Drug Detail:Vyvgart (Efgartigimod alfa [ ef-gar-tig-i-mod-al-fa ])
Drug Class: Immune globulins
Highlights of Prescribing Information
VYVGART® (efgartigimod alfa-fcab) injection, for intravenous use
Initial U.S. Approval: 2021
Indications and Usage for Vyvgart
VYVGART is a neonatal Fc receptor blocker indicated for the treatment of generalized myasthenia gravis (gMG) in adult patients who are anti-acetylcholine receptor (AChR) antibody positive. (1)
Vyvgart Dosage and Administration
- Evaluate the need to administer age-appropriate vaccines according to immunization guidelines before initiation of a new treatment cycle with VYVGART. (2.1)
- The recommended dosage is 10 mg/kg administered as an intravenous infusion over one hour once weekly for 4 weeks. In patients weighing 120 kg or more, the recommended dose is 1200 mg per infusion. (2.2)
- Administer subsequent treatment cycles based on clinical evaluation; the safety of initiating subsequent cycles sooner than 50 days from the start of the previous treatment cycle has not been established. (2.2)
- Must be diluted with 0.9% Sodium Chloride Injection, USP prior to administration. (2.3)
- Administer as an intravenous infusion over one hour via a 0.2 micron in-line filter. (2.3)
Dosage Forms and Strengths
Injection: 400 mg in 20 mL (20 mg/mL) single-dose vial. (3)
Contraindications
None. (4)
Warnings and Precautions
- Infections: Delay administration of VYVGART to patients with an active infection. Monitor for signs and symptoms of infection in patients treated with VYVGART. If serious infection occurs, administer appropriate treatment and consider withholding VYVGART until the infection has resolved. (5.1)
- Hypersensitivity Reactions: Angioedema, dyspnea, and rash have occurred. If a hypersensitivity reaction occurs, discontinue the infusion and institute appropriate therapy. (5.2)
Adverse Reactions/Side Effects
Most common adverse reactions ( ≥10%) in patients treated with gMG are respiratory tract infections, headache, and urinary tract infection. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact argenx at 1-833-argx411 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
Closely monitor for reduced effectiveness of medications that bind to the human neonatal Fc receptor. When concomitant long-term use of such medications is essential for patient care, consider discontinuing VYVGART and using alternative therapies. (7)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 4/2022
Full Prescribing Information
1. Indications and Usage for Vyvgart
VYVGART is indicated for the treatment of generalized myasthenia gravis (gMG) in adult patients who are anti-acetylcholine receptor (AChR) antibody positive.
2. Vyvgart Dosage and Administration
2.1 Recommended Vaccination
Because VYVGART causes transient reduction in IgG levels, immunization with live-attenuated or live vaccines is not recommended during treatment with VYVGART. Evaluate the need to administer age-appropriate immunizations according to immunization guidelines before initiation of a new treatment cycle with VYVGART [see Dosage and Administration (2.2) and Warnings and Precautions (5.1)].
2.2 Recommended Dose and Dose Schedules
Dilute VYVGART prior to administration. Administer via intravenous infusion only [see Dosage and Administration (2.3)].
The recommended dosage of VYVGART is 10 mg/kg administered as an intravenous infusion over one hour once weekly for 4 weeks. In patients weighing 120 kg or more, the recommended dose of VYVGART is 1200 mg (3 vials) per infusion.
Administer subsequent treatment cycles based on clinical evaluation. The safety of initiating subsequent cycles sooner than 50 days from the start of the previous treatment cycle has not been established.
If a scheduled infusion is missed, VYVGART may be administered up to 3 days after the scheduled time point. Thereafter, resume the original dosing schedule until the treatment cycle is completed.
2.3 Preparation and Administration Instructions
Prior to administration, VYVGART single-dose vials require dilution in 0.9% Sodium Chloride Injection, USP, to make a total volume to be administered of 125 mL (see Preparation).
Check that the VYVGART solution is clear to slightly opalescent and colorless to slightly yellow. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Do not use if opaque particles, discoloration, or other foreign particles are present.
Use aseptic technique when preparing the VYVGART diluted solution for intravenous infusion. Each vial is for single-dose only.
Discard any unused portion.
