Drug Detail:Xdemvy (Lotilaner 0.25% ophthalmic solution)
Drug Class: Ophthalmic anti-infectives
Highlights of Prescribing Information
XDEMVY™ (lotilaner ophthalmic solution) 0.25%, for topical ophthalmic use
Initial U.S. Approval: 2023
Indications and Usage for Xdemvy Eye Drops
XDEMVY is an ectoparasiticide (anti-parasitic) indicated for the treatment of Demodex blepharitis. (1)
Xdemvy Eye Drops Dosage and Administration
Instill one drop of XDEMVY in each eye twice daily (approximately 12 hours apart) for 6 weeks. (2)
Dosage Forms and Strengths
Ophthalmic solution containing lotilaner 0.25%. (3)
Contraindications
None. (4)
Adverse Reactions/Side Effects
The most common adverse reaction was instillation site stinging and burning (10%). (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Tarsus Pharmaceuticals at 1-888-421-4002 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 7/2023
Related/similar drugs
Maxitrol, Neo-Poly-Dex, Cortisporin Ophthalmic Suspension, lotilaner ophthalmicFull Prescribing Information
1. Indications and Usage for Xdemvy Eye Drops
XDEMVY is indicated for the treatment of Demodex blepharitis.
2. Xdemvy Eye Drops Dosage and Administration
Instill one drop of XDEMVY in each eye twice daily (approximately 12 hours apart) for 6 weeks. If more than one topical ophthalmic drug is being used, the drugs should be administered at least five (5) minutes apart. If one dose is missed, treatment should continue with the next scheduled dose.
3. Dosage Forms and Strengths
XDEMVY (lotilaner ophthalmic solution) is provided as an ophthalmic solution containing lotilaner 0.25% (2.5 mg/mL).
5. Warnings and Precautions
5.1 Risk of Contamination
Do not allow the tip of the dispensing container to contact the eye, surrounding structures, fingers, or any other surface in order to minimize contamination of the solution. Serious damage to the eye and subsequent loss of vision may result from using contaminated solutions.
6. Adverse Reactions/Side Effects
6.1 Clinical Trials Experience
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
XDEMVY was evaluated in 833 patients with Demodex blepharitis in two randomized, double-masked, vehicle-controlled studies (Saturn-1 and Saturn-2) with 42 days of treatment. The most common ocular adverse reaction observed in controlled clinical studies with XDEMVY was instillation site stinging and burning which was reported in 10% of patients. Other ocular adverse reactions reported in less than 2% of patients were chalazion/hordeolum and punctate keratitis.
8. Use In Specific Populations
11. Xdemvy Eye Drops Description
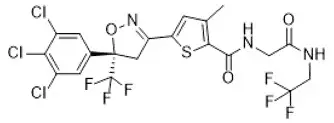
Lotilaner is a member of the isoxazoline family of compounds. Its chemical name is 2-Thiophenecarboxamide, 5-[(5S)-4,5-dihydro-5-(3,4,5-trichlorophenyl)-5-(trifluoromethyl)-3-isoxazolyl]-3-methyl-N-[2-oxo-2-[(2,2,2-trifluoroethyl)amino]ethyl]-2-thiophenecarboxamide. The molecular formula is C20H14Cl3F6N3O3S. The molecular weight is 596.76 g/mol. The chemical structure is:
XDEMVY is a sterile, preserved, multi-dose, slightly yellowish, slightly opalescent, topical ophthalmic solution containing lotilaner, 0.25% as the active ingredient. It is preserved with potassium sorbate and contains the following additional inactive ingredients: edetate disodium, hydroxypropyl methylcellulose (HPMC), polyoxyl 35 castor oil, glycerin, dibasic sodium phosphate, monobasic sodium phosphate, and water for injection.
12. Xdemvy Eye Drops - Clinical Pharmacology
12.1 Mechanism of Action
Lotilaner is a gamma-aminobutyric acid (GABA)-gated chloride channel inhibitor selective for mites. Inhibition of these GABA chloride channels causes a paralytic action in the target organism leading to its death. Lotilaner is not an inhibitor of mammalian GABA mediated chloride channels when tested at up to 30 µM (18 µg/mL) in vitro (approximately 1100 times the RHOD).
