Drug Detail:Xelstrym (Dextroamphetamine [ dex-tro-am-fet-a-meen ])
Drug Class: CNS stimulants
Highlights of Prescribing Information
XELSTRYM ™ (dextroamphetamine) transdermal system, CII
Initial U.S. Approval: 1975
WARNING: ABUSE AND DEPENDENCE
See full prescribing information for complete boxed warning.
- CNS stimulants, including XELSTRYM, other amphetamine-containing products, and methylphenidate, have a high potential for abuse and dependence (5.1, 9.2, 9.3)
- Assess the risk of abuse prior to prescribing and monitor for signs of abuse and dependence while on therapy (5.1, 9.2)
Indications and Usage for Xelstrym Patch
XELSTRYM is a central nervous system (CNS) stimulant indicated for the treatment of Attention Deficit Hyperactivity Disorder (ADHD) in adults and pediatric patients 6 years and older (1)
Limitations of Use:
Pediatric patients with ADHD younger than 6 years of age experienced more long-term weight loss than patients 6 years and older (8.4)
Xelstrym Patch Dosage and Administration
- Pediatric patients (6 to 17 years): Recommended starting dose is 4.5 mg/9 hours. Titrate dosage in weekly increments of 4.5 mg up to a maximum recommended dose of 18 mg/9 hours (2.2)
- Adults: Recommended starting dose is 9 mg/9 hours. Maximum recommended dose is 18 mg/9 hours (2.2)
- Apply one XELSTRYM transdermal system 2 hours before an effect is needed and remove within 9 hours (2.3)
- Apply XELSTRYM to one of the following sites: hip, upper arm, chest, upper back or flank. Change the site of application when applying a new transdermal system (2.3)
- Do not substitute for other amphetamine products on a milligram-per-milligram basis because of different amphetamine base compositions and differing pharmacokinetic profiles (2.5)
- Severe renal impairment: Maximum recommended dose is 13.5 mg/9 hours (2.6)
- End stage renal disease (ESRD): Maximum recommended dose is 9 mg/9 hours (2.6)
Dosage Forms and Strengths
Transdermal system: 4.5 mg/9 hours, 9 mg/9 hours, 13.5 mg/9 hours, 18 mg/9 hours (3)
Contraindications
- Known hypersensitivity to amphetamine products or other ingredients in XELSTRYM (4)
- Use with monoamine oxidase inhibitor (MAOI), or within 14 days of the last MAOI dose (4, 7.1)
Warnings and Precautions
- Serious Cardiovascular Reactions: Sudden death has been reported in association with CNS stimulant treatment at recommended doses in pediatric patients with structural cardiac abnormalities or other serious heart problems. In adults, sudden death, stroke, and myocardial infarction have been reported. Avoid use in patients with known structural cardiac abnormalities, cardiomyopathy, serious heart rhythm abnormalities, or coronary artery disease (5.2)
- Blood Pressure and Heart Rate Increases: Monitor blood pressure and pulse. Consider benefits and risks before use in patients for whom blood pressure increases may be problematic (5.3)
- Psychiatric Adverse Reactions: May cause psychotic or manic symptoms in patients with no prior history, or exacerbation of symptoms in patients with pre-existing psychosis. Evaluate for bipolar disorder prior to XELSTRYM use (5.4)
- Suppression of Growth: Monitor height and weight in pediatric patients during treatment (5.5)
- Peripheral Vasculopathy, including Raynaud’s phenomenon: Stimulants are associated with peripheral vasculopathy, including Raynaud’s phenomenon. Careful observation for digital changes is necessary during treatment with ADHD stimulants (5.6)
- Serotonin Syndrome: Increased risk when co-administered with serotonergic agents (e.g., SSRIs, SNRIs, triptans), but also during overdosage situations. If it occurs, discontinue XELSTRYM and initiate supportive treatment (5.7, 10).
- Contact Sensitization: Use of XELSTRYM may lead to contact sensitization. Discontinue XELSTRYM if contact sensitization is suspected (5.8)
- Application Site Reactions: During wear time or immediately after removal of XELSTRYM, local skin reactions may occur. Select a different application site each day to limit the occurrence of skin reactions (5.9)
- External Heat: Avoid exposing XELSTRYM to external heat sources during wear because both the rate and extent of absorption are increased (5.10)
Adverse Reactions/Side Effects
Most common adverse reactions (incidence ≥2% and greater than the rate for placebo) in pediatric patients 6 to 17 years treated with XELSTRYM were decreased appetite, headache, insomnia, tic, abdominal pain, vomiting, nausea, irritability, blood pressure increased, and heart rate increased (6.1)
Most common adverse reactions (incidence ≥5% and at a rate at least twice placebo) in adults treated with lisdexamfetamine were decreased appetite, insomnia, dry mouth, diarrhea, nausea, and anxiety (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Noven Therapeutics, LLC at 1-877-567-7857 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
- Acidifying and Alkalinizing Agents: Agents that alter GI and urinary pH can alter blood levels of amphetamine. Acidifying agents can decrease amphetamine blood levels, while alkalinizing agents increase amphetamine blood levels (2.7, 7.1)
Use In Specific Populations
- Pregnancy: May cause fetal harm (8.1)
- Lactation: Breastfeeding not recommended (8.3)
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 11/2022
Full Prescribing Information
WARNING: ABUSE AND DEPENDENCE
CNS stimulants, including XELSTRYM, other amphetamine-containing products, and methylphenidate, have high potential for abuse and dependence. Assess the risk of abuse prior to prescribing and monitor for signs of abuse and dependence while on therapy [see WARNINGS AND PRECAUTIONS (5.1), and DRUG ABUSE AND DEPENDENCE (9.2, 9.3)]
1. Indications and Usage for Xelstrym Patch
XELSTRYM™ is indicated for the treatment of Attention Deficit Hyperactivity Disorder (ADHD) in adults and pediatric patients 6 years and older [see CLINICAL STUDIES (14)].
Limitations of Use
Pediatric patients younger than 6 years of age experienced more long-term weight loss than patients 6 years and older [USE IN SPECIFIC POPULATIONS (8.4)].
2. Xelstrym Patch Dosage and Administration
2.1 Important Information Prior to Initiating Treatment
Prior to initiating treatment with XELSTRYM, assess for the presence of cardiac disease (e.g., perform a careful history, family history of sudden death or ventricular arrhythmia, and physical exam) [see WARNINGS AND PRECAUTIONS (5.2)].
Assess the risk of abuse prior to prescribing and monitor for signs of abuse and dependence while on therapy. Maintain careful prescription records, educate patients about abuse, monitor for signs of abuse and overdose, and periodically re-evaluate the need for XELSTRYM use [see WARNINGS AND PRECAUTIONS (5.1) and DRUG ABUSE AND DEPENDENCE (9)].
2.2 Recommended Dosage
Pediatric Patients 6 to 17 years
- Recommended starting dose of XELSTRYM in pediatric patients 6 to 17 years is 4.5 mg/9 hours. Dosage may be adjusted in weekly increments of 4.5 mg up to a maximum recommended dose of 18 mg/9 hours.
Adults
- Recommended starting dose of XELSTRYM in adults is 9 mg/9 hours. Dosage may be adjusted up to a maximum recommended dose of 18 mg/9 hours.
Apply XELSTRYM to the application site 2 hours before an effect is needed and remove within 9 hours after application. Dose titration and final dosage should be individualized depending on clinical response and tolerability.
Pharmacological treatment of ADHD may be needed for an extended period. Periodically re-evaluate the long-term use of XELSTRYM and adjust dosage as needed.
2.3 Important Administration Instructions
- Apply one XELSTRYM transdermal system at a time for not more than 9 hours. Use only one XELSTRYM per 24 hours.
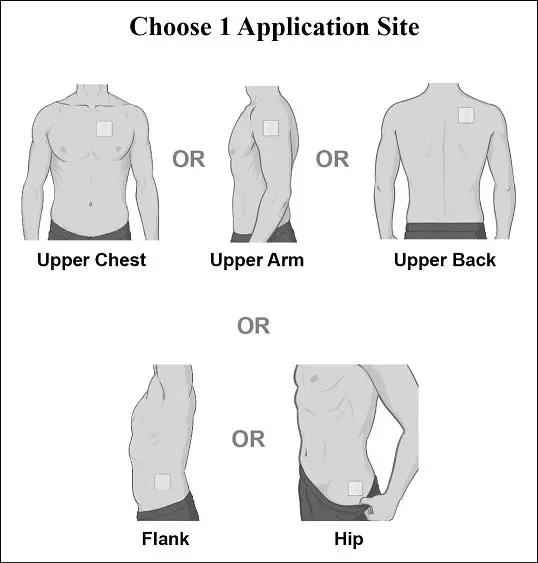
- Apply XELSTRYM to clean (void of lotions, oils, or gels), dry (not wet), and intact skin at the selected application site. Application sites include: hip, upper arm, chest, upper back, or flank. Select a different application site each time a new XELSTRYM transdermal system is applied [see WARNINGS AND PRECAUTIONS (5.9)].
