years of age and older with a 26-week open-label extension, 21.2% of VICTOZA-treated patients (mean age 14.6 years) with type 2 diabetes mellitus, had hypoglycemia with a blood glucose <54 mg/dL with or without symptoms (335 events per 1000 patient years). No severe hypoglycemic episodes occurred in the VICTOZA treatment group (severe hypoglycemia was defined as an episode requiring assistance of another person to actively administer carbohydrate, glucagon, or other resuscitative actions).
Papillary thyroid carcinoma
In adult glycemic control trials of VICTOZA, there were 7 reported cases of papillary thyroid carcinoma in patients treated with VICTOZA and 1 case in a comparator-treated patient (1.5 vs. 0.5 cases per 1000 patient-years). Most of these papillary thyroid carcinomas were <1 cm in greatest diameter and were diagnosed in surgical pathology specimens after thyroidectomy prompted by findings on protocol-specified screening with serum calcitonin or thyroid ultrasound.
Cholelithiasis and cholecystitis
In adult glycemic control trials of VICTOZA, the incidence of cholelithiasis was 0.3% in both VICTOZA-treated and placebo-treated patients. The incidence of cholecystitis was 0.2% in both VICTOZA-treated and placebo-treated patients.
In the LEADER trial [see Clinical Studies (14.3)], the incidence of cholelithiasis was 1.5% (3.9 cases per 1000 patient years of observation) in adult VICTOZA-treated and 1.1% (2.8 cases per 1000 patient years of observation) in placebo-treated patients, both on a background of standard of care. The incidence of acute cholecystitis was 1.1% (2.9 cases per 1000 patient years of observation) in adult VICTOZA-treated and 0.7% (1.9 cases per 1000 patient years of observation) in placebo-treated patients. The majority of events required hospitalization or cholecystectomy.
Laboratory Tests
Bilirubin
In the five adult glycemic control trials of at least 26 weeks duration, mildly elevated serum bilirubin concentrations (elevations to no more than twice the upper limit of the reference range) occurred in 4.0% of VICTOZA-treated patients, 2.1% of placebo-treated patients and 3.5% of active-comparator-treated patients. This finding was not accompanied by abnormalities in other liver tests. The significance of this isolated finding is unknown.
Calcitonin
Calcitonin, a biological marker of MTC, was measured throughout the clinical development program. At the end of the adult glycemic control trials, adjusted mean serum calcitonin concentrations were higher in VICTOZA-treated patients compared to placebo-treated patients but not compared to patients receiving active comparator. Between group differences in adjusted mean serum calcitonin values were approximately 0.1 ng/L or less. Among adult patients with pretreatment calcitonin <20 ng/L, calcitonin elevations to >20 ng/L occurred in 0.7% of VICTOZA-treated patients, 0.3% of placebo-treated patients, and 0.5% of active-comparator-treated patients. The clinical significance of these findings is unknown.
Lipase and Amylase
In one adult glycemic control trial in renal impairment patients, a mean increase of 33% for lipase and 15% for amylase from baseline was observed for VICTOZA-treated patients while placebo-treated patients had a mean decrease in lipase of 3% and a mean increase in amylase of 1%.
In the LEADER trial, serum lipase and amylase were routinely measured. Among adult VICTOZA-treated patients, 7.9% had a lipase value at any time during treatment of greater than or equal to 3 times the upper limit of normal compared with 4.5% of placebo-treated patients, and 1% of VICTOZA-treated patients had an amylase value at any time during treatment of greater than or equal to 3 times the upper limit of normal versus 0.7% of placebo-treated patients.
The clinical significance of elevations in lipase or amylase with VICTOZA is unknown in the absence of other signs and symptoms of pancreatitis [see Warnings and Precautions (5.2)].
Vital signs
VICTOZA did not have adverse effects on blood pressure. Mean increases from baseline in heart rate of 2 to 3 beats per minute have been observed in adult patients treated with VICTOZA compared to placebo.
6.2 Postmarketing Experience
The following additional adverse reactions have been reported during post-approval use of VICTOZA. Because these events are reported voluntarily from a population of uncertain size, it is generally not possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- •
- Gastrointestinal: Acute pancreatitis, hemorrhagic and necrotizing pancreatitis sometimes resulting in death, ileus
- •
- General Disorders and Administration Site Conditions: Allergic reactions: rash and pruritus
- •
- Hepatobiliary: Elevations of liver enzymes, hyperbilirubinemia, cholestasis, cholecystitis, cholelithiasis requiring cholecystectomy, hepatitis
- •
- Immune system: Angioedema and anaphylactic reactions
- •
- Metabolism and nutrition: Dehydration resulting from nausea, vomiting and diarrhea
- •
- Neoplasms: Medullary thyroid carcinoma
- •
- Nervous system: Dysgeusia, dizziness
- •
- Renal and urinary: Increased serum creatinine, acute renal failure or worsening of chronic renal failure, sometimes requiring hemodialysis.
- •
- Skin and subcutaneous tissue: Cutaneous amyloidosis
7. Drug Interactions
7.1 Effects of Delayed Gastric Emptying on Oral Medications
VICTOZA causes a delay of gastric emptying, and thereby has the potential to impact the absorption of concomitantly administered oral medications. In clinical pharmacology trials, VICTOZA did not affect the absorption of the tested orally administered medications to any clinically relevant degree [see Clinical Pharmacology (12.3)]. Nonetheless, caution should be exercised when oral medications are concomitantly administered with VICTOZA.
7.2 Concomitant Use with an Insulin Secretagogue (e.g., Sulfonylurea) or with Insulin
VICTOZA stimulates insulin release in the presence of elevated blood glucose concentrations. Patients receiving VICTOZA in combination with an insulin secretagogue (e.g., sulfonylurea) or insulin may have an increased risk of hypoglycemia, including severe hypoglycemia. When initiating VICTOZA, consider reducing the dose of concomitantly administered insulin secretagogues (such as sulfonylureas) or insulin to reduce the risk of hypoglycemia [see Warnings and Precautions (5.4) and Adverse Reactions (6.1)].
8. Use In Specific Populations
8.1 Pregnancy
Risk Summary
Based on animal reproduction studies, there may be risks to the fetus from exposure to VICTOZA during pregnancy. VICTOZA should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Animal reproduction studies identified increased adverse developmental outcomes from exposure during pregnancy. Liraglutide exposure was associated with early embryonic deaths and an imbalance in some fetal abnormalities in pregnant rats administered liraglutide during organogenesis at doses that approximate clinical exposures at the maximum recommended human dose (MRHD) of 1.8 mg/day. In pregnant rabbits administered liraglutide during organogenesis, decreased fetal weight and an increased incidence of major fetal abnormalities were seen at exposures below the human exposures at the MRHD [see Animal Data].
The estimated background risk of major birth defects for women with uncontrolled pre-gestational diabetes (Hemoglobin A1C >7) is 6 to 10%. The major birth defect rate has been reported to be as high as 20 to 25% in women with a Hemoglobin A1C >10. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Clinical Considerations
Disease-associated maternal and/or embryo/fetal risk
Poorly controlled diabetes in pregnancy increases the maternal risk for diabetic ketoacidosis, pre-eclampsia, spontaneous abortions, preterm delivery, and delivery complications. Poorly controlled diabetes increases the fetal risk for major birth defects, still birth, and macrosomia related morbidity.
Animal Data
Female rats given subcutaneous doses of 0.1, 0.25 and 1.0 mg/kg/day liraglutide beginning 2 weeks before mating through gestation day 17 had estimated systemic exposures 0.8-, 3-, and 11-times the human exposure at the MRHD based on plasma AUC comparison. The number of early embryonic deaths in the 1 mg/kg/day group increased slightly. Fetal abnormalities and variations in kidneys and blood vessels, irregular ossification of the skull, and a more complete state of ossification occurred at all doses. Mottled liver and minimally kinked ribs occurred at the highest dose. The incidence of fetal malformations in liraglutide-treated groups exceeding concurrent and historical controls were misshapen oropharynx and/or narrowed opening into larynx at 0.1 mg/kg/day and umbilical hernia at 0.1 and 0.25 mg/kg/day.
Pregnant rabbits given subcutaneous doses of 0.01, 0.025 and 0.05 mg/kg/day liraglutide from gestation day 6 through day 18 inclusive, had estimated systemic exposures less than the human exposure at the MRHD of 1.8 mg/day at all doses, based on plasma AUC. Liraglutide decreased fetal weight and dose-dependently increased the incidence of total major fetal abnormalities at all doses. The incidence of malformations exceeded concurrent and historical controls at 0.01 mg/kg/day (kidneys, scapula), ≥ 0.01 mg/kg/day (eyes, forelimb), 0.025 mg/kg/day (brain, tail and sacral vertebrae, major blood vessels and heart, umbilicus), ≥ 0.025 mg/kg/day (sternum) and at 0.05 mg/kg/day (parietal bones, major blood vessels). Irregular ossification and/or skeletal abnormalities occurred in the skull and jaw, vertebrae and ribs, sternum, pelvis, tail, and scapula; and dose-dependent minor skeletal variations were observed. Visceral abnormalities occurred in blood vessels, lung, liver, and esophagus. Bilobed or bifurcated gallbladder was seen in all treatment groups, but not in the control group.
In pregnant female rats given subcutaneous doses of 0.1, 0.25 and 1.0 mg/kg/day liraglutide from gestation day 6 through weaning or termination of nursing on lactation day 24, estimated systemic exposures were 0.8-, 3-, and 11-times human exposure at the MRHD of 1.8 mg/day, based on plasma AUC. A slight delay in parturition was observed in the majority of treated rats. Group mean body weight of neonatal rats from liraglutide-treated dams was lower than neonatal rats from control group dams. Bloody scabs and agitated behavior occurred in male rats descended from dams treated with 1 mg/kg/day liraglutide. Group mean body weight from birth to postpartum day 14 trended lower in F2 generation rats descended from liraglutide-treated rats compared to F2 generation rats descended from controls, but differences did not reach statistical significance for any group.
8.2 Lactation
Risk Summary
There are no data on the presence of VICTOZA in human milk, the effects on the breastfed infant, or the effects on milk production. Liraglutide was present in milk of lactating rats [see Data].
Developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for VICTOZA and any potential adverse effects on the breastfed infant from VICTOZA or from the underlying maternal condition.
Data
In lactating rats, liraglutide was present unchanged in milk at concentrations approximately 50% of maternal plasma concentrations.
8.4 Pediatric Use
The safety and effectiveness of VICTOZA as an adjunct to diet and exercise to improve glycemic control in type 2 diabetes mellitus have been established in pediatric patients 10 years of age and older. Use of VICTOZA for this indication is supported by a 26-week placebo-controlled clinical trial and a 26-week open-label extension in 134 pediatric patients 10 to 17 years of age with type 2 diabetes mellitus, a pediatric pharmacokinetic study, and studies in adults with type 2 diabetes mellitus [see Clinical Pharmacology (12.3) and Clinical Studies (14.1,14.2)]. The risk of hypoglycemia was higher with VICTOZA in pediatric patients regardless of insulin and/or metformin use [see Adverse Reactions (6.1)].