Preparation
- Calculate the dose (mg), total drug volume (mL) of VYVGART solution required, and the number of vials needed based on the recommended dose according to the patient's body weight [see Dosage and Administration (2.2)]. Each vial contains a total of 400 mg of VYVGART at a concentration of 20 mg per mL.
- Gently withdraw the calculated dose of VYVGART from the vial(s) with a sterile syringe and needle. Discard any unused portion of the vials.
- Dilute the withdrawn VYVGART with 0.9% Sodium Chloride Injection, USP to make a total volume of 125 mL for intravenous infusion.
- Gently invert the infusion bag containing the diluted VYVGART without shaking to ensure thorough mixing of the product and the diluent.
- The diluted solution can be administered using polyethylene (PE), polyvinyl chloride (PVC), ethylene vinyl acetate (EVA), or ethylene/polypropylene copolymer bags (polyolefins bags), and with PE, PVC, EVA, or polyurethane/polypropylene infusion lines.
3. Dosage Forms and Strengths
Injection: 400 mg/20 mL (20 mg/mL) as a colorless to slightly yellow, clear to slightly opalescent solution, in a single-dose vial.
5. Warnings and Precautions
5.1 Infections
VYVGART may increase the risk of infection. The most common infections observed in Study 1 were urinary tract infection (10% of VYVGART-treated patients compared to 5% of placebo-treated patients) and respiratory tract infections (33% of VYVGART-treated patients compared to 29% of placebo-treated patients) [see Adverse Reactions (6.1) and Clinical Studies (14)]. A higher frequency of patients who received VYVGART compared to placebo were observed to have below normal levels for white blood cell counts (12% versus 5%, respectively), lymphocyte counts (28% versus 19%, respectively), and neutrophil counts (13% versus 6%, respectively). The majority of infections and hematologic abnormalities were mild to moderate in severity. Delay VYVGART administration in patients with an active infection until the infection is resolved. During treatment with VYVGART, monitor for clinical signs and symptoms of infections. If serious infection occurs, administer appropriate treatment and consider withholding VYVGART until the infection has resolved.
5.2 Hypersensitivity Reactions
Hypersensitivity reactions, including rash, angioedema, and dyspnea were observed in VYVGART-treated patients. In clinical trials, hypersensitivity reactions were mild or moderate, occurred within one hour to three weeks of administration, and did not lead to treatment discontinuation. Monitor patients during administration and for 1 hour thereafter for clinical signs and symptoms of hypersensitivity reactions [see Dosage and Administration (2.3)]. If a hypersensitivity reaction occurs during administration, discontinue VYVGART infusion and institute appropriate supportive measures if needed.
6. Adverse Reactions/Side Effects
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Infections [see Warnings and Precautions (5.1)]
- Hypersensitivity Reactions [see Warnings and Precautions (5.2)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In clinical studies, the safety of VYVGART has been evaluated in 246 patients who received at least one dose of VYVGART, including 57 patients exposed to at least 7 treatment cycles and 8 patients exposed to at least 10 treatment cycles.
In a placebo-controlled study (Study 1) in patients with gMG, 84 patients received VYVGART 10 mg/kg [see Clinical Studies (14)]. Of these 84 patients, approximately 75% were female, 82% were White, 11% were Asian, and 8% were of Hispanic or Latino ethnicity. The mean age at study entry was 46 years (range 19 to 78).
The minimum time between treatment cycles, specified by study protocol, was 50 days. On average, VYVGART-treated patients received 2 cycles in Study 1. The mean and median times to the second treatment cycle were 94 days and 72 days from the initial infusion of the first treatment cycle, respectively, for VYVGART-treated patients.
Adverse reactions reported in at least 5% of patients treated with VYVGART and more frequently than placebo are summarized in Table 1. The most common adverse reactions (reported in at least 10% of VYVGART-treated patients) were respiratory tract infection, headache, and urinary tract infection.
| Adverse reaction | VYVGART (N=84) % | Placebo (N=83) % |
|---|---|---|
|
||
| Respiratory tract infection | 33 | 29 |
| Headache* | 32 | 29 |
| Urinary tract infection | 10 | 5 |
| Paraesthesia† | 7 | 5 |
| Myalgia | 6 | 1 |
6.2 Immunogenicity
As with all therapeutic proteins, there is potential for immunogenicity. The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies to VYVGART in the studies described below with the incidence of antibodies in other studies or to other products may be misleading.