- Avoid touching the adhesive side of XELSTRYM in order to avoid absorption of amphetamine. If the adhesive side is touched, immediately wash hands with soap and water.
- If the XELSTRYM transdermal system lifts at the edges, reattach XELSTRYM by pressing firmly and smoothing down the edges of the system. If XELSTRYM comes off completely, apply a new XELSTRYM transdermal system. XELSTRYM should not be applied or re-applied with dressings, tape or other common adhesives. Follow recommendations for discarding used and unused XELSTRYM [see DOSAGE AND ADMINISTRATION (2.4)].
- Avoid exposing the application site to direct external heat sources, such as hair dryers, heating pads, electric blankets, heated water beds, etc., while wearing XELSTRYM [see WARNINGS AND PRECAUTIONS (5.10)]. When heat is applied to XELSTRYM after application, both the rate and the extent of absorption are increased [see CLINICAL PHARMACOLOGY (12.3)].
2.4 Disposal Instructions
Upon removal of XELSTRYM, used transdermal systems should be folded so that the adhesive side of the system adheres to itself and placed in a lidded container. Used XELSTRYM should not be flushed down the toilet.
If the patient stops using the prescription, the patient should comply with local laws and regulations on drug disposal of CNS stimulants [see HOW SUPPLIED/STORAGE AND HANDLING (16)].
2.5 Switching from Other Amphetamine Products
For patients switching from another medication or any other amphetamine product, discontinue that treatment, and titrate with XELSTRYM using the titration schedule [see DOSAGE AND ADMINISTRATION (2.2)].
Do not substitute for other amphetamine products on a milligram-per-milligram basis because of different amphetamine base compositions and differing pharmacokinetic profiles [see CLINICAL PHARMACOLOGY (12.3)].
2.6 Dosage in Patients with Renal Impairment
In patients with severe renal impairment (GFR 15 to < 30 mL/min/1.73 m2), the maximum dose should not exceed 13.5 mg/9 hours. The maximum recommended dose in end stage renal disease (GFR < 15 mL/min/1.73 m2) patients is 9 mg/9 hours [see USE IN SPECIFIC POPULATIONS (8.6)].
3. Dosage Forms and Strengths
XELSTRYM is a translucent transdermal system with a printed backing on one side and a release liner on the other that is available in four strengths:
- 4.5 mg dextroamphetamine/9 hours
- 9 mg dextroamphetamine/9 hours
- 13.5 mg dextroamphetamine/9 hours
- 18 mg dextroamphetamine/9 hours
4. Contraindications
XELSTRYM is contraindicated in patients with:
- known hypersensitivity to amphetamine products or other components of XELSTRYM. Anaphylactic reactions, Stevens-Johnson Syndrome, angioedema, and urticaria have been observed in postmarketing reports [see ADVERSE REACTIONS (6.2)]
- patients taking monoamine oxidase inhibitors (MAOI), or within 14 days of stopping MAOIs (including MAOIs such as linezolid or intravenous methylene blue), because of an increased risk of hypertensive crisis [see WARNINGS AND PRECAUTIONS (5.7) and DRUG INTERACTIONS (7.1)]
5. Warnings and Precautions
5.1 Potential for Abuse and Dependence
CNS stimulants, including XELSTRYM, other amphetamine-containing products, and methylphenidate, have a high potential for abuse and dependence. Assess the risk of abuse prior to prescribing, and monitor for signs of abuse and dependence while on therapy [see DRUG ABUSE AND DEPENDENCE (9.2, 9.3)].
5.2 Serious Cardiovascular Reactions
Sudden death, stroke and myocardial infarction have been reported in adults with CNS stimulant treatment at recommended doses. Sudden death has been reported in pediatric patients with structural cardiac abnormalities and other serious heart problems taking CNS stimulants at recommended doses for ADHD. Avoid use in patients with known structural cardiac abnormalities, cardiomyopathy, serious heart arrhythmia, coronary artery disease, and other serious heart problems. Further evaluate patients who develop exertional chest pain, unexplained syncope, or arrhythmias during XELSTRYM treatment.
5.3 Blood Pressure and Heart Rate Increases
CNS stimulants cause an increase in blood pressure (mean increase about 2 to 4 mm Hg) and heart rate (mean increase about 3 to 6 bpm). Monitor all patients for potential tachycardia and hypertension.
5.4 Psychiatric Adverse Reactions
Exacerbation of Pre-existing Psychosis
CNS stimulants may exacerbate symptoms of behavior disturbance and thought disorder in patients with a pre-existing psychotic disorder.
Induction of a Manic Episode in Patients with Bipolar Disorder
CNS stimulants may induce a mixed/manic episode in patients with bipolar disorder. Prior to initiating treatment, screen patients for risk factors for developing a manic episode (e.g., comorbid or history of depressive symptoms or a family history of suicide, bipolar disorder, and depression).
New Psychotic or Manic Symptoms
CNS stimulants, at recommended doses, may cause psychotic or manic symptoms (e.g., hallucinations, delusional thinking, or mania) in patients without a prior history of psychotic illness or mania. If such symptoms occur, consider discontinuing XELSTRYM. In a pooled analysis of multiple short-term, placebo-controlled studies of CNS stimulants, psychotic or manic symptoms occurred in 0.1% of CNS stimulant-treated patients compared to 0% in placebo-treated patients.
5.5 Suppression of Growth
CNS stimulants have been associated with weight loss and slowing of growth rate in pediatric patients. Closely monitor growth (weight and height) in pediatric patients treated with CNS stimulants, including XELSTRYM. In a 7-week trial with a dose-optimization phase and a placebo-controlled phase of XELSTRYM in pediatric patients 6 to 17 years old with ADHD, there was a mean decrease in weight while taking XELSTRYM. Additionally, in studies of another CNS stimulant, there was slowing of the increase in height [see ADVERSE REACTIONS (6.1)].
Patients who are not growing or gaining height or weight as expected may need to have their treatment interrupted. XELSTRYM is not approved for use in pediatric patients below 6 years of age [see USE IN SPECIFIC POPULATIONS (8.4)].
5.6 Peripheral Vasculopathy, including Raynaud’s Phenomenon
Stimulants, including XELSTRYM, are associated with peripheral vasculopathy, including Raynaud's phenomenon. Signs and symptoms are usually intermittent and mild; however, very rare sequelae include digital ulceration and/or soft tissue breakdown. Effects of peripheral vasculopathy, including Raynaud's phenomenon, were observed in post-marketing reports at different times and at therapeutic doses in all age groups throughout the course of treatment. Signs and symptoms generally improve after reduction in dose or discontinuation of drug. Careful observation for digital changes is necessary during treatment with stimulants. Further clinical evaluation (e.g., rheumatology referral) may be appropriate for certain patients.
5.7 Serotonin Syndrome
Serotonin syndrome, a potentially life-threatening reaction, may occur when amphetamines are used in combination with other drugs that affect the serotonergic neurotransmitter systems such as monoamine oxidase inhibitors (MAOIs), selective serotonin reuptake inhibitors (SSRIs), serotonin norepinephrine reuptake inhibitors (SNRIs), triptans, tricyclic antidepressants, fentanyl, lithium, tramadol, tryptophan, buspirone, and St. John's Wort [see DRUG INTERACTIONS (7.1)]. The co-administration with cytochrome P450 2D6 (CYP2D6) inhibitors may also increase the risk with increased exposure to XELSTRYM. In these situations, consider an alternative non-serotonergic drug or an alternative drug that does not inhibit CYP2D6 [see DRUG INTERACTIONS (7.1)].
Serotonin syndrome symptoms may include mental status changes (e.g., agitation, hallucinations, delirium, and coma), autonomic instability (e.g., tachycardia, labile blood pressure, dizziness, diaphoresis, flushing, hyperthermia), neuromuscular symptoms (e.g., tremor, rigidity, myoclonus, hyperreflexia, incoordination), seizures, and/or gastrointestinal symptoms (e.g., nausea, vomiting, diarrhea).