The safety and effectiveness of VICTOZA have not been established in pediatric patients less than 10 years of age.
8.5 Geriatric Use
In the VICTOZA treatment arms of the glycemic control trials, a total of 832 (19.3%) of the patients were 65 to 74 years of age and 145 (3.4%) were 75 years of age and over [see Clinical Studies (14.1)]. In the VICTOZA treatment arm of the LEADER trial [see Clinical Studies (14.3)], a total of 1738 (37.2%) patients were 65 to 74 years of age, 401 (8.6%) were 75 to 84 years of age, and 17 (0.4%) were 85 years of age or older at baseline.
No overall differences in safety or effectiveness for VICTOZA have been observed between patients 65 years of age and older and younger patients.
8.6 Renal Impairment
No dose adjustment of VICTOZA is recommended for patients with renal impairment [see Clinical Pharmacology (12.3)]. The safety and efficacy of VICTOZA was evaluated in a 26-week clinical study that included patients with moderate renal impairment (eGFR 30 to 60 mL/min/1.73m2) [see Clinical Studies (14.1)].
In the VICTOZA treatment arm of the LEADER trial [see Clinical Studies (14.3)], 1932 (41.4%) patients had mild renal impairment, 999 (21.4%) patients had moderate renal impairment and 117 (2.5%) patients had severe renal impairment at baseline. No overall differences in safety or efficacy were seen in these patients compared to patients with normal renal function.
There is limited experience with VICTOZA in patients with end stage renal disease. There have been postmarketing reports of acute renal failure and worsening of chronic renal failure, which may sometimes require hemodialysis [see Warnings and Precautions (5.5) and Adverse Reactions (6.2)]. Use caution in patients who experience dehydration.
8.7 Hepatic Impairment
There is limited experience in patients with mild, moderate or severe hepatic impairment. Therefore, VICTOZA should be used with caution in this patient population. No dose adjustment of VICTOZA is recommended for patients with hepatic impairment [see Clinical Pharmacology (12.3)].
10. Overdosage
Overdoses have been reported in clinical trials and post-marketing use of VICTOZA. Observed effects have included severe nausea, severe vomiting, and severe hypoglycemia. In the event of overdosage, appropriate supportive treatment should be initiated according to the patient’s clinical signs and symptoms.
11. Victoza Description
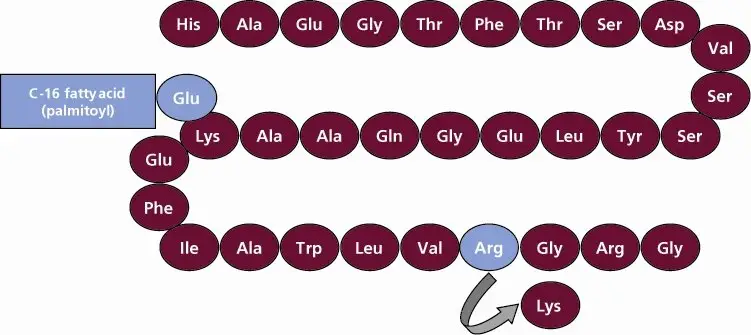
VICTOZA contains liraglutide, an analog of human GLP-1 and acts as a GLP-1 receptor agonist. The peptide precursor of liraglutide, produced by a process that includes expression of recombinant DNA in Saccharomyces cerevisiae, has been engineered to be 97% homologous to native human GLP-1 by substituting arginine for lysine at position 34. Liraglutide is made by attaching a C-16 fatty acid (palmitic acid) with a glutamic acid spacer on the remaining lysine residue at position 26 of the peptide precursor. The molecular formula of liraglutide is C172H265N43O51 and the molecular weight is 3751.2 Daltons. The structural formula (Figure 1) is:
Figure 1 Structural Formula of liraglutide
VICTOZA injection is a sterile, aqueous, clear, colorless or almost colorless solution for subcutaneous use. Each 1 mL of VICTOZA solution contains 6 mg of liraglutide and the following inactive ingredients: disodium phosphate dihydrate, 1.42 mg; propylene glycol, 14 mg; phenol, 5.5 mg; and water for injection. VICTOZA has a pH of approximately 8.15, hydrochloric acid or sodium hydroxide may be added to adjust pH. Each pre-filled pen contains a 3 mL solution of VICTOZA equivalent to 18 mg liraglutide (free-base, anhydrous).
12. Victoza - Clinical Pharmacology
12.1 Mechanism of Action
Liraglutide is an acylated human Glucagon-Like Peptide-1 (GLP-1) receptor agonist with 97% amino acid sequence homology to endogenous human GLP-1(7-37). GLP-1(7-37) represents <20% of total circulating endogenous GLP-1. Like GLP-1(7-37), liraglutide activates the GLP-1 receptor, a membrane-bound cell-surface receptor coupled to adenylyl cyclase by the stimulatory G-protein, Gs, in pancreatic beta cells. Liraglutide increases intracellular cyclic AMP (cAMP) leading to insulin release in the presence of elevated glucose concentrations. This insulin secretion subsides as blood glucose concentrations decrease and approach euglycemia. Liraglutide also decreases glucagon secretion in a glucose-dependent manner. The mechanism of blood glucose lowering also involves a delay in gastric emptying.
GLP-1(7-37) has a half-life of 1.5-2 minutes due to degradation by the ubiquitous endogenous enzymes, dipeptidyl peptidase IV (DPP-IV) and neutral endopeptidases (NEP). Unlike native GLP-1, liraglutide is stable against metabolic degradation by both peptidases and has a plasma half-life of 13 hours after subcutaneous administration. The pharmacokinetic profile of liraglutide, which makes it suitable for once daily administration, is a result of self-association that delays absorption, plasma protein binding and stability against metabolic degradation by DPP-IV and NEP.
12.2 Pharmacodynamics
VICTOZA’s pharmacodynamic profile is consistent with its pharmacokinetic profile observed after single subcutaneous administration as VICTOZA lowered fasting, premeal and postprandial glucose throughout the day [see Clinical Pharmacology (12.3)].
Fasting and postprandial glucose was measured before and up to 5 hours after a standardized meal after treatment to steady state with 0.6, 1.2 and 1.8 mg VICTOZA or placebo. Compared to placebo, the postprandial plasma glucose AUC0-300min was 35% lower after VICTOZA 1.2 mg and 38% lower after VICTOZA 1.8 mg.
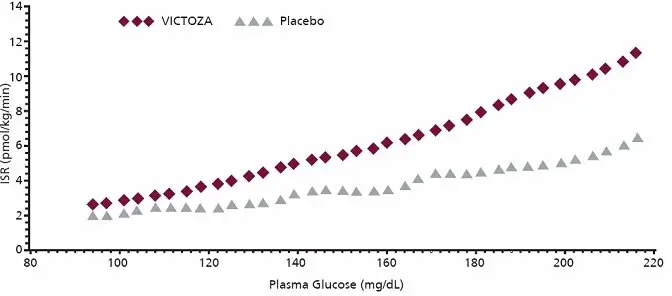
Glucose-dependent insulin secretion
The effect of a single dose of 7.5 mcg/kg (~ 0.7 mg) VICTOZA on insulin secretion rates (ISR) was investigated in 10 patients with type 2 diabetes mellitus during graded glucose infusion. In these patients, on average, the ISR response was increased in a glucose-dependent manner (Figure 2).
Figure 2 Mean Insulin Secretion Rate (ISR) versus Glucose Concentration Following Single-Dose VICTOZA 7.5 mcg/kg (~ 0.7 mg) or Placebo in Patients with Type 2 Diabetes Mellitus (N=10) During Graded Glucose Infusion
Glucagon secretion
VICTOZA lowered blood glucose by stimulating insulin secretion and lowering glucagon secretion. A single dose of VICTOZA 7.5 mcg/kg (~ 0.7 mg) did not impair glucagon response to low glucose concentrations.
Gastric emptying
VICTOZA causes a delay of gastric emptying, thereby reducing the rate at which postprandial glucose appears in the circulation.
Cardiac Electrophysiology (QTc)
The effect of VICTOZA on cardiac repolarization was tested in a QTc study. VICTOZA at steady state concentrations with daily doses up to 1.8 mg did not produce QTc prolongation.
12.3 Pharmacokinetics
Absorption
Following subcutaneous administration, maximum concentrations of liraglutide are achieved at 8-12 hours post dosing. The mean peak (Cmax) and total (AUC) exposures of liraglutide were 35 ng/mL and 960 ng·h/mL, respectively, for a subcutaneous single dose of 0.6 mg. After subcutaneous single dose administrations, Cmax and AUC of liraglutide increased proportionally over the therapeutic dose range of 0.6 mg to 1.8 mg. At 1.8 mg VICTOZA, the average steady state concentration of liraglutide over 24 hours was approximately 128 ng/mL. AUC0-∞ was equivalent between upper arm and abdomen, and between upper arm and thigh. AUC0-∞ from thigh was 22% lower than that from abdomen. However, liraglutide exposures were considered comparable among these three subcutaneous injection sites. Absolute bioavailability of liraglutide following subcutaneous administration is approximately 55%.
Distribution
The mean apparent volume of distribution after subcutaneous administration of VICTOZA 0.6 mg is approximately 13 L. The mean volume of distribution after intravenous administration of VICTOZA is 0.07 L/kg. Liraglutide is extensively bound to plasma protein (>98%).
Elimination
The mean apparent clearance following subcutaneous administration of a single dose of liraglutide is approximately 1.2 L/h with an elimination half-life of approximately 13 hours.
Metabolism
During the initial 24 hours following administration of a single [3H]-liraglutide dose to healthy subjects, the major component in plasma was intact liraglutide. Liraglutide is endogenously metabolized in a similar manner to large proteins without a specific organ as a major route of elimination.
Excretion
Following a [3H]-liraglutide dose, intact liraglutide was not detected in urine or feces. Only a minor part of the administered radioactivity was excreted as liraglutide-related metabolites in urine or feces (6% and 5%, respectively). The majority of urine and feces radioactivity was excreted during the first 6-8 days.
Specific Populations
Geriatric Patients
Age had no effect on the pharmacokinetics of VICTOZA based on a pharmacokinetic study in healthy elderly subjects (65 to 83 years) and population pharmacokinetic analyses of patients 18 to 80 years of age [see Use in Specific Populations (8.5)].
Pediatric Patients
A population pharmacokinetic analysis was conducted for VICTOZA using data from 72 pediatric patients (10 to 17 years of age) with type 2 diabetes mellitus. The pharmacokinetic profile of VICTOZA in the pediatric patients was consistent with that in adults.
Male and Female Patients
Based on the results of population pharmacokinetic analyses, females have 25% lower weight-adjusted clearance of VICTOZA compared to males.