14. Clinical Studies
The safety and efficacy of XDEMVY for the treatment of Demodex blepharitis was evaluated in a total of 833 patients (415 of which received XDEMVY) in two 6-week, randomized, multicenter, double-masked, vehicle-controlled studies (Saturn-1 and Saturn-2). Patients with Demodex blepharitis were randomized to either XDEMVY or Vehicle at a 1:1 ratio dosed twice daily in each eye.
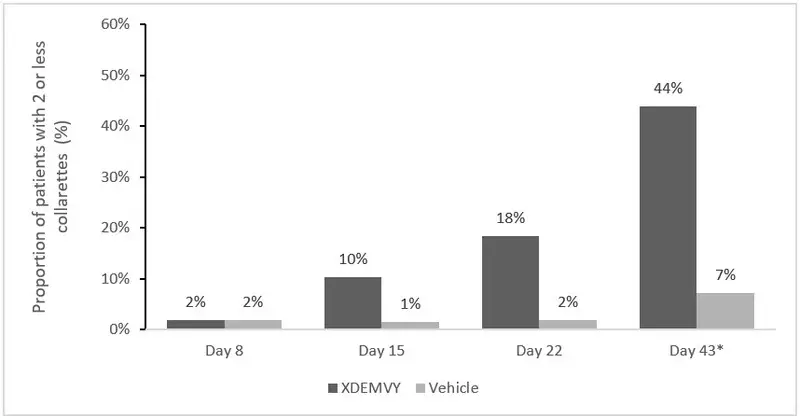
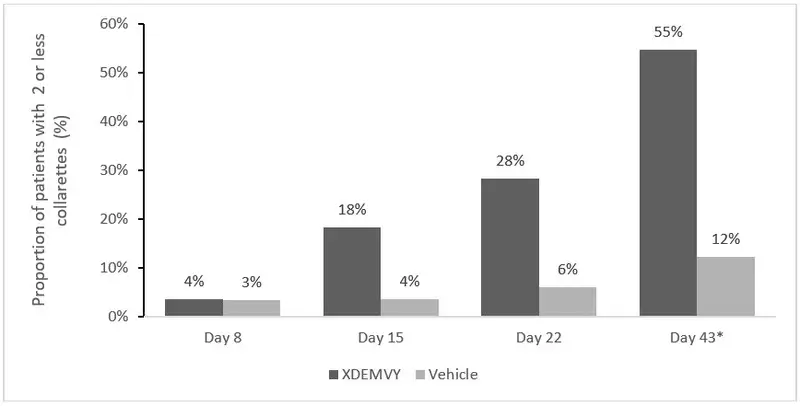
Efficacy was demonstrated by improvement in lids (reduction of collarettes to no more than 2 collarettes per upper lid) in each study (Saturn-1 and Saturn-2) (see Figure 1 and Figure 2) by Day 43.
Figure 1. Saturn-1: Proportion of patients with 2 or less collarettes for the upper eyelid
*Day 43 Primary Endpoint; XDEMVY N=209, Vehicle N=204, p-value <0.01
Figure 2. Saturn-2: Proportion of patients with 2 or less collarettes for the upper eyelid
*Day 43 Primary Endpoint; XDEMVY N=193, Vehicle N=200, p-value <0.01
The endpoints of mite eradication (mite density of 0 mites/lash) and erythema cure (Grade 0) of XDEMVY vs. Vehicle demonstrated statistically significant improvement at Day 43 across both Saturn-1 (Table 1) and Saturn-2 (Table 2) studies.
| XDEMVY (N=212) | Vehicle (N=209) | p-value | |
|---|---|---|---|
| Mite Eradication | 68% | 17% | < 0.01 |
| Erythema Cure | 19% | 7% | < 0.01 |
| XDEMVY (N=203) | Vehicle (N=209) | p-value | |
|---|---|---|---|
| Mite Eradication | 50% | 14% | < 0.01 |
| Erythema Cure | 30% | 9% | < 0.01 |
16. How is Xdemvy Eye Drops supplied
XDEMVY is supplied as a sterile ophthalmic solution in a white, opaque, low-density polyethylene (LDPE) multiple-dose bottle with a LDPE dropper tip and tan high-density polyethylene (HDPE) cap.
| 10 mL fill in a 11 mL container | NDC # 81942-125-01 |
| XDEMVY
lotilaner ophthalmic solution solution/ drops |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Tarsus Pharmaceuticals, Inc. (081222566) |