Concomitant use of XELSTRYM with MAOI drugs is contraindicated [see CONTRAINDICATIONS (4)].
Discontinue treatment with XELSTRYM and any concomitant serotonergic agents immediately if symptoms of serotonin syndrome occur, and initiate supportive symptomatic treatment. If concomitant use of XELSTRYM with other serotonergic drugs or CYP2D6 inhibitors is clinically warranted, initiate XELSTRYM with lower doses, monitoring patients for the emergence of serotonin syndrome during drug initiation or titration, and inform patients of the increased risk for serotonin syndrome.
5.8 Contact Sensitization
Use of XELSTRYM may lead to contact sensitization (allergic contact dermatitis). Erythema is commonly seen with use of XELSTRYM and is not by itself an indication of sensitization. However, contact sensitization should be suspected if erythema is accompanied by evidence of a more intense local reaction (edema, papules, vesicles) that does not significantly improve within 48 hours or spreads beyond the application site. Confirmation of a diagnosis of contact sensitization may require further diagnostic testing [see CONTRAINDICATIONS (4)].
Manifestations of systemic sensitization may include a flare-up of previous dermatitis or of prior positive patch-test sites, or generalized skin eruptions in previously unaffected skin. Other systemic reactions may include headache, fever, malaise, arthralgia, diarrhea, or vomiting. No cases of systemic sensitization have been observed in clinical trials of XELSTRYM.
Patients who develop contact sensitization to XELSTRYM and require oral treatment with amphetamine should be initiated on oral medication under close medical supervision. Discontinue XELSTRYM if contact sensitization is suspected. It is possible that some patients sensitized to amphetamine by exposure to XELSTRYM may not be able to take amphetamine in any form.
5.9 Application Site Reactions
Local skin reactions, such as pain, pruritus, burning sensation, erythema, discomfort, edema, and/or swelling were reported during the wear time or immediately after removal of XELSTRYM [see ADVERSE REACTIONS (6.1)]. Patients who experienced discomfort and/or pain during the wear time reported resolution within 2 to 4 hours after application.
The potential for application site reactions and increased skin irritation, discomfort or pain may occur with XELSTRYM if the same application site is used repeatedly. Patients should select a different application site each day to minimize skin reactions.
5.10 Use of External Heat
When heat is applied to XELSTRYM after application, both the rate and extent of absorption are increased. After application of a heating pad, amphetamine exposure (AUC0-9h) was about 1.5-times greater than without heating pad application [see CLINICAL PHARMACOLOGY (12.3)]. Advise patients to avoid exposing XELSTRYM to direct external heat sources such as hair dryers, heating pads, electric blankets, heated water beds, etc., while wearing XELSTRYM.
6. Adverse Reactions/Side Effects
The following adverse reactions are discussed in greater detail in other sections of the labeling
- Known hypersensitivity to amphetamine products or other ingredients of XELSTRYM [see CONTRAINDICATIONS (4)]
- Hypertensive Crisis When Used Concomitantly with Monoamine Oxidase Inhibitors [see CONTRAINDICATIONS (4) and DRUG INTERACTIONS (7.1)]
- Drug Dependence [see BOXED WARNING, WARNINGS AND PRECAUTIONS (5.1) and DRUG ABUSE AND DEPENDENCE (9.2, 9.3)]
- Serious Cardiovascular Reactions [see WARNINGS AND PRECAUTIONS (5.2)]
- Blood Pressure and Heart Rate Increases [see WARNINGS AND PRECAUTIONS (5.3)]
- Psychiatric Adverse Reactions [see WARNINGS AND PRECAUTIONS (5.4)]
- Suppression of Growth [see WARNINGS AND PRECAUTIONS (5.5)]
- Peripheral Vasculopathy, including Raynaud's phenomenon [see WARNINGS AND PRECAUTIONS (5.6)]
- Serotonin Syndrome [see WARNINGS AND PRECAUTIONS (5.7)]
- Contact Sensitization [see WARNINGS AND PRECAUTIONS (5.8)]
- Application Site Reactions [see WARNINGS AND PRECAUTIONS (5.9)]
- Use of External Heat [see WARNINGS AND PRECAUTIONS (5.10)]
6.1 Clinical Trial Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
The safety of XELSTRYM for the treatment of ADHD in adults and pediatric patients 6 to 17 years is based on a study with XELSTRYM in pediatric patients (presented below) and adequate and well-controlled studies of lisdexamfetamine in adult and pediatric patients with ADHD.
XELSTRYM was studied in pediatric patients 6 to 17 years with ADHD. The safety data are from a 7-week study including a 5-week open-label dose optimization phase (n=110) followed by a 2-week randomized, parallel-group, crossover, placebo-controlled double-blind treatment phase (n=105) [see CLINICAL TRIALS (14)].
Adverse Reactions Leading to Discontinuation of Treatment
In the dose-optimization phase (no placebo comparator in this phase), 2.7% (3/110) of patients treated with XELSTRYM discontinued due to adverse reactions. These adverse reactions reported in one patient each were abdominal pain (0.9%), irritability (0.9%) and decreased appetite (0.9%). There were no discontinuations due to adverse reactions during the double-blind phase.
Adverse Reactions Occurring at an Incidence of 5% or More in XELSTRYM Treated Pediatric Patients Ages 6 to 17 Years During Dose-optimized Treatment
Adverse reactions (incidence of ≥ 5%) that occurred during the dose-optimization phase of the clinical study include: decreased appetite (54%), insomnia1 (32%), headache (21%), irritability (16%), abdominal pain2 (16%) affect lability3 (16%), application site pain4 (13%), nausea (9%), application site pruritus (7%), and fatigue (5%).
1insomnia includes insomnia, delayed sleep phase, initial insomnia, middle insomnia, and terminal insomnia
2abdominal pain includes abdominal pain and abdominal pain upper
3affect lability includes affect lability, emotional disorder, mood swings, and mood altered
4application site pain includes application site pain and application site burn
Adverse Reactions Occurring at an Incidence of 2% or More of XELSTRYM Treated Pediatric Patients Ages 6 to 17 Years During Double-blind Treatment
Adverse reactions (incidence of ≥ 2% and incidence greater than placebo) that occurred during the double-blind, placebo-controlled phase of the clinical study are shown in Table 1.
| *The following terms were combined: insomnia includes insomnia, delayed sleep phase, initial insomnia, middle insomnia, and terminal insomnia abdominal pain includes abdominal pain and abdominal pain upper affect lability includes affect lability, emotional disorder, mood swings, and mood altered application site pain includes application site pain and application site burn |
||
| System Organ Class Preferred Term | XELSTRYM All Doses (n = 105) % | Placebo (n = 105) % |
| Metabolism and nutrition disorders | ||
| Decreased appetite | 12 | 2 |
| Nervous system disorders | ||
| Headache | 6 | 4 |
| Psychiatric disorders | ||
| Insomnia | 8 | 6 |
| Affect lability | 3 | 0 |
| Tic | 2 | 0 |
| Gastrointestinal Disorders | ||
| Vomiting | 4 | 0 |
| Abdominal pain* | 4 | 2 |
| Nausea | 3 | 1 |
| General disorders and administration site conditions | ||
| Iritability | 2 | 1 |
| Investigations/Cardiac Disorders | ||
| Blood pressure increased* | 2 | 1 |
| Heart rate increased* | 2 | 0 |
Application Site Reactions
Based on daily patient diaries and dermal reaction scales at clinic assessments, local skin reactions were reported with XELSTRYM. During the wear time or immediately after removal of XELSTRYM, patients experienced pain, pruritus, burning sensation, erythema, discomfort, edema, and swelling.
Patients who experienced discomfort and pain at the application site during the wear time reported resolution within 2 to 4 hours after XELSTRYM application. Most dermal irritation was limited to the site of application. All patients who reported application site reactions in the 7- week pediatric classroom study continued to use XELSTRYM, and there were no discontinuations from the study due to application site reactions.
During the dose-optimization phase of the clinical study, 45% of patients reported application site discomfort associated with the use of XELSTRYM in daily patient diaries; 72% of patients reported discomfort at clinic visit assessments; and 13% of patients reported severe discomfort at clinic visit assessments. XELSTRYM 4.5 mg was the starting dose for all patients undergoing titration during the dose optimization phase and the majority of application site discomfort was reported at this starting dose. During the dose-optimization phase, 73% of patients reported application site irritation.
Application site reactions that occurred during the double-blind phase of the clinical study are presented in Table 2.