Race or Ethnic Groups
Race and ethnicity had no effect on the pharmacokinetics of VICTOZA based on the results of population pharmacokinetic analyses that included White, Black or African American, Asian and Hispanic or Latino/Non-Hispanic or Latino subjects.
Body Weight
Body weight significantly affects the pharmacokinetics of VICTOZA based on results of population pharmacokinetic analyses. The exposure of liraglutide decreases with an increase in baseline body weight. However, the 1.2 mg and 1.8 mg daily doses of VICTOZA provided adequate systemic exposures over the body weight range of 40 – 160 kg evaluated in the clinical trials. Liraglutide was not studied in patients with body weight >160 kg.
Patients with Renal Impairment
The single-dose pharmacokinetics of VICTOZA were evaluated in patients with varying degrees of renal impairment. Patients with mild (estimated creatinine clearance 50-80 mL/min) to severe (estimated creatinine clearance <30 mL/min) renal impairment and subjects with end-stage renal disease requiring dialysis were included in the trial. Compared to healthy subjects, liraglutide AUC in mild, moderate, and severe renal impairment and in end-stage renal disease was on average 35%, 19%, 29% and 30% lower, respectively [see Use in Specific Populations (8.6)].
Patients with Hepatic Impairment
The single-dose pharmacokinetics of VICTOZA were evaluated in patients with varying degrees of hepatic impairment. Patients with mild (Child Pugh score 5-6) to severe (Child Pugh score > 9) hepatic impairment were included in the trial. Compared to healthy subjects, liraglutide AUC in patients with mild, moderate and severe hepatic impairment was on average 11%, 14% and 42% lower, respectively [see Use in Specific Populations (8.7)].
Drug Interaction Studies
In vitro assessment of drug-drug interactions
VICTOZA has low potential for pharmacokinetic drug-drug interactions related to cytochrome P450 (CYP) and plasma protein binding.
In vivo assessment of drug-drug interactions
The drug-drug interaction studies were performed at steady state with VICTOZA 1.8 mg/day. Before administration of concomitant treatment, subjects underwent a 0.6 mg weekly dose increase to reach the maximum dose of 1.8 mg/day. Administration of the interacting drugs was timed so that Cmax of VICTOZA (8-12 h) would coincide with the absorption peak of the co-administered drugs.
Digoxin
A single dose of digoxin 1 mg was administered 7 hours after the dose of VICTOZA at steady state. The concomitant administration with VICTOZA resulted in a reduction of digoxin AUC by 16%; Cmax decreased by 31%. Digoxin median time to maximal concentration (Tmax) was delayed from 1 h to 1.5 h.
Lisinopril
A single dose of lisinopril 20 mg was administered 5 minutes after the dose of VICTOZA at steady state. The co-administration with VICTOZA resulted in a reduction of lisinopril AUC by 15%; Cmax decreased by 27%. Lisinopril median Tmax was delayed from 6 h to 8 h with VICTOZA.
Atorvastatin
VICTOZA did not change the overall exposure (AUC) of atorvastatin following a single dose of atorvastatin 40 mg, administered 5 hours after the dose of VICTOZA at steady state. Atorvastatin Cmax was decreased by 38% and median Tmax was delayed from 1 h to 3 h with VICTOZA.
Acetaminophen
VICTOZA did not change the overall exposure (AUC) of acetaminophen following a single dose of acetaminophen 1000 mg, administered 8 hours after the dose of VICTOZA at steady state. Acetaminophen Cmax was decreased by 31% and median Tmax was delayed up to 15 minutes.
Griseofulvin
VICTOZA did not change the overall exposure (AUC) of griseofulvin following co-administration of a single dose of griseofulvin 500 mg with VICTOZA at steady state. Griseofulvin Cmax increased by 37% while median Tmax did not change.
Oral Contraceptives
A single dose of an oral contraceptive combination product containing 0.03 mg ethinylestradiol and 0.15 mg levonorgestrel was administered under fed conditions and 7 hours after the dose of VICTOZA at steady state. VICTOZA lowered ethinylestradiol and levonorgestrel Cmax by 12% and 13%, respectively. There was no effect of VICTOZA on the overall exposure (AUC) of ethinylestradiol. VICTOZA increased the levonorgestrel AUC0-∞ by 18%. VICTOZA delayed Tmax for both ethinylestradiol and levonorgestrel by 1.5 h.
Insulin Detemir
No pharmacokinetic interaction was observed between VICTOZA and insulin detemir when separate subcutaneous injections of insulin detemir 0.5 Unit/kg (single-dose) and VICTOZA 1.8 mg (steady state) were administered in patients with type 2 diabetes mellitus.
12.6 Immunogenicity
The observed incidence of anti-drug antibodies is highly dependent on the sensitivity and specificity of the assay. Differences in assay methods preclude meaningful comparisons of the incidence of anti-drug antibodies in the studies described below with the incidence of anti-drug antibodies in other studies, including those with VICTOZA or other liraglutide products.
A subset of VICTOZA-treated patients (1104 of 2501, 44%) in five adult double-blind clinical trials of 26 weeks duration or longer were tested for the presence of anti-liraglutide antibodies at the end of treatment [see Clinical Studies (14.1)] and 102/1104 (9%) of VICTOZA-treated patients developed anti-liraglutide antibodies. Of these 102 VICTOZA-treated patients, 56 (5%) patients developed antibodies that cross-reacted with native GLP-1. These cross-reacting antibodies were not tested for neutralizing effect against native GLP-1, and thus the potential for clinically significant neutralization of native GLP-1 was not assessed. Antibodies that had a neutralizing effect on liraglutide in an in vitro assay occurred in 12 (1%) of the VICTOZA-treated patients. There was no identified clinically significant effect of anti-liraglutide antibodies on effectiveness of VICTOZA.
In five double-blind adult glycemic control trials of VICTOZA, events from a composite of adverse events potentially related to immunogenicity (e.g., urticaria, angioedema) occurred among 0.8% of VICTOZA-treated patients and among 0.4% of comparator-treated patients. Urticaria accounted for approximately one-half of the events in this composite for VICTOZA-treated patients. Patients who developed anti-liraglutide antibodies were not more likely to develop events from the immunogenicity events composite than were patients who did not develop anti-liraglutide antibodies.
In the LEADER trial [see Clinical Studies (14.3)], anti-liraglutide antibodies were detected in 11 out of the 1247 (0.9%) adult VICTOZA-treated patients with antibody measurements. Of the 11 adult VICTOZA-treated patients who developed anti-liraglutide antibodies, none were observed to develop neutralizing antibodies to liraglutide, and 5 patients (0.4%) developed cross-reacting antibodies against native GLP-1.
In a clinical trial with pediatric patients aged 10 years and older [see Clinical Studies (14.2)], anti-liraglutide antibodies were detected in 1 (2%) VICTOZA treated patient at week 26 and 5 (9%) VICTOZA treated patients at week 53. None of the 5 patients had antibodies cross reactive to native GLP-1 or had neutralizing antibodies.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
A 104-week carcinogenicity study was conducted in male and female CD-1 mice at doses of 0.03, 0.2, 1.0, and 3.0 mg/kg/day liraglutide administered by bolus subcutaneous injection yielding systemic exposures 0.2-, 2-, 10- and 45-times the human exposure, respectively, at the MRHD of 1.8 mg/day based on plasma AUC comparison. A dose-related increase in benign thyroid C-cell adenomas was seen in the 1.0 and the 3.0 mg/kg/day groups with incidences of 13% and 19% in males and 6% and 20% in females, respectively. C-cell adenomas did not occur in control groups or 0.03 and 0.2 mg/kg/day groups. Treatment-related malignant C-cell carcinomas occurred in 3% of females in the 3.0 mg/kg/day group. Thyroid C-cell tumors are rare findings during carcinogenicity testing in mice. A treatment-related increase in fibrosarcomas was seen on the dorsal skin and subcutis, the body surface used for drug injection, in males in the 3 mg/kg/day group. These fibrosarcomas were attributed to the high local concentration of drug near the injection site. The liraglutide concentration in the clinical formulation (6 mg/mL) is 10-times higher than the concentration in the formulation used to administer 3 mg/kg/day liraglutide to mice in the carcinogenicity study (0.6 mg/mL).
A 104-week carcinogenicity study was conducted in male and female Sprague Dawley rats at doses of 0.075, 0.25 and 0.75 mg/kg/day liraglutide administered by bolus subcutaneous injection with exposures 0.5-, 2- and 8-times the human exposure, respectively, resulting from the MRHD based on plasma AUC comparison. A treatment-related increase in benign thyroid C-cell adenomas was seen in males in 0.25 and 0.75 mg/kg/day liraglutide groups with incidences of 12%, 16%, 42%, and 46% and in all female liraglutide-treated groups with incidences of 10%, 27%, 33%, and 56% in 0 (control), 0.075, 0.25, and 0.75 mg/kg/day groups, respectively. A treatment-related increase in malignant thyroid C-cell carcinomas was observed in all male liraglutide-treated groups with incidences of 2%, 8%, 6%, and 14% and in females at 0.25 and 0.75 mg/kg/day with incidences of 0%, 0%, 4%, and 6% in 0 (control), 0.075, 0.25, and 0.75 mg/kg/day groups, respectively. Thyroid C-cell carcinomas are rare findings during carcinogenicity testing in rats.
Studies in mice demonstrated that liraglutide-induced C-cell proliferation was dependent on the GLP-1 receptor and that liraglutide did not cause activation of the REarranged during Transfection (RET) proto-oncogene in thyroid C-cells.
Human relevance of thyroid C-cell tumors in mice and rats is unknown and has not been determined by clinical studies or nonclinical studies [see Boxed Warning and Warnings and Precautions (5.1)].
Liraglutide was negative with and without metabolic activation in the Ames test for mutagenicity and in a human peripheral blood lymphocyte chromosome aberration test for clastogenicity. Liraglutide was negative in repeat-dose in vivo micronucleus tests in rats.
In rat fertility studies using subcutaneous doses of 0.1, 0.25 and 1.0 mg/kg/day liraglutide, males were treated for 4 weeks prior to and throughout mating and females were treated 2 weeks prior to and throughout mating until gestation day 17. No direct adverse effects on male fertility was observed at doses up to 1.0 mg/kg/day, a high dose yielding an estimated systemic exposure 11- times the human exposure at the MRHD, based on plasma AUC. In female rats, an increase in early embryonic deaths occurred at 1.0 mg/kg/day. Reduced body weight gain and food consumption were observed in females at the 1.0 mg/kg/day dose.
14. Clinical Studies
14.1 Glycemic Control Trials in Adults with Type 2 Diabetes Mellitus
In glycemic control trials in adults, VICTOZA has been studied as monotherapy and in combination with one or two oral anti-diabetic medications or basal insulin. VICTOZA was also studied in a cardiovascular outcomes trial (LEADER trial).