In up to 26 weeks of treatment in Study 1, 20% (17/83) of patients developed antibodies to VYVGART. Seven percent (6/83) of patients developed neutralizing antibodies.
Because few patients tested positive for anti-efgartigimod alfa-fcab antibodies and neutralizing antibodies, the available data are too limited to make definitive conclusions regarding immunogenicity and the effect on pharmacokinetics, safety, or efficacy of VYVGART.
7. Drug Interactions
7.1 Effect of VYVGART on Other Drugs
Concomitant use of VYVGART with medications that bind to the human neonatal Fc receptor (FcRn) (e.g., immunoglobulin products, monoclonal antibodies, or antibody derivates containing the human Fc domain of the IgG subclass) may lower systemic exposures and reduce effectiveness of such medications. Closely monitor for reduced effectiveness of medications that bind to the human neonatal Fc receptor. When concomitant long-term use of such medications is essential for patient care, consider discontinuing VYVGART and using alternative therapies.
8. Use In Specific Populations
8.5 Geriatric Use
Clinical studies of VYVGART did not include sufficient numbers of patients aged 65 and older to determine whether they respond differently from younger adult patients.
8.6 Renal Impairment
No dose adjustment of VYVGART is needed for patients with mild renal impairment. There are insufficient data to evaluate the impact of moderate renal impairment (eGFR 30-59 mL/min/1.73 m2) and severe renal impairment (eGFR <30 mL/min/1.73 m2) on pharmacokinetic parameters of efgartigimod alfa-fcab [see Clinical Pharmacology (12.3)].
11. Vyvgart Description
Efgartigimod alfa-fcab is a human immunoglobulin G1 (IgG1)-derived Fc fragment (fragment, crystallized) of the za allotype. The efgartigimod alfa-fcab Fc fragment is a homodimer consisting of two identical peptide chains each consisting of 227 amino acids linked together by two interchain disulfide bonds with affinity for FcRn.The molecular weight of efgartigimod alfa-fcab is approximately 54 kDa.
VYVGART (efgartigimod alfa-fcab) injection is a sterile, preservative free, clear to slightly opalescent, colorless to slightly yellow solution supplied in a single-dose vial for infusion after dilution.
Each 20 mL single-dose vial contains 400 mg of efgartigimod alfa-fcab at a concentration of 20 mg/mL. In addition, each mL of solution contains L-arginine hydrochloride (31.6 mg), polysorbate 80 (0.2 mg), sodium chloride (5.8 mg), sodium phosphate dibasic anhydrous (2.4 mg), sodium phosphate monobasic monohydrate (1.1 mg) and water for injection, USP, at a pH of 6.7.
12. Vyvgart - Clinical Pharmacology
12.1 Mechanism of Action
Efgartigimod alfa-fcab is a human IgG1 antibody fragment that binds to the neonatal Fc receptor (FcRn), resulting in the reduction of circulating IgG.
12.2 Pharmacodynamics
In Study 1 [see Clinical Studies (14)], the pharmacological effect of efgartigimod alfa-fcab was assessed by measuring the decrease in serum IgG levels and AChR autoantibody levels. In patients testing positive for AChR antibodies and who were treated with VYVGART, there was a reduction in total IgG levels relative to baseline. Decrease in AChR autoantibody levels followed a similar pattern.
12.3 Pharmacokinetics
Efgartigimod alfa-fcab exhibits linear pharmacokinetics, and following single doses of efgartigimod alfa-fcab, exposures increase proportionally up to 50 mg/kg (5 times the recommended dosage).
14. Clinical Studies
The efficacy of VYVGART for the treatment of generalized myasthenia gravis (gMG) in adults who are AChR antibody positive was established in a 26-week, multicenter, randomized, double-blind, placebo-controlled trial (Study 1; NCT03669588).
Study 1 enrolled patients who met the following criteria at screening:
- -
- Myasthenia Gravis Foundation of America (MGFA) clinical classification class II to IV
- -
- MG-Activities of Daily Living (MG-ADL) total score of ≥ 5
- -
- On stable dose of MG therapy prior to screening, that included acetylcholinesterase (AChE) inhibitors, steroids, or non-steroidal immunosuppressive therapies (NSISTs), either in combination or alone
- -
- IgG levels of at least 6 g/L
A total of 167 patients were enrolled in Study 1 and were randomized to receive either VYVGART 10 mg/kg (1200 mg for those weighing 120 kg or more) (n=84) or placebo (n=83). Baseline characteristics were similar between treatment groups. Patients had a median age of 46 years at screening (range: 19 to 81 years) and a median time since diagnosis of 9 years. Seventy-one percent were female, and 84% were White. Median MG-ADL total score was 9, and median Quantitative Myasthenia Gravis (QMG) total score was 16. The majority of patients (n=65 for VYVGART; n=64 for placebo) were positive for AChR antibodies.