Table 2: Summary Application Site Reactions During the Double-Blind Phase
| XELSTRYM n/N | Placebo n/N |
|
| Discomfort | ||
| Reported in patient diaries | 8/96 (8%) | 8/98 (8%) |
| Clinic assessments | ||
| Any discomfort | 72/104 (69%) | 9/101 (9%) |
| Severe discomfort | 10/104 (10%) | 4/101 (4%) |
| Irritation | ||
| Reported in patient diaries | 64/103 (62%) | 41/105 (39%) |
| Reported at Clinic assessments | 97/103 (94%) | 55/101 (54%) |
Weight Loss and Slowing Growth Rate
In a 7-week trial of XELSTRYM with a 5-week dose optimization phase and a 2-week crossover placebo-controlled phase in pediatric patients ages 6 to 17 years, patients had a mean weight loss from baseline of -3.1 pounds after 5 weeks of XELSTRYM.
Leukopenia and Neutropenia
In the 2-week crossover phase of the 7-week trial of XELSTRYM in pediatric patients ages 6 to 17 years, shifts in WBCs from normal to low occurred in 10% of patients treated with XELSTRYM and 2% of patients treated with placebo. Shifts in neutrophils from normal to low occurred in 14% of patients treated with XELSTRYM and 6% of patients treated with placebo.
Weight Loss and Slowing Growth Rate in Pediatric Patients with ADHD with Lisdexamfetamine and Other Stimulants
Lisdexamfetamine
The long-term safety of XELSTRYM for the treatment of ADHD relies on information from adequate and well-controlled studies of lisdexamfetamine. In a controlled trial of lisdexamfetamine in pediatric patients 6 to 12 years, mean weight loss from baseline after 4 weeks of therapy was -0.9, -1.9, and -2.5 pounds, respectively, for patients receiving 30 mg, 50 mg, and 70 mg of lisdexamfetamine, compared to 1 pound weight gain for patients receiving placebo. Higher doses were associated with greater weight loss with 4 weeks of treatment. Careful follow-up for weight in pediatric patients 6 to 12 years who received lisdexamfetamine over 12 months suggests that consistently medicated pediatric patients (i.e., treatment for 7 days per week throughout the year) have a slowing in growth rate, measured by body weight as demonstrated by an age- and sex-normalized mean change from baseline in percentile, of -13.4 over 1 year (average percentiles at baseline and 12 months were 60.9 and 47.2, respectively). In a 4-week controlled trial of lisdexamfetamine in pediatric patients 13 to 17 years, mean weight loss from baseline to endpoint was -2.7, -4.3, and -4.8 pounds, respectively, for patients receiving 30 mg, 50 mg, and 70 mg of lisdexamfetamine, compared to a 2 pound weight gain for patients receiving placebo.
Other CNS stimulants
Careful follow-up of weight and height in pediatric patients 7 to 10 years who were randomized to either methylphenidate or non-medication treatment groups over 14 months, as well as in naturalistic subgroups of newly methylphenidate-treated and non-medication treated pediatric patients over 36 months (to the ages of 10 to 13 years), suggests that consistently medicated pediatric patients 7 to 13 years (i.e., treatment for 7 days per week throughout the year) have a temporary slowing in growth rate (on average, a total of about 2 cm less growth in height and 2.7 kg less growth in weight over 3 years), without evidence of growth rebound during this period of development. In a controlled trial of amphetamine (d- and l-enantiomer ration of 3:1) in pediatric patients 13 to 17 years, mean weight change from baseline within the initial 4 weeks of therapy was -1.1 pounds and -2.8 pounds, respectively, for patients receiving 10 mg and 20 mg of amphetamine. Higher doses were associated with greater weight loss within the initial 4 weeks of treatment [see WARNINGS AND PRECAUTIONS (5.5)].
Clinical Trials Experience in Adult Patients with ADHD Treated with Lisdexamfetamine
Adverse Reactions Associated with Discontinuation of Treatment in Adult ADHD Clinical Trials
In a controlled trial of lisdexamfetamine in adults with ADHD, 6% (21/358) of lisdexamfetamine-treated patients discontinued due to adverse reactions compared to 2% (1/62) of placebo-treated patients. The most frequently reported adverse reactions (1% or more and twice rate of placebo) were insomnia (8/358; 2%), tachycardia (3/358; 1%), irritability (2/358; 1%), hypertension (4/358; 1%), headache (2/358; 1%), anxiety (2/358; 1%), and dyspnea (3/358; 1%). Less frequently reported adverse reactions (less than 1% or less than twice rate of placebo) included palpitations, diarrhea, nausea, decreased appetite, dizziness, agitation, depression, paranoia and restlessness.
Adverse Reactions Occurring at an Incidence of ≥5% or More Among Lisdexamfetamine-Treated Patients with ADHD in Clinical Trials
The most common adverse reactions (incidence ≥5% and at a rate at least twice placebo) were: Decreased appetite, insomnia, dry mouth, diarrhea, nausea, and anxiety.
In addition, in the adult population, erectile dysfunction was observed in 2.6% of males on lisdexamfetamine and 0% on placebo; decreased libido was observed in 1.4% of subjects on lisdexamfetamine and 0% on placebo.
Weight Loss in Adults with ADHD
In a controlled adult trial of lisdexamfetamine, mean weight loss after 4 weeks of therapy was 2.8 pounds, 3.1 pounds, and 4.3 pounds, for patients receiving final doses of 30 mg, 50 mg, and 70 mg of lisdexamfetamine, respectively, compared to a mean weight gain of 0.5 pounds for patients receiving placebo.
Adverse Reactions with Other Amphetamine Products in Pediatric Patients and Adults with ADHD
Cardiac Disorders: Palpitations, tachycardia, and chest pain.
Gastrointestinal Disorders: Dry mouth, abdominal pain upper, dyspepsia, diarrhea, constipation, vomiting, nausea, and tooth disorder (e.g., teeth clenching, tooth infection).
General Disorders and Administration Site Conditions: Asthenia, fatigue, pyrexia, and feeling jittery.
Infections and Infestations: Infection, urinary tract infection.
Injury, Poisoning, and Procedural Complications: Accidental injury.
Investigations: Weight decreased, blood pressure increased, and ECG voltage criteria for ventricular hypertrophy.
Metabolism and Nutrition Disorders: Loss of appetite.
Musculoskeletal and Connective Tissue Disorders: Muscle twitching, growth retardation.
Nervous System Disorders: Somnolence, insomnia, tremor, dizziness, headache, tics, speech disorder (e.g., stuttering, excessive speech), psychomotor hyperactivity, and agitation.
Psychiatric Disorders: Depression, anxiety, dermatillomania, mood swings, anger, affect lability, logorrhea, irritability, nervousness, paranoia, and restlessness.
Reproductive System and Breast Disorders: Impotence, libido decreased, erectile dysfunction, and dysmenorrhea.
Respiratory, Thoracic, and Mediastinal Disorders: Dyspnea, rhinitis allergic.
Skin and Subcutaneous Tissue Disorders: Rash, photosensitivity reaction, and hyperhidrosis.
Vascular Disorders: Hypertension, epistaxis.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of amphetamines. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Cardiac Disorders: Palpitations, chest pain, sudden death, and myocardial infarction. There have been isolated reports of cardiomyopathy associated with chronic amphetamine use.
Eye Disorders: Vision blurred, diplopia, difficulties with visual accommodation, and mydriasis.
Gastrointestinal Disorders: Dysgeusia, constipation, intestinal ischemia and other gastrointestinal disturbances.
Hepatobiliary Disorders: Eosinophilic hepatitis.
Immune System Disorders: Urticaria, rash, hypersensitivity reactions including angioedema and anaphylactic reaction. Serious skin rashes, including Stevens-Johnson Syndrome and toxic epidermal necrolysis, have been reported.
Musculoskeletal and Connective Tissue Disorders: Rhabdomyolysis.
Nervous System Disorders: Seizures, overstimulation, restlessness, dyskinesia, tremor, tics, and paresthesia (including formication).
Psychiatric Disorders: Psychotic episodes at recommended doses, depression, logorrhea, aggression, anger, dermatillomania, bruxism, dysphoria, euphoria, and irritability.
Reproductive System and Breast Disorders: Impotence, changes in libido, and frequent or prolonged erections.
Skin and Subcutaneous Tissue Disorders: Alopecia.
Vascular Disorders: Raynaud's phenomenon.