In each of the placebo controlled trials, treatment with VICTOZA produced clinically and statistically significant improvements in hemoglobin A1c and fasting plasma glucose (FPG) compared to placebo.
All VICTOZA-treated patients started at 0.6 mg/day. The dose was increased in weekly intervals by 0.6 mg to reach 1.2 mg or 1.8 mg for patients randomized to these higher doses. VICTOZA 0.6 mg is not effective for glycemic control and is intended only as a starting dose to reduce gastrointestinal intolerance [see Dosage and Administration (2)].
Monotherapy
In this 52-week trial, 746 adult patients with type 2 diabetes mellitus were randomized to VICTOZA 1.2 mg, VICTOZA 1.8 mg, or glimepiride 8 mg. Patients who were randomized to glimepiride were initially treated with 2 mg daily for two weeks, increasing to 4 mg daily for another two weeks, and finally increasing to 8 mg daily. Treatment with VICTOZA 1.8 mg and 1.2 mg resulted in a statistically significant reduction in HbA1c compared to glimepiride (Table 3). The percentage of patients who discontinued due to ineffective therapy was 3.6% in the VICTOZA 1.8 mg treatment group, 6.0% in the VICTOZA 1.2 mg treatment group, and 10.1% in the glimepiride-treatment group.
The mean age of participants was 53 years, and the mean duration of diabetes was 5 years. Participants were 49.7% male, 77.5% White, 12.6% Black or African American and 35.0% of Hispanic or Latino ethnicity. The mean BMI was 33.1 kg/m2.
Table 3 Results of a 52-week Monotherapy Trial in Adults with Type 2 Diabetes Mellitusa
|
VICTOZA 1.8 mg |
VICTOZA 1.2 mg |
|
|
|
Intent-to-Treat Population (N) |
246 |
251 |
|
|
HbA1c (%) (Mean) | |||
|
Baseline |
8.2 |
8.2 |
|
|
Change from baseline (adjusted mean) b |
-1.1 |
-0.8 |
|
|
Difference from glimepiride arm (adjusted mean) b |
-0.6** |
-0.3* | |
|
95% Confidence Interval |
(-0.8, -0.4) |
(-0.5, -0.1) | |
|
Percentage of patients achieving HbA1c <7% |
51 |
43 |
|
|
Fasting Plasma Glucose (mg/dL) (Mean) | |||
|
Baseline |
172 |
168 |
|
|
Change from baseline (adjusted mean) b |
-26 |
-15 |
|
|
Difference from glimepiride arm (adjusted mean) b |
-20** |
-10* | |
|
95% Confidence Interval |
(-29, -12) |
(-19, -1) | |
|
Body Weight (kg) (Mean) | |||
|
Baseline |
92.6 |
92.1 |
|
|
Change from baseline (adjusted mean) b |
-2.5 |
-2.1 |
|
|
Difference from glimepiride arm (adjusted mean) b |
-3.6** |
-3.2** | |
|
95% Confidence Interval |
(-4.3, -2.9) |
(-3.9, -2.5) |
aIntent-to-treat population using last observation on study
bLeast squares mean adjusted for baseline value
*p-value <0.05
**p-value <0.0001
Figure 3 Mean HbA1c for Adult Patients with Type 2 Diabetes Mellitus who Completed the 52-week Trial and for the Last Observation Carried Forward (LOCF, intent-to-treat) data at Week 52 (Monotherapy)
Combination Therapy
Add-on to Metformin
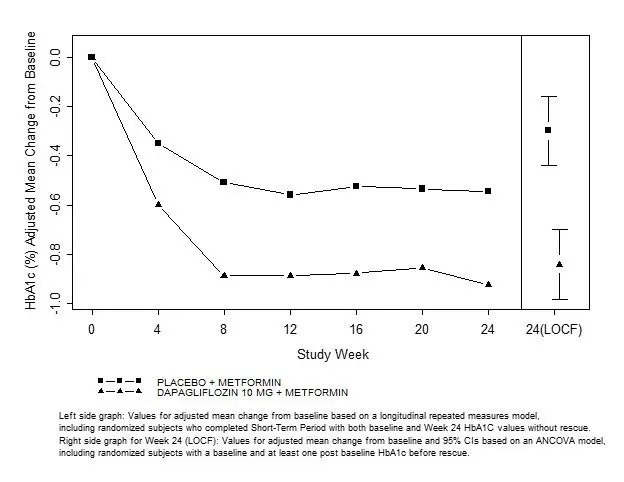
In this 26-week trial, 1,091 adult patients with type 2 diabetes mellitus were randomized to VICTOZA 0.6 mg, VICTOZA 1.2 mg, VICTOZA 1.8 mg, placebo, or glimepiride 4 mg (one-half of the maximal approved dose in the United States), all as add-on to metformin. Randomization occurred after a 6-week run-in period consisting of a 3-week initial forced metformin titration period followed by a maintenance period of another 3 weeks. During the titration period, doses of metformin were increased up to 2000 mg/day. Treatment with VICTOZA 1.2 mg and 1.8 mg as add-on to metformin resulted in a significant mean HbA1c reduction relative to placebo add-on to metformin and resulted in a similar mean HbA1c reduction relative to glimepiride 4 mg add-on to metformin (Table 4). The percentage of patients who discontinued due to ineffective therapy was 5.4% in the VICTOZA 1.8 mg + metformin treatment group, 3.3% in the VICTOZA 1.2 mg + metformin treatment group, 23.8% in the placebo + metformin treatment group, and 3.7% in the glimepiride + metformin treated group.
The mean age of participants was 57 years, and the mean duration of diabetes was 7 years. Participants were 58.2% male, 87.1% White and 2.4% Black or African American. The mean BMI was 31.0 kg/m2.
Table 4 Results of a 26-week Trial of VICTOZA as Add-on to Metformin in Adults with Type 2 Diabetes Mellitusa
|
VICTOZA 1.8 mg + Metformin |
VICTOZA 1.2 mg + Metformin |
|
Glimepiride 4 mg† +
|
|
|
Intent-to-Treat Population (N) |
242 |
240 |
|
|
|
HbA1c (%) (Mean) | ||||
|
Baseline |
8.4 |
8.3 |
|
|
|
Change from baseline (adjusted mean) b |
-1.0 |
-1.0 |
|
|
|
Difference from placebo + metformin arm (adjusted mean) b |
-1.1** |
-1.1** | ||
|
95% Confidence Interval |
(-1.3, -0.9) |
(-1.3, -0.9) | ||
|
Difference from glimepiride + metformin arm (adjusted mean) b |
0.0 |
0.0 | ||
|
95% Confidence Interval |
(-0.2, 0.2) |
(-0.2, 0.2) | ||
|
Percentage of patients achieving HbA1c <7% |
42 |
35 |
|
|
|
Fasting Plasma Glucose (mg/dL) (Mean) | ||||
|
Baseline |
181 |
179 |
|
|
|
Change from baseline (adjusted mean) b |
-30 |
-30 |
|
|
|
Difference from placebo + metformin arm (adjusted mean) b |
-38** |
-37** | ||
|
95% Confidence Interval |
(-48, -27) |
(-47, -26) | ||
|
Difference from glimepiride + metformin arm (adjusted mean) b |
-7 |
-6 | ||
|
95% Confidence Interval |
(-16, 2) |
(-15, 3) | ||
|
Body Weight (kg) (Mean) | ||||
|
Baseline |
88.0 |
88.5 |
|
|
|
Change from baseline (adjusted mean) b |
-2.8 |
-2.6 |
|
|
|
Difference from placebo + metformin arm (adjusted mean) b 95% Confidence Interval |
-1.3* (-2.2, -0.4) |
-1.1* (-2.0, -0.2) | ||
|
Difference from glimepiride + metformin arm (adjusted mean) b |
-3.8** |
-3.5** | ||
|
95% Confidence Interval |
(-4.5, -3.0) |
(-4.3, -2.8) | ||
|
aIntent-to-treat population using last observation on study bLeast squares mean adjusted for baseline value † For glimepiride, one-half of the maximal approved United States dose. *p-value <0.05 **p-value <0.0001 |
||||
VICTOZA Compared to Sitagliptin, Both as Add-on to Metformin
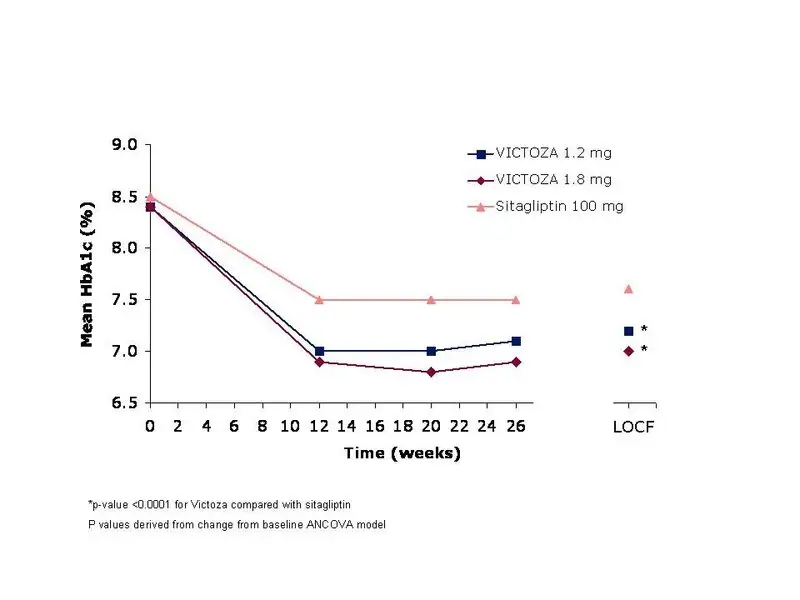
In this 26–week, open-label trial, 665 adult patients with type 2 diabetes mellitus on a background of metformin ≥1,500 mg per day were randomized to VICTOZA 1.2 mg once daily, VICTOZA 1.8 mg once daily or sitagliptin 100 mg once daily, all dosed according to approved labeling. Patients were to continue their current treatment on metformin at a stable, pre-trial dose level and dosing frequency.
The mean age of participants was 56 years, and the mean duration of diabetes was 6 years. Participants were 52.9% male, 86.6% White, 7.2% Black or African American and 16.2% of Hispanic or Latino ethnicity. The mean BMI was 32.8 kg/m2.
The primary endpoint was the change in HbA1c from baseline to Week 26. Treatment with VICTOZA 1.2 mg and VICTOZA 1.8 mg resulted in statistically significant reductions in HbA1c relative to sitagliptin 100 mg (Table 5). The percentage of patients who discontinued due to ineffective therapy was 3.1% in the VICTOZA 1.2 mg group, 0.5% in the VICTOZA 1.8 mg treatment group, and 4.1% in the sitagliptin 100 mg treatment group. From a mean baseline body weight of 94 kg, there was a mean reduction of 2.7 kg for VICTOZA 1.2 mg, 3.3 kg for VICTOZA 1.8 mg, and 0.8 kg for sitagliptin 100 mg.