At baseline, over 80% of patients in each group received AChE inhibitors, over 70% in each treatment group received steroids, and approximately 60% in each treatment group received NSISTs, at stable doses.
Patients were treated with VYVGART at the recommended dosage regimen [see Dosage and Administration (2.2)].
The efficacy of VYVGART was measured using the Myasthenia Gravis-Specific Activities of Daily Living scale (MG-ADL) which assesses the impact of gMG on daily functions of 8 signs or symptoms that are typically affected in gMG. Each item is assessed on a 4-point scale where a score of 0 represents normal function and a score of 3 represents loss of ability to perform that function. A total score ranges from 0 to 24, with the higher scores indicating more impairment. In this study, an MG-ADL responder was defined as a patient with a 2-point or greater reduction in the total MG-ADL score compared to the treatment cycle baseline for at least 4 consecutive weeks, with the first reduction occurring no later than 1 week after the last infusion of the cycle.
The primary efficacy endpoint was the comparison of the percentage of MG-ADL responders during the first treatment cycle between treatment groups in the AChR-Ab positive population. A statistically significant difference favoring VYVGART was observed in the MG-ADL responder rate during the first treatment cycle [67.7% in the VYVGART-treated group vs 29.7% in the placebo-treated group (p <0.0001)].
The efficacy of VYVGART was also measured using the Quantitative Myasthenia Gravis (QMG) total score which is a 13-item categorical grading system that assesses muscle weakness. Each item is assessed on a 4-point scale where a score of 0 represents no weakness and a score of 3 represents severe weakness. A total possible score ranges from 0 to 39, where higher scores indicate more severe impairment. In this study, a QMG responder was defined as a patient who had a 3-point or greater reduction in the total QMG score compared to the treatment cycle baseline for at least 4 consecutive weeks, with the first reduction occurring no later than 1 week after last infusion of the cycle.
The secondary endpoint was the comparison of the percentage of QMG responders during the first treatment cycle between both treatment groups in the AChR-Ab positive patients. A statistically significant difference favoring VYVGART was observed in the QMG responder rate during the first treatment cycle [63.1% in the VYVGART-treated group vs 14.1% in the placebo-treated group (p <0.0001)].
The results are presented in Table 2.
| VYVGART n=65 % | Placebo n=64 % | P-value | Odds Ratio (95% CI) | |
|---|---|---|---|---|
| MG-ADL=Myasthenia Gravis Activities of Daily Living; QMG =Quantitative Myasthenia Gravis; mITT=modified intent-to-treat; n=number of patients for whom the observation was reported; CI = confidence interval; Logistic regression stratified for AChR-Ab status (if applicable), Japanese/Non-Japanese and standard of care, with baseline MG-ADL as covariate / QMG as covariates Two-sided exact p-value |
||||
| MG-ADL Responders | 67.7 | 29.7 | < 0.0001 | 4.951 (2.213, 11.528) |
| QMG Responders | 63.1 | 14.1 | < 0.0001 | 10.842 (4.179, 31.200) |
Figure 1 shows the mean change from baseline on the MG-ADL during cycle 1.