7. Drug Interactions
7.1 Drugs Having Clinically Important Interactions with Amphetamine
Table 3: Drugs Having Clinically Important Interactions with Amphetamines
| MAO Inhibitors (MAOI) | |
| Clinical Impact | MAOI antidepressants slow amphetamine metabolism, increasing amphetamine’s effect on the release of norepinephrine and other monoamines from adrenergic nerve endings causing headaches and other signs of hypertensive crisis. Toxic neurological effects and malignant hyperpyrexia can occur, sometimes with fatal results. |
| Intervention | Do not administer XELSTRYM during or within 14 days following the administration of MAOI [see CONTRAINDICATIONS (4) and WARNINGS AND PRECAUTIONS (5.7)]. |
| Serotonergic Drugs | |
| Clinical Impact | The concomitant use of XELSTRYM and serotonergic drugs increases the risk of serotonin syndrome. |
| Intervention | Initiate with lower doses and monitor patients for signs and symptoms of serotonin syndrome, particularly during XELSTRYM initiation or dosage increase. If serotonin syndrome occurs, discontinue XELSTRYM and the concomitant serotonergic drug(s) [see WARNINGS AND PRECAUTIONS (5.7)]. |
| CYP2D6 Inhibitors | |
| Clinical Impact | The concomitant use of XELSTRYM and CYP2D6 inhibitors may increase the exposure of XELSTRYM compared to the use of the drug alone, and increase the risk of serotonin syndrome. |
| Intervention | Initiate with lower doses and monitor patients for signs and symptoms of serotonin syndrome, particularly during XELSTRYM initiation and after a dosage increase. If serotonin syndrome occurs, discontinue XELSTRYM and the CYP2D6 inhibitor [seeWARNINGS AND PRECAUTIONS (5.7) and OVERDOSAGE (10)]. |
| Alkalinizing Agents | |
| Clinical Impact | Urinary alkalinizing agents can increase blood levels and potentiate the action of amphetamine |
| Intervention | Co-administration of XELSTRYM and urinary alkalinizing agents should be avoided. |
| Acidifying Agents | |
| Clinical Impact | Urinary acidifying agents can lower blood levels and efficacy of amphetamines. |
| Intervention | Increase dose based on clinical response. |
| Tricyclic Antidepressants | |
| Clinical Impact | May enhance the activity of tricyclic or sympathomimetic agents causing striking and sustained increases in the concentration of dextroamphetamine in the brain; cardiovascular effects can be potentiated. |
| Intervention | Monitor frequently and adjust or use alternative therapy based on clinical response. |
7.2 Drugs Having No Clinically Important Interactions with Amphetamine
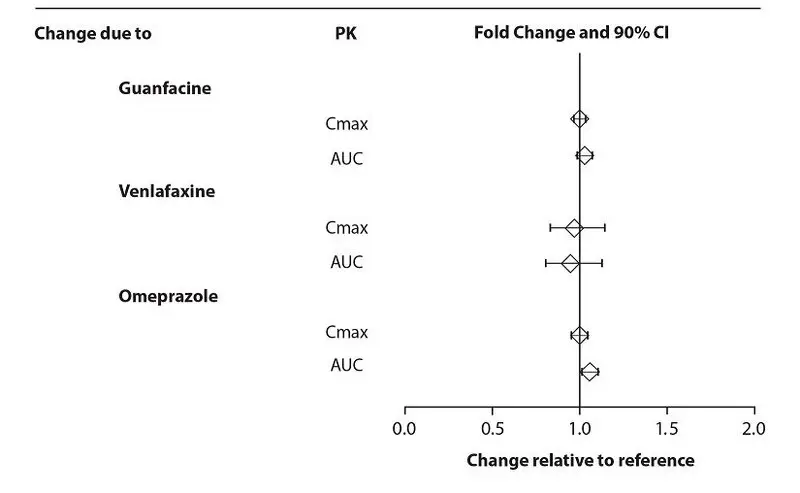
From a pharmacokinetic perspective, no dose adjustment of XELSTRYM is necessary when XELSTRYM is co-administered with guanfacine, venlafaxine, or omeprazole. In addition, no dose adjustment of guanfacine or venlafaxine is needed when XELSTRYM is co-administered [see CLINICAL PHARMACOLOGY (12.3)].
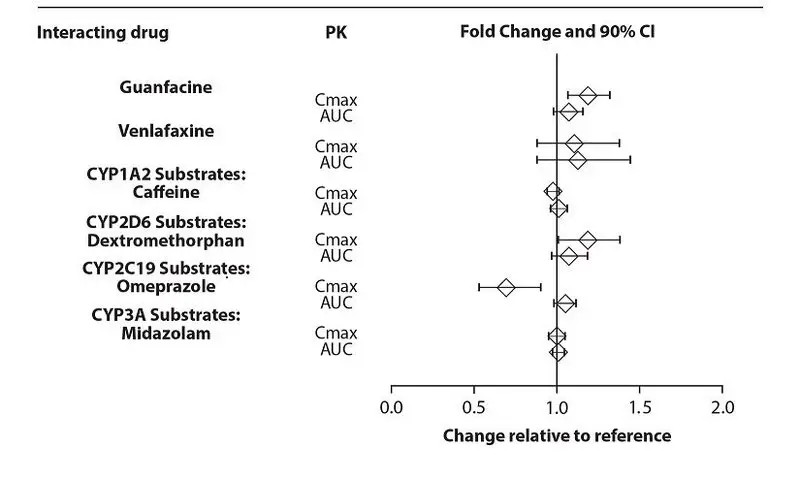
From a pharmacokinetic perspective, no dose adjustment for drugs that are substrates of CYP1A2 (e.g. theophylline, duloxetine, melatonin), CYP2D6 (e.g. atomoxetine, desipramine, venlafaxine), CYP2C19 (e.g. omeprazole, lansoprazole, clobazam), and CYP3A4 (e.g. midazolam, pimozide, simvastatin) is necessary when XELSTRYM is co-administered [see CLINICAL PHARMACOLOGY (12.3)].
8. Use In Specific Populations
8.1 Pregnancy
Pregnancy Exposure Registry
There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to ADHD medications, including XELSTRYM, during pregnancy. Healthcare providers are encouraged to register patients by calling the National Pregnancy Registry for Psychiatric Medications at 1-866-961-2388 or visiting online at https://womensmentalhealth.org/research/pregnancyregistry/adhd-medications/.
Risk Summary
Available data from published epidemiologic studies and postmarketing reports on use of prescription amphetamine in pregnant women have not identified a drug-associated risk of major birth defects and miscarriage (see DATA). Adverse pregnancy outcomes, including premature delivery and low birth weight, have been seen in infants born to mothers taking amphetamines during pregnancy [see CLINICAL CONSIDERATIONS].
No apparent effects on morphological development were observed in embryo-fetal development studies, with oral administration of amphetamine to rats and rabbits during organogenesis. However, in a pre- and post-natal development study, amphetamine (d- to l- ratio of 3:1) administered orally to pregnant rats during gestation and lactation caused a decrease in pup survival and a decrease in pup body weight that correlated with a delay in developmental landmarks at clinically relevant doses of amphetamine. In addition, adverse effects on reproductive performance were observed in pups whose mothers were treated with amphetamine. Long-term neurochemical and behavioral effects have also been reported in animal developmental studies using clinically relevant doses of amphetamine (see DATA).
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Fetal/Neonatal Adverse Reactions
Amphetamines, such as XELSTRYM, cause vasoconstriction and thereby may decrease placental perfusion. In addition, amphetamines can stimulate uterine contractions, increasing the risk of premature delivery. Infants born to mothers taking amphetamines during pregnancy have an increased risk of premature delivery and low birth weight.
Monitor infants born to mothers taking amphetamines for symptoms of withdrawal such as feeding difficulties, irritability, agitation, and excessive drowsiness.
Animal Data
Amphetamine (d- to l- enantiomer ratio of 3:1) had no apparent effects on embryofetal morphological development or survival when administered orally to pregnant rats and rabbits throughout the period of organogenesis at doses of up to 6 and 16 mg/kg/day, respectively. Fetal malformations and death have been reported in mice following parenteral administration of amphetamine doses of 50 mg/kg/day or greater to pregnant animals. Administration of these doses was also associated with severe maternal toxicity.