Table 5 Results of a 26-week Open-label Trial of VICTOZA Compared to Sitagliptin (both in combination with metformin) in Adults with Type 2 Diabetes Mellitusa
|
VICTOZA 1.8 mg + Metformin |
VICTOZA 1.2 mg + Metformin |
Sitagliptin 100 mg + Metformin |
|
|
Intent-to-Treat Population (N) |
218 |
221 |
|
|
HbA1c (%) (Mean) | |||
|
Baseline |
8.4 |
8.4 |
|
|
Change from baseline (adjusted mean) |
-1.5 |
-1.2 |
|
|
Difference from sitagliptin arm (adjusted mean) b 95% Confidence Interval |
-0.6** (-0.8, -0.4) |
-0.3** (-0.5, -0.2) | |
|
Percentage of patients achieving HbA1c <7% |
56 |
44 |
|
|
Fasting Plasma Glucose (mg/dL) (Mean) | |||
|
Baseline |
179 |
182 |
|
|
Change from baseline (adjusted mean) |
-39 |
-34 |
|
|
Difference from sitagliptin arm (adjusted mean) b 95% Confidence Interval |
-24** (-31, -16) |
-19** (-26, -12) |
aIntent-to-treat population using last observation on study
bLeast squares mean adjusted for baseline value
**p-value <0.0001
Figure 4 Mean HbA1c for Adult Patients with Type 2 Diabetes Mellitus who Completed the 26-week Trial and for the Last Observation Carried Forward (LOCF, intent-to-treat) data at Week 26
Combination Therapy with Metformin and Insulin
This 26-week open-label trial enrolled 988 adult patients with type 2 diabetes mellitus with inadequate glycemic control (HbA1c 7-10%) on metformin (≥1,500 mg/day) alone or inadequate glycemic control (HbA1c 7-8.5%) on metformin (≥1500 mg/day) and a sulfonylurea. Patients who were on metformin and a sulfonylurea discontinued the sulfonylurea then all patients entered a 12-week run-in period during which they received add-on therapy with VICTOZA titrated to 1.8 mg once-daily. At the end of the run-in period, 498 patients (50%) achieved HbA1c <7% with VICTOZA 1.8 mg and metformin and continued treatment in a non-randomized, observational arm. Another 167 patients (17%) withdrew from the trial during the run-in period with approximately one-half of these patients doing so because of gastrointestinal adverse reactions [see Adverse Reactions (6.1)]. The remaining 323 patients with HbA1c ≥7% (33% of those who entered the run-in period) were randomized to 26 weeks of once-daily insulin detemir administered in the evening as add-on therapy (N=162) or to continued, unchanged treatment with VICTOZA 1.8 mg and metformin (N=161). The starting dose of insulin detemir was 10 units/day and the mean dose at the end of the 26-week randomized period was 39 units/day. During the 26 week randomized treatment period, the percentage of patients who discontinued due to ineffective therapy was 11.2% in the group randomized to continued treatment with VICTOZA 1.8 mg and metformin and 1.2% in the group randomized to add-on therapy with insulin detemir.
The mean age of participants was 57 years, and the mean duration of diabetes was 8 years. Participants were 55.7% male, 91.3% White, 5.6% Black or African American and 12.5% of Hispanic or Latino ethnicity. The mean BMI was 34.0 kg/m2.
Treatment with insulin detemir as add-on to VICTOZA 1.8 mg + metformin resulted in statistically significant reductions in HbA1c and FPG compared to continued, unchanged treatment with VICTOZA 1.8 mg + metformin alone (Table 6). From a mean baseline body weight of 96 kg after randomization, there was a mean reduction of 0.3 kg in the patients who received insulin detemir add-on therapy compared to a mean reduction of 1.1 kg in the patients who continued on unchanged treatment with VICTOZA 1.8 mg + metformin alone.
Table 6 Results of a 26-week Open-label Trial of Insulin detemir as add on to VICTOZA + Metformin Compared to Continued Treatment with VICTOZA + Metformin alone in Adult Patients with Type 2 Diabetes Mellitus not Achieving HbA1c < 7% after 12 weeks of Metformin and VICTOZAa
|
Insulin detemir + VICTOZA + Metformin |
VICTOZA + Metformin |
|
|
Intent-to-Treat Population (N) |
162 |
157 |
|
HbA1c (%) (Mean) | ||
|
Baseline (week 0) |
7.6 |
7.6 |
|
Change from baseline (adjusted mean) |
-0.5 |
0 |
|
-0.5** | |
|
(-0.7, -0.4) | |
|
Percentage of patients achieving HbA1c <7% |
43 |
17 |
|
Fasting Plasma Glucose (mg/dL) (Mean) | ||
|
Baseline (week 0) |
166 |
159 |
|
Change from baseline (adjusted mean) |
-39 |
-7 |
|
-31** | |
|
(-39, -23) |
aIntent-to-treat population using last observation on study
bLeast squares mean adjusted for baseline value
**p-value <0.0001
Add-on to Sulfonylurea
In this 26-week trial, 1,041 adult patients with type 2 diabetes mellitus were randomized to VICTOZA 0.6 mg, VICTOZA 1.2 mg, VICTOZA 1.8 mg, placebo, or rosiglitazone 4 mg (one-half of the maximal approved dose in the United States), all as add-on to glimepiride. Randomization occurred after a 4-week run-in period consisting of an initial, 2-week, forced-glimepiride titration period followed by a maintenance period of another 2 weeks. During the titration period, doses of glimepiride were increased to 4 mg/day. The doses of glimepiride could be reduced (at the discretion of the investigator) from 4 mg/day to 3 mg/day or 2 mg/day (minimum) after randomization, in the event of unacceptable hypoglycemia or other adverse events.
The mean age of participants was 56 years, and the mean duration of diabetes was 8 years. Participants were 49.4% male, 64.4% White and 2.8% Black or African American. The mean BMI was 29.9 kg/m2.
Treatment with VICTOZA 1.2 mg and 1.8 mg as add-on to glimepiride resulted in a statistically significant reduction in mean HbA1c compared to placebo add-on to glimepiride (Table 7). The percentage of patients who discontinued due to ineffective therapy was 3.0% in the VICTOZA 1.8 mg + glimepiride treatment group, 3.5% in the VICTOZA 1.2 mg + glimepiride treatment group, 17.5% in the placebo + glimepiride treatment group, and 6.9% in the rosiglitazone + glimepiride treatment group.
Table 7 Results of a 26-week Trial of VICTOZA as add-on to Sulfonylurea in Adult Patients with Type 2 Diabetes Mellitusa
|
VICTOZA 1.8 mg + Glimepiride |
VICTOZA 1.2 mg + Glimepiride |
|
|
|
|
Intent-to-Treat Population (N) |
234 |
228 |
|
|
|
HbA1c (%) (Mean) | ||||
|
8.5 |
8.5 |
|
|
|
-1.1 |
-1.1 |
|
|
|
-1.4** |
-1.3** | ||
|
(-1.6, -1.1) |
(-1.5, -1.1) | ||
|
Percentage of patients achieving HbA1c <7% |
42 |
35 |
|
|
|
Fasting Plasma Glucose (mg/dL) (Mean) | ||||
|
174 |
177 |
|
|
|
-29 |
-28 |
|
|
|
-47** |
-46** | ||
|
(-58, -35) |
(-58, -35) | ||
| ||||
|
83.0 |
80.0 |
|
|
|
-0.2 |
+0.3 |
|
|
|
-0.1 |
0.4 | ||
|
(-0.9, 0.6) |
(-0.4, 1.2) |
aIntent-to-treat population using last observation on study
bLeast squares mean adjusted for baseline value
† For rosiglitazone, one-half of the maximal approved United States dose.
**p-value <0.0001
Add-on to Metformin and Sulfonylurea
In this 26-week trial, 581 adult patients with type 2 diabetes mellitus were randomized to VICTOZA 1.8 mg, placebo, or insulin glargine, all as add-on to metformin and glimepiride. Randomization took place after a 6-week run-in period consisting of a 3-week forced metformin and glimepiride titration period followed by a maintenance period of another 3 weeks. During the titration period, doses of metformin and glimepiride were to be increased up to 2,000 mg/day and 4 mg/day, respectively. After randomization, patients randomized to VICTOZA 1.8 mg underwent a 2 week period of titration with VICTOZA. During the trial, the VICTOZA and metformin doses were fixed, although glimepiride and insulin glargine doses could be adjusted. Patients titrated glargine twice-weekly during the first 8 weeks of treatment based on self-measured fasting plasma glucose on the day of titration. After Week 8, the frequency of insulin glargine titration was left to the discretion of the investigator, but, at a minimum, the glargine dose was to be revised, if necessary, at Weeks 12 and 18. Only 20% of glargine-treated patients achieved the pre-specified target fasting plasma glucose of ≤100 mg/dL. Therefore, optimal titration of the insulin glargine dose was not achieved in most patients.
The mean age of participants was 58 years, and the mean duration of diabetes was 9 years. Participants were 56.5% male, 75.0% White and 3.6% Black or African American. The mean BMI was 30.5 kg/m2.
Treatment with VICTOZA as add-on to glimepiride and metformin resulted in a statistically significant mean reduction in HbA1c compared to placebo add-on to glimepiride and metformin (Table 8). The percentage of patients who discontinued due to ineffective therapy was 0.9% in the VICTOZA 1.8 mg + metformin + glimepiride treatment group, 0.4% in the insulin glargine + metformin + glimepiride treatment group, and 11.3% in the placebo + metformin + glimepiride treatment group.
Table 8 Results of a 26-week Trial of VICTOZA as Add-on to Metformin and Sulfonylurea in Adult Patients with Type 2 Diabetes Mellitusa
|
VICTOZA 1.8 mg + Metformin + Glimepiride |
Placebo + Metformin + Glimepiride |
Insulin glargine† + Metformin + Glimepiride |
|
|
Intent-to-Treat Population (N) |
230 |
114 |
|
|
HbA1c (%) (Mean) | |||
|
Baseline |
8.3 |
8.3 |
|
|
Change from baseline (adjusted mean) b |
-1.3 |
-0.2 |
|
|
-1.1** | ||
|
(-1.3, -0.9) | ||
|
Percentage of patients achieving HbA1c <7% |
53 |
15 |
|
|
Fasting Plasma Glucose (mg/dL) (Mean) | |||
|
Baseline |
165 |
170 |
|
|
Change from baseline (adjusted mean) b |
-28 |
+10 |
|
|
-38** | ||
|
(-46, -30) | ||
|
Body Weight (kg) (Mean) | |||
|
Baseline |
85.8 |
85.4 |
|
|
Change from baseline (adjusted mean) b |
-1.8 |
-0.4 |
|
|
-1.4* | ||
|
(-2.1, -0.7) |
aIntent-to-treat population using last observation on study
bLeast squares mean adjusted for baseline value
† For insulin glargine, optimal titration regimen was not achieved for 80% of patients.