| Figure 1: Mean Change in Total MG-ADL From Cycle 1 Baseline Over Time in AChR-Ab Positive Patients (mITT Analysis Set) |
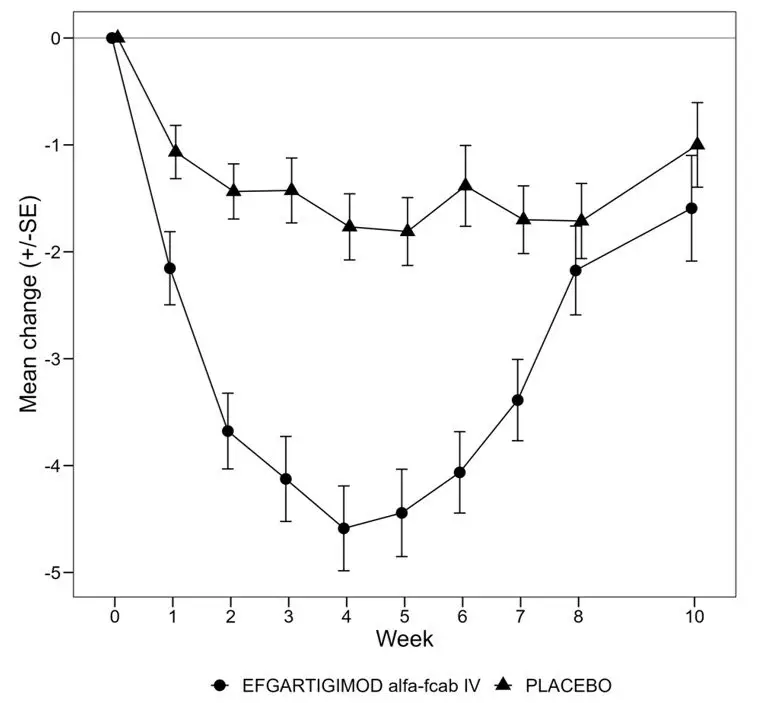 |
Figure 2 shows the distribution of response on the MG-ADL and QMG during cycle 1, four weeks after the first infusion with VYVGART.
| Figure 2: Percentage of Patients with MG-ADL and QMG Total Score Change 4 Weeks Post Initial Infusion of the First Cycle in AChR-Ab Positive Population |
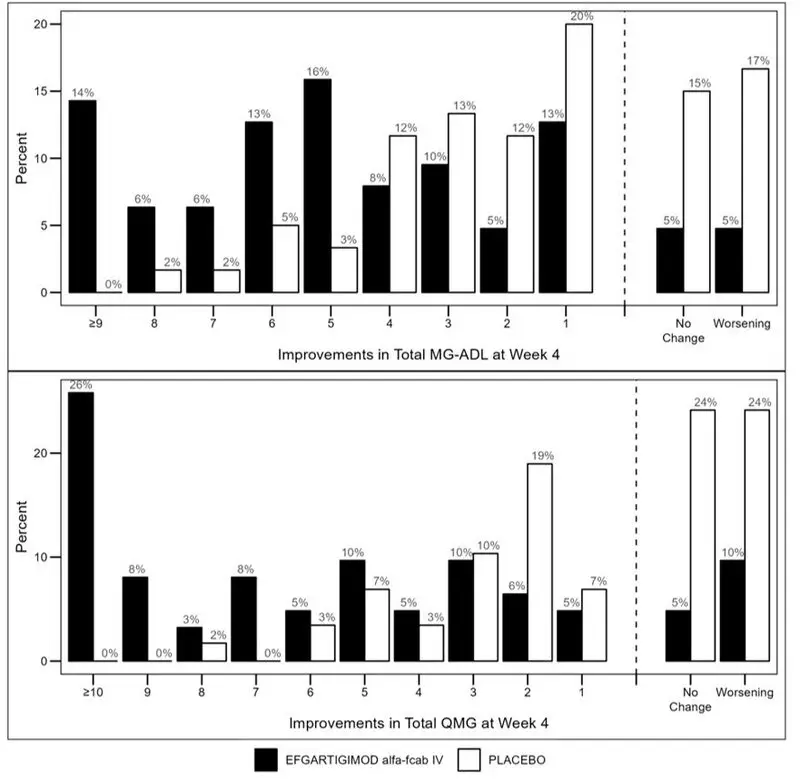 |
16. How is Vyvgart supplied
VYVGART (efgartigimod alfa-fcab) injection is a preservative free, sterile, colorless to slightly yellow, clear to slightly opalescent solution supplied as 400 mg/20 mL (20 mg/mL) in one single-dose vial per carton (NDC 73475-3041-5).
| VYVGART
efgartigimod alfa injection |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - argenx US (116702819) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Vetter (DP) | 316126754 | MANUFACTURE(73475-3041) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Lonza Biologics, plc. | 230794810 | API MANUFACTURE(73475-3041) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Lonza Biologics Tuas Pte. Ltd. | 936939342 | API MANUFACTURE(73475-3041) | |