A study was conducted in which pregnant rats received daily oral doses of amphetamine (d- to l- enantiomer ratio of 3:1) of 2, 6, and 10 mg/kg from gestation day 6 to lactation day 20. All doses caused hyperactivity and decreased weight gain in the dams. A decrease in pup survival was seen at all doses. A decrease in pup body weight was seen at 6 and 10 mg/kg which correlated with delays in developmental landmarks, such as preputial separation and vaginal opening. Increased pup locomotor activity was seen at 10 mg/kg on day 22 postpartum but not at 5 weeks postweaning. When pups were tested for reproductive performance at maturation, gestational weight gain, number of implantations, and number of delivered pups were decreased in the group whose mothers had been given 10 mg/kg.
A number of studies from the literature in rodents indicate that prenatal or early postnatal exposure to amphetamine (d- or d, l-) at doses similar to those used clinically can result in long-term neurochemical and behavioral alterations. Reported behavioral effects include learning and memory deficits, altered locomotor activity, and changes in sexual function.
8.2 Lactation
Risk Summary
Based on limited case reports in published literature, amphetamine (d- or d, l-) is present in human milk, at relative infant doses of 2% to 13.8% of the maternal weight-adjusted dosage and a milk/plasma ratio ranging between 1.9 and 7.5. There are no reports of adverse effects on the breastfed infant. Long-term neurodevelopmental effects on infants from amphetamine exposure are unknown. It is possible that large dosages of amphetamine might interfere with milk production, especially in women whose lactation is not well established. Because of the potential for serious adverse reactions in nursing infants, including serious cardiovascular reactions, blood pressure and heart rate increase, suppression of growth, and peripheral vasculopathy, advise patients that breastfeeding is not recommended during treatment with XELSTRYM.
8.4 Pediatric Use
Safety and effectiveness of XELSTRYM have been established in pediatric patients with ADHD ages 6 to 17 years [see ADVERSE REACTIONS (6.1), CLINICAL PHARMACOLOGY (12.3), and CLINICAL STUDIES (14)].
Safety and effectiveness of XELSTRYM have not been established in pediatric patients below the age of 6 years.
Safety and efficacy of lisdexamfetamine were evaluated in a double-blind, randomized, parallel-group, placebo-controlled, fixed-dose study in pediatric patients 4 to 5 years with ADHD, followed by a 1-year open-label extension study. In these studies, patients experienced elevated rates of adverse reactions, including weight loss, decreased BMI, decreased appetite, insomnia, infections (upper respiratory and nasopharyngitis), irritability, and affect lability.
Growth Suppression
Growth should be monitored during treatment with stimulants, including XELSTRYM, and pediatric patients who are not growing or gaining weight as expected may need to have their treatment interrupted [see WARNINGS AND PRECAUTIONS (5.5) and ADVERSE REACTIONS (6.1)].
Juvenile Animal Data
Juvenile rats treated with mixed amphetamine salts early in the postnatal period through sexual maturation demonstrated transient changes in motor activity. Learning and memory were impaired. No recovery was seen following a drug free period. A delay in sexual maturation was observed, although there was no effect on fertility.
In a juvenile developmental study, rats received daily oral doses of amphetamine (d- to l- enantiomer ratio of 3:1) of 2, 6, or 20 mg/kg on days 7-13 of age; from day 14 to approximately day 60 of age, these doses were given twice daily for total daily doses of 4, 12, or 40 mg/kg. Post dosing hyperactivity was seen at all doses; motor activity measured prior to the daily dose was decreased during the dosing period but the decreased motor activity was largely absent after an 18 day drug-free recovery period. Performance in the Morris water maze test for learning and memory was impaired at the 40 mg/kg dose, and sporadically at the lower doses, when measured prior to the daily dose during the treatment period; no recovery was seen after a 19 day drug-free period. A delay in the developmental milestones of vaginal opening and preputial separation was seen at 40 mg/kg but there was no effect on fertility.
8.5 Geriatric Use
Clinical studies of XELSTRYM did not include subjects over 65 years to determine whether they respond differently from younger subjects. Other reported clinical experience and pharmacokinetic data [see CLINICAL PHARMACOLOGY (12.3)] have not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should start at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
8.6 Renal Impairment
Due to reduced clearance in patients with severe renal impairment (GFR 15 to < 30 mL/min/1.73 m2), the maximum XELSTRYM dose should not exceed 13.5 mg/9 hours. The maximum recommended dose in end stage renal disease (GFR < 15 mL/min/1.73 m2) patients is 9 mg/9 hours XELSTRYM.
Dextroamphetamine is not dialyzable.
9. Drug Abuse and Dependence
9.2 Abuse
CNS stimulants, including XELSTRYM, other amphetamines, and methylphenidate-containing products have a high potential for abuse. Abuse is the intentional non-therapeutic use of a drug, even once, to achieve a desired psychological or physiological effect. Drug addiction is a cluster of behavioral, cognitive, and physiological phenomena that may include a strong desire to take the drug, difficulties in controlling drug use (e.g., continuing drug use despite harmful consequences, giving a higher priority to drug use than other activities and obligations), and possible tolerance or physical dependence. Both abuse and misuse may lead to addiction, and some individuals may develop addiction even when taking XELSTRYM as prescribed.
Signs and symptoms of CNS stimulant abuse may include increased heart rate, respiratory rate, blood pressure, and/or sweating, dilated pupils, hyperactivity, restlessness, insomnia, decreased appetite, loss of coordination, tremors, flushed skin, vomiting, and/or abdominal pain. Anxiety, psychosis, hostility, aggression, and suicidal or homicidal ideation have also been observed. Individuals who abuse CNS stimulants may chew, snort, inject, or use other unapproved routes of administration, which can result in overdose and death [see OVERDOSAGE(10)].
To reduce the abuse of XELSTRYM, assess the risk of abuse prior to prescribing. After prescribing, keep careful prescription records, educate patients and their families about abuse and on proper storage and disposal of CNS stimulants, monitor for signs of abuse while on therapy, and re-evaluate the need for XELSTRYM use.
9.3 Dependence
Physical Dependence
XELSTRYM may produce physical dependence from continued therapy. Physical dependence is a state that develops as a result of physiological adaptation in response to repeated drug use, manifested by withdrawal signs and symptoms after abrupt discontinuation or a significant dose reduction of a drug. Withdrawal symptoms after abrupt cessation following prolonged high-dosage administration of CNS stimulants include dysphoric mood; depression; fatigue; vivid, unpleasant dreams; insomnia or hypersomnia; increased appetite; and psychomotor retardation or agitation.
Tolerance
XELSTRYM may produce tolerance from continued therapy. Tolerance is a physiological state characterized by a reduced response to a drug after repeated administration (i.e., a higher dose of a drug is required to produce the same effect that was once obtained at a lower dose).
10. Overdosage
Manifestations of amphetamine overdose include restlessness, tremor, hyperreflexia, rapid respiration, confusion, assaultiveness, hallucinations, panic states, hyperpyrexia, and rhabdomyolysis. Fatigue and depression usually follow the central nervous system stimulation. Serotonin syndrome has been reported with amphetamine use. Cardiovascular effects include arrhythmias, hypertension or hypotension and circulatory collapse. Gastrointestinal symptoms include nausea, vomiting, diarrhea, and abdominal cramps. Fatal poisoning is usually preceded by convulsions and coma.
Dextroamphetamine is not dialyzable.
Remove all transdermal systems immediately and cleanse the area(s) to remove any remaining adhesive. The continuing absorption of dextroamphetamine from the skin, even after removal of the transdermal system, should be considered when treating patients with overdose.
Consider contacting a Poison Center (1-800-222-1222) or a medical toxicologist for additional management recommendations. Individual patient response to amphetamines varies widely. Toxic symptoms may occur idiosyncratically at low doses.
11. Xelstrym Patch Description
XELSTRYM (dextroamphetamine) transdermal system, contains dextroamphetamine, a CNS stimulant.
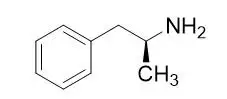
Dextroamphetamine is the dextro isomer of the compound d,l-amphetamine. The chemical name for dextroamphetamine is (2S)-1-phenylpropan-2-amine. It is a clear to slightly amber colored liquid. Molecular weight of dextroamphetamine is 135.21 g/mol and the molecular formula is C9H13N. The chemical structure is:
XELSTRYM is provided in four strengths: 4.5 mg/9 hours, 9 mg/9 hours, 13.5 mg/9 hours, and 18 mg/9 hours. The composition per unit area of all dosage strengths is identical. Inactive ingredients include: acrylic adhesives, green ink, polyester/polyurethane backing, and polyester release liner.