*p-value <0.05
**p-value <0.0001
VICTOZA Compared to Exenatide, Both as Add-on to Metformin and/or Sulfonylurea Therapy
In this 26–week, open-label trial, 464 adult patients with type 2 diabetes mellitus on a background of metformin monotherapy, sulfonylurea monotherapy or a combination of metformin and sulfonylurea were randomized to once daily VICTOZA 1.8 mg or exenatide 10 mcg twice daily. Maximally tolerated doses of background therapy were to remain unchanged for the duration of the trial. Patients randomized to exenatide started on a dose of 5 mcg twice-daily for 4 weeks and then were escalated to 10 mcg twice daily.
The mean age of participants was 57 years, and the mean duration of diabetes was 8 years. Participants were 51.9% male, 91.8% White, 5.4% Black or African American and 12.3% of Hispanic or Latino ethnicity. The mean BMI was 32.9 kg/m2.
Treatment with VICTOZA 1.8 mg resulted in statistically significant reductions in HbA1c and FPG relative to exenatide (Table 9). The percentage of patients who discontinued for ineffective therapy was 0.4% in the VICTOZA treatment group and 0% in the exenatide treatment group. Both treatment groups had a mean decrease from baseline in body weight of approximately 3 kg.
Table 9 Results of a 26-week Open-label trial of VICTOZA versus Exenatide (both in combination with metformin and/or sulfonylurea) in Adult Patients with Type 2 Diabetes Mellitusa
|
VICTOZA 1.8 mg once daily + metformin and/or sulfonylurea |
Exenatide 10 mcg twice daily + metformin and/or sulfonylurea |
|
|
Intent-to-Treat Population (N) |
233 |
231 |
|
HbA1c (%) (Mean) | ||
|
Baseline |
8.2 |
8.1 |
|
Change from baseline (adjusted mean) b |
-1.1 |
-0.8 |
|
Difference from exenatide arm (adjusted mean) b 95% Confidence Interval |
-0.3** (-0.5, -0.2) | |
|
Percentage of patients achieving HbA1c <7% |
54 |
43 |
|
Fasting Plasma Glucose (mg/dL) (Mean) | ||
|
Baseline |
176 |
171 |
|
Change from baseline (adjusted mean) b |
-29 |
-11 |
|
Difference from exenatide arm (adjusted mean) b 95% Confidence Interval |
-18** (-25, -12) |
aIntent-to-treat population using last observation carried forward
bLeast squares mean adjusted for baseline value
**p-value <0.0001
Add-on to Metformin and Thiazolidinedione
In this 26-week trial, 533 adult patients with type 2 diabetes mellitus were randomized to VICTOZA 1.2 mg, VICTOZA 1.8 mg or placebo, all as add-on to rosiglitazone (8 mg) plus metformin (2,000 mg). Patients underwent a 9 week run-in period (3-week forced dose escalation followed by a 6-week dose maintenance phase) with rosiglitazone (starting at 4 mg and increasing to 8 mg/day within 2 weeks) and metformin (starting at 500 mg with increasing weekly increments of 500 mg to a final dose of 2,000 mg/day). Only patients who tolerated the final dose of rosiglitazone (8 mg/day) and metformin (2000 mg/day) and completed the 6-week dose maintenance phase were eligible for randomization into the trial.
The mean age of participants was 55 years, and the mean duration of diabetes was 9 years. Participants were 61.6% male, 84.2% White, 10.2% Black or African American and 16.4% of Hispanic or Latino ethnicity. The mean BMI was 33.9 kg/m2.
Treatment with VICTOZA as add-on to metformin and rosiglitazone produced a statistically significant reduction in mean HbA1c compared to placebo add-on to metformin and rosiglitazone (Table 10). The percentage of patients who discontinued due to ineffective therapy was 1.7% in the VICTOZA 1.8 mg + metformin + rosiglitazone treatment group, 1.7% in the VICTOZA 1.2 mg + metformin + rosiglitazone treatment group, and 16.4% in the placebo + metformin + rosiglitazone treatment group.
Table 10 Results of a 26-week Trial of VICTOZA as Add-on to Metformin and Thiazolidinedione in Adult Patients with Type 2 Diabetes Mellitusa
|
VICTOZA 1.8 mg + Metformin + Rosiglitazone |
VICTOZA 1.2 mg + Metformin + Rosiglitazone |
Placebo + Metformin +
|
|
|
Intent-to-Treat Population (N) |
178 |
177 |
|
|
HbA1c (%) (Mean) | |||
|
Baseline |
8.6 |
8.5 |
|
|
Change from baseline (adjusted mean) b |
-1.5 |
-1.5 |
|
|
-0.9** |
-0.9** | |
|
(-1.1, -0.8) |
(-1.1, -0.8) | |
|
Percentage of patients achieving HbA1c <7% |
54 |
57 |
|
|
Fasting Plasma Glucose (mg/dL) (Mean) | |||
|
Baseline |
185 |
181 |
|
|
Change from baseline (adjusted mean) b |
-44 |
-40 |
|
|
-36** |
-32** | |
|
(-44, -27) |
(-41, -23) | |
|
Body Weight (kg) (Mean) | |||
|
Baseline |
94.9 |
95.3 |
|
|
Change from baseline (adjusted mean) b |
-2.0 |
-1.0 |
|
|
-2.6** |
-1.6** | |
|
(-3.4, -1.8) |
(-2.4, -1.0) |
aIntent-to-treat population using last observation on study
bLeast squares mean adjusted for baseline value
**p-value <0.0001
VICTOZA Compared to Placebo Both With or Without metformin and/or Sulfonylurea and/or Pioglitazone and/or Basal or Premix insulin in Patients with Type 2 Diabetes Mellitus and Moderate Renal Impairment
In this 26-week, double-blind, randomized, placebo-controlled, parallel-group trial in adult patients with type 2 diabetes mellitus, 279 patients with moderate renal impairment, as per MDRD formula (eGFR 30−59 mL/min/1.73 m2), were randomized to VICTOZA or placebo once daily. VICTOZA was added to the patient’s stable pre-trial antidiabetic regimen (insulin therapy and/or metformin, pioglitazone, or sulfonylurea). The dose of VICTOZA was escalated according to approved labeling to achieve a dose of 1.8 mg per day. The insulin dose was reduced by 20% at randomization for patients with baseline HbA1c ≤ 8% and fixed until liraglutide dose escalation was complete. Dose reduction of insulin and SU was allowed in case of hypoglycemia; up titration of insulin was allowed but not beyond the pre-trial dose.
The mean age of participants was 67 years, and the mean duration of diabetes was 15 years. Participants were 50.5% male, 92.3% White, 6.6% Black or African American, and 7.2% of Hispanic or Latino ethnicity. The mean BMI was 33.9 kg/m2. Approximately half of patients had an eGFR between 30 and <45mL/min/1.73 m2.
Treatment with VICTOZA resulted in a statistically significant reduction in HbA1c from baseline at Week 26 compared to placebo (see Table 11). 123 patients reached the 1.8 mg dose of VICTOZA.
Table 11 Results of a 26-week Trial of VICTOZA Compared to Placebo in Adult Patients with Type 2 Diabetes Mellitus and Moderate Renal Impairmenta
|
VICTOZA 1.8 mg + insulin and/or OAD |
Placebo + insulin and/or OAD |
|
|
Intent to Treat Population (N) |
140 |
137 |
|
HbA1c (%) | ||
|
Baseline (mean) |
8.1 |
8.0 |
|
Change from baseline (estimated mean) b, c |
-0.9 |
-0.4 |
|
Difference from placebob, c 95% Confidence Interval |
-0.6* (-0.8, -0.3) | |
|
Proportion achieving HbA1c < 7% d |
39.3 |
19.7 |
|
FPG (mg/dL) | ||
|
Baseline (mean) |
171 |
167 |
|
Change from baseline (estimated mean) e |
-22 |
-10 |
|
Difference from placeboe 95% Confidence Interval |
-12** (-23, -0.8) |
a Intent-to-treat population
b Estimated using a mixed model for repeated measurement with treatment, country, stratification groups as factors and baseline as a covariate, all nested within visit. Multiple imputation method modeled “wash out” of the treatment effect for patients having missing data who discontinued treatment.
c Early treatment discontinuation, before week 26, occurred in 25% and 22% of VICTOZA and placebo patients, respectively.
d Based on the known number of subjects achieving HbA1c < 7%. When applying the multiple imputation method described in b) above, the estimated percents achieving HbA1c < 7% are 47.6% and 24.9% for VICTOZA and placebo, respectively.
e Estimated using a mixed model for repeated measurement with treatment, country, stratification groups as factors and baseline as a covariate, all nested within visit.
*p-value <0.0001
**p-value <0.05
14.2 Glycemic Control Trial in Pediatric Patients Aged 10 Years and Older with Type 2 Diabetes Mellitus
VICTOZA was evaluated in a 26-week, double-blind, randomized, parallel group, placebo controlled multi-center trial (NCT01541215), in 134 pediatric patients with type 2 diabetes mellitus aged 10 years and older. Patients were randomized to VICTOZA once-daily or placebo once-daily in combination with metformin with or without basal insulin treatment. All patients were on a metformin dose of 1000 to 2000 mg prior to randomization. The basal insulin dose was decreased by 20% at randomization and VICTOZA was titrated weekly by 0.6 mg for 2 to 3 weeks based on tolerability and an average fasting plasma glucose goal of <110 mg/dL.
The mean age was 14.6 years: 29.9% were ages 10-14 years, and 70.1% were greater than 14 years of age. 38.1% were male, 64.9% were White, 13.4% were Asian, 11.9% were Black or African American; 29.1% were of Hispanic or Latino ethnicity. The mean BMI was 33.9 kg/m2 and the mean BMI SDS was 2.9. 18.7% of patients were using basal insulin at baseline. The mean duration of diabetes was 1.9 years and the mean HbA1c was 7.8%.
At week 26, treatment with VICTOZA was superior in reducing HbA1c from baseline versus placebo. The estimated treatment difference in HbA1c reduction from baseline between VICTOZA and placebo was
-1.06% with a 95% confidence interval of [-1.65%; -0.46%] (see Table 12).
Table 12 Results at week 26 in a trial comparing VICTOZA in combination with metformin with or without basal insulin versus Placebo in combination with metformin with or without basal insulin in Pediatric Patients Aged 10 Years and Older with Type 2 Diabetes Mellitus
|
VICTOZA+metformin±basal insulin |
Placebo+metformin±basal insulin |
|
|
N |
66 |
68 |
|
HbA1c (%) | ||
|
Baseline |
7.9 |
7.7 |
|
End of 26 weeks |
7.1 |
8.2 |
|
Adjusted mean change from baseline after 26 weeksa |
-0.64 |
0.42 |
|
Treatment difference [95% CI] VICTOZA vs Placebo |
-1.06 [-1.65; -0.46]* |
|
|
Percentage of patients achieving HbA1c <7%b |
63.7 |
36.5 |
|
FPG (mg/dL) | ||
|
Baseline |
157 |
147 |
|
End of 26 weeks |
132 |
166 |
|
Adjusted mean change from baseline after 26 weeksa |
-19.4 |
14.4 |
|
Treatment difference [95% CI] VICTOZA vs Placebo |
-33.83 [-55.74 ; -11.92] |
|
a The change from baseline to end of treatment visit in HbA1c and FPG was analyzed using a pattern mixture model with multiple imputation. Missing observations (10.6% in the VICTOZA, 14.5% in the placebo) were imputed from the placebo arm based on multiple (x10,000) imputations. The data for week 26 was then analyzed with an ANCOVA model containing treatment, sex and age group as fixed effects and baseline value as covariate.
b Categories are derived from continuous measurements of HbA1c using a pattern mixture model with multiple imputation for missing observations.
* p-value <0.001
14.3 Cardiovascular Outcomes Trial in Adult Patients with Type 2 Diabetes Mellitus and Atherosclerotic Cardiovascular Disease
The LEADER trial (NCT01179048) was a multi-national, multi-center, placebo-controlled, double-blind trial. In this study, 9,340 adult patients with inadequately controlled type 2 diabetes mellitus and atherosclerotic cardiovascular disease (CVD) were randomized to VICTOZA 1.8 mg or placebo for a median duration of 3.5 years. The study compared the risk of major adverse cardiovascular events between VICTOZA and placebo when these were added to, and used concomitantly with, background standard of care treatments for type 2 diabetes mellitus. The primary endpoint, major adverse cardiac events (MACE), was the time to first occurrence of a three part composite outcome which included; cardiovascular death, non-fatal myocardial infarction and non-fatal stroke.
Patients eligible to enter the trial were; 50 years of age or older and had established, stable, cardiovascular, cerebrovascular, peripheral artery disease, chronic kidney disease or New York Heart Association (NYHA) class II and III heart failure (80% of the enrolled population) or were 60 years of age or older and had other specified risk factors for cardiovascular disease (20% of the enrolled population).
At baseline, demographic and disease characteristics were balanced. The mean age was 64 years and the population was 64.3% male, 77.5% White, 10.0% Asian, and 8.3% Black or African American. In the study, 12.1% of the population identified as Hispanic or Latino ethnicity. The mean duration of type 2 diabetes mellitus was 12.8 years, the mean HbA1c was 8.7% and the mean BMI was 32.5 kg/m2. A history of previous myocardial infarction was reported in 31% of randomized individuals, a prior revascularization procedure in 39%, a prior ischemic stroke in 11%, documented symptomatic coronary disease in 9%, documented asymptomatic cardiac ischemia in 26%, and a diagnosis of NYHA class II to III heart failure in 14%. The mean eGFR at baseline was 79 mL/min/1.73 m2 and 41.8% of patients had mild renal impairment (eGFR 60 to 90 mL/min/1.73m2), 20.7% had moderate renal impairment (eGFR 30 to 60 mL/min/1.73m2) and 2.4% of patients had severe renal impairment (eGFR < 30 mL/min/1.73m2).
At baseline, patients treated their diabetes with; diet and exercise only (3.9%), oral antidiabetic drugs only (51.5%), oral antidiabetic drugs and insulin (36.7%) or insulin only (7.9%). The most common background antidiabetic drugs used at baseline and in the trial were metformin, sulfonylurea and insulin. Use of DPP-4 inhibitors and other GLP-1 receptor agonists was excluded by protocol and sodium-glucose cotransporter-2 (SGLT-2) inhibitors were either not approved or not widely available. At baseline, cardiovascular disease and risk factors were managed with; non-diuretic antihypertensives (92.4%), diuretics (41.8%), statin therapy (72.1%) and platelet aggregation inhibitors (66.8%). During the trial, investigators could modify anti-diabetic and cardiovascular medications to achieve local standard of care treatment targets with respect to blood glucose, lipid, and blood pressure, and manage patients recovering from an acute coronary syndrome or stroke event per local treatment guidelines.
For the primary analysis, a Cox proportional hazards model was used to test for non-inferiority against the pre-specified risk margin of 1.3 for the hazard ratio of MACE and to test for superiority on MACE if non-inferiority was demonstrated. Type 1 error was controlled across multiple tests.
VICTOZA significantly reduced the occurrence of MACE. The estimated hazard ratio (95% CI) for time to first MACE was 0.87 (0.78, 0.97). Refer to Figure 5 and Table 13.
Vital status was available for 99.7% of subjects in the trial. A total of 828 deaths were recorded during the LEADER trial. A majority of the deaths in the trial were categorized as cardiovascular deaths and non-cardiovascular deaths were balanced between the treatment groups (3.5% in patients treated with VICTOZA and 3.6% in patients treated with placebo). The estimated hazard ratio of time to all-cause death for VICTOZA compared to placebo was 0.85 (0.74, 0.97).
Figure 5 Kaplan-Meier: Time to First Occurrence of a MACE in the LEADER Trial (Patients with Type 2 Diabetes Mellitus and Atherosclerotic CVD)
Table 13 Treatment Effect for the Primary Composite Endpoint, MACE, and its Components in the LEADER Trial (Patients with Type 2 Diabetes Mellitus and Atherosclerotic CVD)a
|
VICTOZA N=4668 |
Placebo N=4672 |
Hazard Ratio (95% CI)b |
|
|
Composite of cardiovascular death, non-fatal myocardial infarction, non-fatal stroke (MACE) (time to first occurrence) c |
608 (13.0%) |
694 (14.9%) |
0.87 (0.78; 0.97) |
|
Non-fatal myocardial infarctiond |
281 (6.0%) |
317 (6.8%) |
0.88 (0.75;1.03) |
|
Non-fatal stroked |
159 (3.4%) |
177 (3.8%) |
0.89 (0.72;1.11) |
|
Cardiovascular deathd |
219 (4.7%) |
278 (6%) |
0.78 (0.66;0.93) |
aFull analysis set (all randomized patients)
bCox-proportional hazards model with treatment as a factor
cp-value for superiority (2-sided) 0.011
dNumber and percentage of first events
16. How is Victoza supplied
16.1 How Supplied
VICTOZA Injection: 18 mg/3 mL (6 mg/mL) clear, colorless solution in a pre-filled, single-patient-use pen that delivers doses of 0.6 mg, 1.2 mg, or 1.8 mg is available in the following package sizes:
2 x VICTOZA pen NDC 0169-4060-12
3 x VICTOZA pen NDC 0169-4060-13
16.2 Recommended Storage
Prior to first use, VICTOZA should be stored in a refrigerator between 36ºF to 46ºF (2ºC to 8ºC). Do not store in the freezer or directly adjacent to the refrigerator cooling element. Do not freeze VICTOZA and do not use VICTOZA if it has been frozen.
After first use of the VICTOZA pen, the pen can be stored for 30 days at controlled room temperature (59°F to 86°F; 15°C to 30°C) or in a refrigerator (36°F to 46°F; 2°C to 8°C). Keep the pen cap on when not in use. Protect VICTOZA from excessive heat and sunlight. Always remove and safely discard the needle after each injection and store the VICTOZA pen without an injection needle attached. This will reduce the potential for contamination, infection, and leakage while also ensuring dosing accuracy. Always use a new needle for each injection to prevent contamination.
17. Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Risk of Thyroid C-cell Tumors
Inform patients that liraglutide causes benign and malignant thyroid C-cell tumors in mice and rats and that the human relevance of this finding is unknown. Counsel patients to report symptoms of thyroid tumors (e.g., a lump in the neck, hoarseness, dysphagia, or dyspnea) to their physician [see Boxed Warning and Warnings and Precautions (5.1)].
Pancreatitis
Inform patients of the potential risk for pancreatitis. Explain that persistent severe abdominal pain that may radiate to the back and which may or may not be accompanied by vomiting, is the hallmark symptom of acute pancreatitis. Instruct patients to discontinue VICTOZA promptly and contact their physician if persistent severe abdominal pain occurs [see Warnings and Precautions (5.2)].
Never Share a VICTOZA Pen Between Patients
Advise patients that they must never share a VICTOZA pen with another person, even if the needle is changed, because doing so carries a risk for transmission of blood-borne pathogens [see Warnings and Precautions (5.3)].
Hypoglycemia
Inform patients that hypoglycemia has been reported when VICTOZA is used with insulin secretagogues or insulin and may occur in pediatric patients regardless of concomitant antidiabetic treatment. Educate patients or caregivers on the signs and symptoms of hypoglycemia [see Warnings and Precautions (5.4)].
Acute Kidney Injury
Advise patients of the potential risk of dehydration due to gastrointestinal adverse reactions and to take precautions to avoid fluid depletion. Inform patients of the potential risk for worsening renal function, which in some cases may require dialysis [see Warnings and Precautions (5.5)].
Hypersensitivity Reactions
Inform patients that serious hypersensitivity reactions have been reported during postmarketing use of VICTOZA. Advise patients on the symptoms of hypersensitivity reactions and instruct them to stop taking VICTOZA and seek medical advice promptly if such symptoms occur [see Warnings and Precautions (5.6)].
Acute Gallbladder Disease
Inform patients of the potential risk for cholelithiasis or cholecystitis. Instruct patients to contact their physician if cholelithiasis or cholecystitis is suspected for appropriate clinical follow-up [see Warnings and Precautions (5.7)].
Missed Dose
Inform patients not to take an extra dose of VICTOZA to make up for a missed dose. If a dose is missed, the once-daily regimen should be resumed as prescribed with the next scheduled dose. If more than 3 days have elapsed since the last dose, advise the patient to reinitiate VICTOZA at 0.6 mg to mitigate any gastrointestinal symptoms associated with reinitiation of treatment. VICTOZA should be titrated at the discretion of the healthcare provider [see Dosage and Administration (2.2)].
Manufactured by:
Novo Nordisk A/S
DK-2880 Bagsvaerd, Denmark
Version: 16
VICTOZA® is a registered trademark of Novo Nordisk A/S.
PATENT Information: http://novonordisk-us.com/products/product-patents.html
© 2023 Novo Nordisk
For information about VICTOZA contact:
Novo Nordisk Inc.
800 Scudders Mill Road
Plainsboro, NJ 08536
1-877-484-2869
Medication Guide
|
VICTOZA® (VIC-tow-za)
|
|
Read this Medication Guide before you start using VICTOZA and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or your treatment. |
|
What is the most important information I should know about VICTOZA?
|
|
What is VICTOZA? VICTOZA is an injectable prescription medicine used:
VICTOZA is not for use in people with type 1 diabetes. It should not be used with other medicines that contain liraglutide. It is not known if VICTOZA is safe and effective to lower blood sugar (glucose) in children under 10 years of age. |
|
Who should not use VICTOZA?
|
|
What should I tell my healthcare provider before using VICTOZA?