Table 4: XELSTRYM (dextroamphetamine) transdermal system
| Dosage Strength (dextroamphetamine) | Dextroamphetamine Content per Transdermal System | Transdermal System Size |
| 4.5 mg / 9 hours | 5 mg | 4.76 cm2 |
| 9 mg / 9 hours | 10 mg | 9.52 cm2 |
| 13.5 mg / 9 hours | 15 mg | 14.29 cm2 |
| 18 mg / 9 hours | 20 mg | 19.05 cm2 |
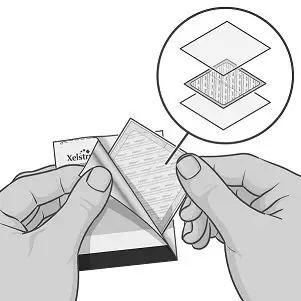
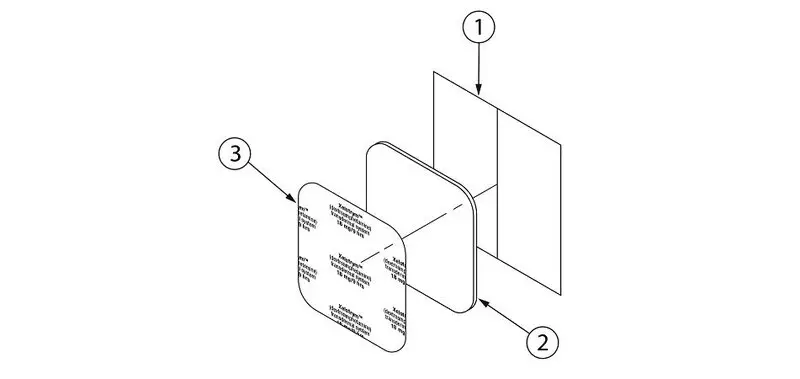
Transdermal System Components
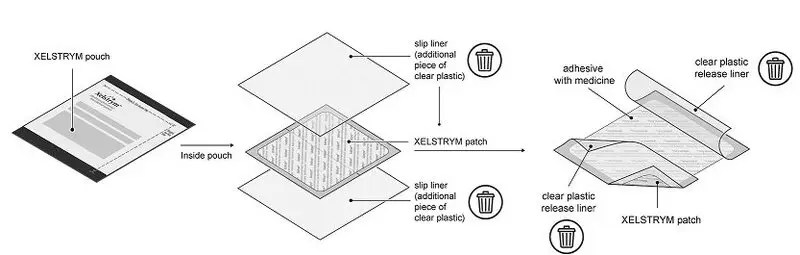
XELSTRYM consists of three layers (Figure 1). The layers are (1) oversized protective silicone-coated polyester release liner that is removed and discarded prior to application (2) acrylic adhesive matrix containing dextroamphetamine, and (3) polyester and polyurethane laminate film (backing).
Figure 1: XELSTRYM Transdermal System (Exploded View)
12. Xelstrym Patch - Clinical Pharmacology
12.1 Mechanism of Action
Amphetamines are non-catecholamine sympathomimetic amines with CNS stimulant activity. The exact mode of therapeutic action in ADHD is not known.
12.2 Pharmacodynamics
Amphetamines block the reuptake of norepinephrine and dopamine into the presynaptic neuron and increase the release of these monoamines into the extraneuronal space.
12.3 Pharmacokinetics
Following a single 9-hour application of XELSTRYM in pediatric patients 6 to 12 years with ADHD, the Cmax and AUC of dextroamphetamine were dose-proportional over the dose range of 4.5 mg/9 hours to 18 mg/9 hours.
After a single dose of 18 mg/9 hours of XELSTRYM or 70 mg of lisdexamfetamine in adults, the peak plasma concentration (Cmax) of dextroamphetamine were 44.6 ng/mL and 67.6 ng/mL, respectively; and area under concentration curve (AUCinf) of dextroamphetamine were 996 ng*h/mL and 1260 ng*h/mL, respectively. Median time-to-peak concentrations (tmax) was 9 hours for XELSTRYM and 4 hours for lisdexamfetamine.
The plasma PK profiles of dextroamphetamine following administration of XELSTRYM and lisdexamfetamine are shown in Figure 2.
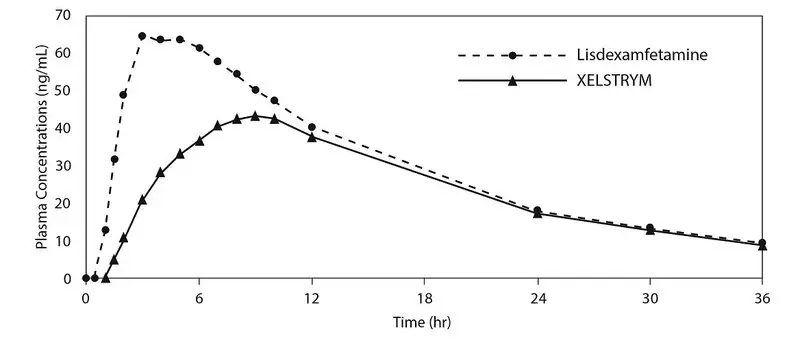
After multiple dosing of 18 mg/9 hours of XELSTRYM with application site rotation in adults or 70 mg of lisdexamfetamine (simulated), Cmax of dextroamphetamine were 68.8 ng/mL and 84.5 ng/mL, respectively; AUC0-24 of dextroamphetamine were 1150 ng*h/mL and 1248 ng*h/mL, respectively.
Absorption
The amount of dextroamphetamine absorbed systemically is a function of both wear time and transdermal system size. Peak plasma levels of dextroamphetamine were typically reached at 6 to 9 hours after single application and 6 hours after repeat applications of XELSTRYM when worn up to 9 hours.
On average, approximately 90% of dextroamphetamine is delivered from the transdermal system over 9 hours. Inter-individual variability for XELSTRYM as coefficient of variation (%CV) for the dextroamphetamine Cmax and AUC was generally about 20% to 30%.
After repeat applications of XELSTRYM for 4 weeks in adults with ADHD, there was 46% increase in Cmax and 54% increase in AUC0-24 when applied with rotating application sites for each transdermal system. There was 86% increase in Cmax and 104% increase in AUC0-24 when XELSTRYM was applied on the same site for 28 days.
Application of a heating pad on XELSTRYM for 6 consecutive hours led to a faster absorption rate (median Tmax about 6.5 hours) as compared with XELSTRYM without a heating pad (median Tmax about 8.5 hours). Geometric least square mean ratios for dextroamphetamine exposure, calculated as Cmax and AUC0-9h, were about 116% and 150%, respectively, compared with XELSTRYM without a heating pad, indicating the apparent heat effect on dextroamphetamine absorption.
The application of XELSTYRM to different sites (hip, upper arm, chest, upper back and flank) did not alter dextroamphetamine PK.
Elimination
When XELSTRYM is removed after 9 hours wear time, the mean apparent elimination half-life of dextroamphetamine ranged from 6.4 to 11.5 hours in the pediatric and adult population, respectively.
Metabolism
Amphetamine is reported to be oxidized at the 4 position of the benzene ring to form 4-hydroxyamphetamine, or on the side chain α or β carbons to form alpha-hydroxy-amphetamine or norephedrine, respectively. Norephedrine and 4-hydroxy-amphetamine are both active and each is subsequently oxidized to form 4-hydroxy-norephedrine. Alpha-hydroxy-amphetamine undergoes deamination to form phenylacetone, which ultimately forms benzoic acid and its glucuronide and the glycine conjugate hippuric acid. Although the enzymes involved in amphetamine metabolism have not been clearly defined, CYP2D6 is known to be involved with formation of 4-hydroxy-amphetamine. Since CYP2D6 is genetically polymorphic, population variations in amphetamine metabolism are a possibility.
Excretion
With normal urine pHs, approximately half of an administered oral dose of amphetamine is recoverable in urine as derivatives of alpha-hydroxy-amphetamine and approximately another 30-40% of the dose is recoverable in urine as amphetamine itself. Since amphetamine has a pKa of 9.9, urinary recovery of amphetamine is highly dependent on pH and urine flow rates. Alkaline urine pHs result in less ionization and reduced renal elimination, and acidic pHs and high flow rates result in increased renal elimination with clearances greater than glomerular filtration rates, indicating the involvement of active secretion. Urinary recovery of amphetamine has been reported to range from 1% to 75%, depending on urinary pH, with the remaining fraction of the dose hepatically metabolized. Consequently, both hepatic and renal dysfunction have the potential to inhibit the elimination of amphetamine and result in prolonged exposures. In addition, drugs that affect urinary pH are known to alter the elimination of amphetamine, and any decrease in amphetamine's metabolism that might occur due to drug interactions or genetic polymorphisms is more likely to be clinically significant when renal elimination is decreased [see DRUG INTERACTIONS (7)].