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. VICTOZA may affect the way some medicines work and some medicines may affect the way VICTOZA works. Before using VICTOZA, talk to your healthcare provider about low blood sugar and how to manage it. Tell your healthcare provider if you are taking other medicines to treat diabetes, including insulin or sulfonylureas. Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine. |
|
How should I use VICTOZA?
Your dose of VICTOZA and other diabetes medicines may need to change because of:
|
|
What are the possible side effects of VICTOZA?
The most common side effects of VICTOZA may include nausea, diarrhea, vomiting, decreased appetite, indigestion and constipation. Talk to your healthcare provider about any side effects that bothers you or does not go away. These are not all the possible side effects of VICTOZA. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1‑800‑FDA‑1088. |
|
General information about the safe and effective use of VICTOZA. Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use VICTOZA for a condition for which it was not prescribed. Do not give VICTOZA to other people, even if they have the same symptoms that you have. It may harm them. If you would like more information, talk with your healthcare provider. You can ask your pharmacist or healthcare provider for information about VICTOZA that is written for health professionals. |
|
What are the ingredients in VICTOZA?
|
|
Manufactured by: Novo Nordisk A/S, DK-2880 Bagsvaerd, Denmark Victoza® is a registered trademark of Novo Nordisk A/S. For more information, go to victoza.com or call 1-877-484-2869. PATENT Information: http://novonordisk-us.com/patients/products/product-patents.html © 2022 Novo Nordisk This Medication Guide has been approved by the U.S. Food and Drug Administration Revised: 06/2022 |
INSTRUCTIONS FOR USE
Victoza (liraglutide) injection
First read the Medication Guide that comes with your Victoza single-patient use pen and then read this Patient Instructions for Use for information about how to use your Victoza pen the right way.
These instructions do not take the place of talking with your healthcare provider about your medical condition or your treatment.
Do not share your Victoza Pen with other people, even if the needle has been changed. You may give other people a serious infection, or get a serious infection from them.
Your Victoza pen is a disposable single-patient-use prefilled pen injector that contains 3 mL of Victoza and will deliver doses of 0.6 mg, 1.2 mg or 1.8 mg. The number of doses that you can take with a Victoza pen depends on the dose of medicine that is prescribed for you. Your healthcare provider will tell you how much Victoza to take.
Victoza pen should be used with Novo Nordisk disposable needles. Talk to your healthcare provider or pharmacist for more information about needles for your Victoza pen.
Important Information
- Δ Always use a new needle for each injection to prevent contamination.
- Δ Always remove the needle after each injection, and store your pen without the needle
- attached. This reduces the risk of contamination, infection, leakage of liraglutide,
- blocked needles and inaccurate dosing.
- Δ Keep your Victoza pen and all medicines out of the reach of children.
- Δ If you drop your Victoza pen, repeat “First Time Use For Each New Pen” (steps A
- through D).
- Δ Be careful not to bend or damage the needle.
- Δ Do not use the cartridge scale to measure how much Victoza to inject.
- Δ Be careful when handling used needles to avoid needle stick injuries.
- Δ You can use your Victoza pen for up to 30 days after you use it the first time.
First Time Use for Each New Pen
Step A. Check the Pen
- •
- Take your new Victoza pen out of the refrigerator.
- •
- Wash hands with soap and water before use.
- •
- Check pen label before each use to make sure it is your Victoza pen.
- •
- Pull off pen cap (See Figure A).
- •
- Check Victoza in the cartridge. The liquid should be clear, colorless and free of particles. If not, do not use.
- •
- Wipe the rubber stopper with an alcohol swab.
Step B. Attach the Needle
- •
- Remove protective tab from outer needle cap (See Figure B).
- •
- Push outer needle cap containing the needle straight onto the pen, then screw needle on until secure.
- •
- Pull off outer needle cap (See Figure C). Do not throw away
- •
- Pull off inner needle cap and throw away (See Figure D). A small drop of liquid may appear. This is normal.
Step C. Dial to the Flow Check Symbol
This step is done only Once for each new pen and is Only required the first time you use a new pen.
- •
- Turn dose selector until flow check symbol (--) lines up with pointer (See Figure E). The flow check symbol does not administer the dose as prescribed by your healthcare provider.
- •
- To select the dose prescribed by your healthcare provider, continue to Step G under “Routine Use”.
Step D. Prepare the Pen
- •
- Hold pen with needle pointing up.
- •
- Tap cartridge gently with your finger a few times to bring any air bubbles to the top of the cartridge (See Figure F).
- •
- Keep needle pointing up and press dose button until 0 mg lines up with pointer (See Figure G). Repeat steps C and D, up to 6 times, until a drop of Victoza appears at the needle tip.
If you still see no drop of Victoza, use a new pen and contact Novo Nordisk at 1-877-484-2869.
Continue to Step G under "Routine Use" →
Routine Use
Step E. Check the Pen
- •
- Take your Victoza pen from where it is stored.
- •
- Wash hands with soap and water before use.
- •
- Check pen label before each use to make sure it is your Victoza pen.
- •
- Pull off pen cap (See Figure H).
- •
- Check Victoza in the cartridge. The liquid should be clear, colorless and free of particles. If not, do not use.
- •
- Wipe the rubber stopper with an alcohol swab.
Step F. Attach the Needle
- •
- Remove protective tab from outer needle cap.
- •
- Push outer needle cap containing the needle straight onto the pen, then screw needle on until secure (See Figure I).
- •
- Pull off outer needle cap. Do not throw away (See Figure J).
- •
- Pull off inner needle cap and throw away (See Figure K). A small drop of liquid may appear. This is normal.
Step G. Dial the Dose
- •
- Victoza pen can give a dose of 0.6 mg (starting dose), 1.2 mg or 1.8 mg. Be sure that you know the dose of Victoza that is prescribed for you.
- •
- Turn the dose selector until your needed dose lines up with the pointer (0.6 mg, 1.2 mg or 1.8 mg) (See Figure L).
- •
- You will hear a "click" every time you turn the dose selector. Do not set the dose by counting the number of clicks you hear.
- •
- If you select a wrong dose, change it by turning the dose selector backwards or forwards until the correct dose lines up with the pointer. Be careful not to press the dose button when turning the dose selector. This may cause Victoza to come out.
Step H. Injecting the Dose
- •
- Insert needle into your skin in the stomach (abdomen), thigh or upper arm. Use the injection technique shown to you by your healthcare provider. Do not inject Victoza into a vein or muscle.
- •
- Press down on the center of the dose button to inject until 0 mg lines up with the pointer (See Figure M).
- •
- Be careful not to touch the dose display with your other fingers. This may block the injection.
- •
- Keep the dose button pressed down and make sure that you keep the needle under the skin for a full count of 6 seconds to make sure the full dose is injected. Keep your thumb on the injection button until you remove the needle from your skin (See Figure N).
- •
- Change (rotate) your injection sites within the area you choose for each dose. Do not use the same injection site for each injection.
Step I. Withdraw Needle
- •
- You may see a drop of Victoza at the needle tip. This is normal and it does not affect the dose you just received. If blood appears after you take the needle out of your skin, apply light pressure, but do not rub the area (See Figure O).
Step J. Remove and Dispose of the Needle
- •
- Carefully put the outer needle cap over the needle (See Figure P). Unscrew the needle.
- •
- Safely remove the needle from your Victoza pen after each use.
- •
- Put your used VICTOZA pen and needles in a FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) loose needles and pens in your household trash.
- •
- If you do not have a FDA-cleared sharps disposal container, you may use a household container that is:
- o
- made of a heavy-duty plastic
- o
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out
- o
- upright and stable during use
- o
- leak-resistant
- o
- properly labeled to warn of hazardous waste inside the container
- •
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. Do not reuse or share your needles with other people. For more information about the safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA’s website at: http://www.fda.gov/safesharpsdisposal.
- •
- Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
Caring for your Victoza pen
- •
- After removing the needle, put the pen cap on your Victoza pen and store your Victoza pen without the needle attached (See Figure Q).
- •
- Do not try to refill your Victoza pen - it is prefilled and is disposable.
- •
- Do not try to repair your pen or pull it apart.
- •
- Keep your Victoza pen away from dust, dirt and liquids.
- •
- If cleaning is needed, wipe the outside of the pen with a clean, damp cloth.
How should I store Victoza?
Before use:
- •
- Store your new, unused Victoza pen in the refrigerator at 36ºF to 46ºF (2ºC to 8ºC).
- •
- If Victoza is stored outside of refrigeration (by mistake) prior to first use, it should be used or thrown away within 30 days.
- •
- Do not freeze Victoza or use Victoza if it has been frozen. Do not store Victoza near the refrigerator cooling element.
Pen in use:
- •
- Use a Victoza pen for only 30 days. Throw away a used Victoza pen 30 days after you start using it, even if some medicine is left in the pen.
- •
- Store your Victoza pen at 59ºF to 86ºF (15ºC to 30ºC), or in a refrigerator at 36ºF to 46ºF (2°C to 8°C).
- •
- When carrying the pen away from home, store the pen at a temperature between 59ºF to 86ºF (15ºC to 30ºC).
- •
- If Victoza has been exposed to temperatures above 86ºF (30°C), it should be thrown away.
- •
- Protect your Victoza pen from heat and sunlight.
- •
- Keep the pen cap on when your Victoza pen is not in use.
- •
- Always remove the needle after each injection and store your pen without the needle attached. This reduces the risk of contamination, infection, leakage and inaccurate dosing.
Principal Display Panel - 3 Victoza Pens
NDC 0169-4060-13
List 406013
VICTOZA®
(liraglutide) injection
For Single Patient Use Only
18 mg/3 mL (6 mg/mL)
Each pen delivers doses of 0.6 mg, 1.2 mg or 1.8 mg
Subcutaneous use only
Discard pen 30 days after first use
REFRIGERATE - DO NOT FREEZE
Rx Only
Contains: 3 Victoza Pens, Product Literature
Dispense the enclosed Medication Guide to each patient
Intended for use with Novo Nordisk disposable needles
3 Pens
Principal Display Panel - 2 Victoza Pens
NDC 0169-4060-12
List 406012
VICTOZA®
(liraglutide) injection
For Single Patient Use Only
18 mg/3 mL (6 mg/mL)
Each pen delivers doses of 0.6 mg, 1.2 mg or 1.8 mg
Subcutaneous use only
Discard pen 30 days after first use
REFRIGERATE - DO NOT FREEZE
Rx Only
Contains: 2 Victoza Pens, Product Literature
Dispense the enclosed Medication Guide to each patient
Intended for use with Novo Nordisk disposable needles
2 Pens
| VICTOZA
liraglutide injection |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Novo Nordisk (622920320) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Novo Nordisk A/S | 305914798 | MANUFACTURE(0169-4060) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Novo Nordisk Pharmaceutical Industries, LP | 622920320 | MANUFACTURE(0169-4060) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Novo Nordisk A/S | 305156788 | API MANUFACTURE(0169-4060) | |