Specific Populations
The shapes of pharmacokinetic profiles after XELSTRYM were generally similar between the pediatric and the adult population. Based on population PK, the median Cmax in pediatric patients 6 to 17 years are predicted to be 120% and 180%, respectively, the median AUC in pediatric patients are predicted to be 112% and 148%, respectively, of those in adults with a dose of 18 mg/9 hours.
Exposures of dextroamphetamine in specific populations evaluated with lisdexamfetamine are summarized in Figure 3.
Figure 3: Dextroamphetamine Exposures in Specific Populations
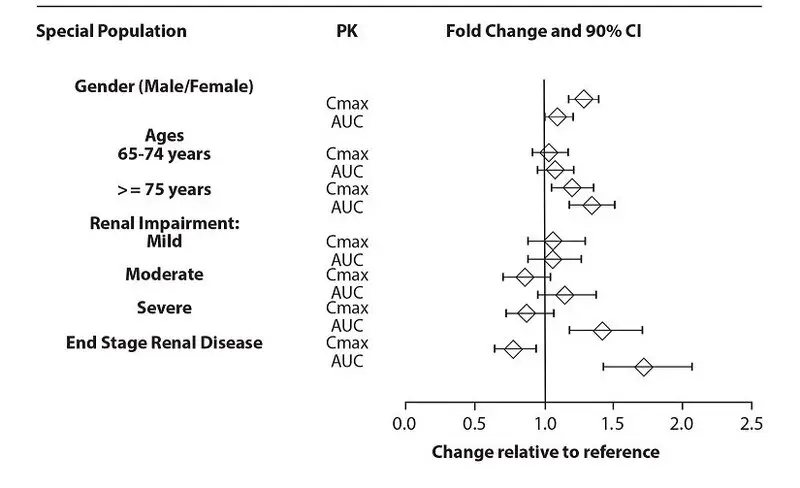
*Figure 3 shows the geometric mean ratios and the 90% confidence limits for Cmax and AUC of dextroamphetamine. Comparison for gender uses males as the reference. Comparison for age uses 55-64 years as the reference
Drug Interaction Studies
Effects of other drugs on the exposures of dextroamphetamine evaluated with lisdexamfetamine are summarized in Figure 4.
Figure 4: Effect of Other Drugs on Dextroamphetamine
The effects of dextroamphetamine on the exposures of other drugs evaluated with lisdexamfetamine are summarized in Figure 5.
Figure 5: Effects of Dextroamphetamine on Other Drugs
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
No evidence of carcinogenicity was found in studies in which d,l-amphetamine (enantiomer ratio of 1:1) was administered to mice and rats in the diet for 2 years at doses of up to 30 mg/kg/day in male mice, 19 mg/kg/day in female mice, and 5 mg/kg/day in male and female rats. Dermal carcinogenicity studies with dextroamphetamine were not conducted.
Mutagenesis
Amphetamine, in the enantiomer ratio d- to l- ratio of 3:1, was not clastogenic in the mouse bone marrow micronucleus test in vivo and was negative when tested in the E. coli component of the Ames test in vitro. d,l-Amphetamine (1:1 enantiomer ratio) has been reported to produce a positive response in the mouse bone marrow micronucleus test, an equivocal response in the Ames test, and negative responses in the in vitro sister chromatid exchange and chromosomal aberration assays.
Impairment of Fertility
Amphetamine, in the enantiomer ratio d- to l- ratio of 3:1, did not adversely affect fertility or early embryonic development in the rat at doses of up to 20 mg/kg/day.
14. Clinical Studies
The efficacy of XELSTRYM for the treatment of ADHD in adults and pediatric patients 6 to 17 years was established in a study with XELSTRYM in pediatric patients (presented below) and also based on adequate and well-controlled studies of lisdexamfetamine in pediatric and adult patients. Efficacy of lisdexamfetamine in the treatment of ADHD has been established in three short-term trials in pediatric patients 6 to 12 years, one short-term trial in pediatric patients 13 to 17 years, one short-term trial in pediatric patients 6 to 17 years, two short-term trials in adults 18 to 55 years, and two randomized withdrawal trials in pediatric patients 6 to 17 years and adults 18 to 55 years.
Pediatric Patients 6 to 17 years with ADHD
The efficacy of XELSTRYM for the treatment of ADHD in pediatric patients 6 to 17 years was evaluated in a multi-center, randomized, double-blind, placebo-controlled, cross-over design, modified analog classroom study (Study 1; NCT01711021). The study was conducted in 110 patients who met DSM-IV-TR criteria for ADHD.
Following a 5-week open-label, dose optimization phase with XELSTRYM (4.5 mg/9 hours, 9 mg/9 hours, 13.5 mg/9 hours and 18 mg/9 hours), patients were randomized to one of two treatment sequences: 1) XELSTRYM (optimized dose) followed by placebo, each for one week, or 2) placebo followed by XELSTRYM (optimized dose), each for one week. Efficacy was assessed at the end of each week using the Swanson, Kotkin, Agler, M.Flynn, and Pelham (SKAMP) total score, a validated 13-item rating scale to assess manifestations of ADHD in a classroom setting. Items are specific to place (classroom setting) and time (during a typical classroom period), and the scale is used to assess multiple ratings taken within a day.
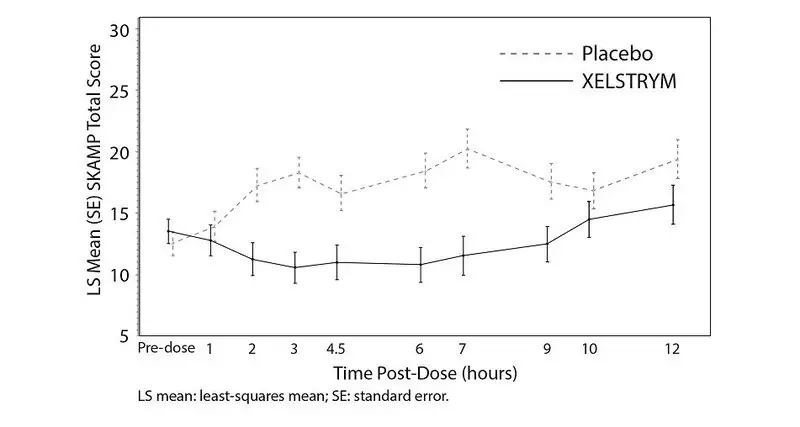
Efficacy was solely based on data from Period 1, which was the first week of the two-week double-blind, placebo-controlled, crossover treatment phase. A statistically significant separation from placebo was observed with use of XELSTRYM in Period 1 (Table 5). Changes in SKAMP total scores assessed at pre-dose (-0.5 hours) and at 1, 2, 3, 4.5, 6, 7, 9, 10, and 12 hours post-application are presented in Figure 6.
| SD: standard deviation; SE: standard error; LS Mean: least-squares mean; CI: confidence interval. aStatistically significant to placebo. bPre-dose score on Period 1 classroom day. cLS mean over hours 1, 2, 3, 4.5, 6, 7, 9, 10, and 12 hours post-dose on Period 1 classroom day. dDifference (drug minus placebo) in least-squares means on Period 1 classroom day. |
||||
| Study Number | Treatment Group | Pre-Dose Score on Classroom Dayb Mean (SD) | LS Meanc
(SE) | Placebo-subtracted Differenced
(95% CI) |
| Study 1 | XELSTRYMa | 13.6 (5.9) | 12.4 (1.2) | -4.7 (-8.0, -1.4) |
| Placebo | 12.7 (7.9) | 17.1 (1.2) | --- | |
Adhesion
Based on a clinical study in adult subjects wearing XELSTRYM 18 mg/9 hours, 233 of 238 transdermal systems (98%) exhibited 75% or greater surface area adhesion at all timepoints evaluated (every hour) throughout the 9-hour wear period. In another study in which pediatric patients 6 to 17 years and adult patients wearing XELSTRYM 4.5 mg/9 hours or 18 mg/9 hours were not confined to the clinical unit, 50 out of 58 transdermal systems (86%) exhibited 75% or greater surface area adhesion at 9 hours; 3 transdermal systems (5%) were reported as fully detached during the study.
16. How is Xelstrym Patch supplied
How Supplied
XELSTRYM (dextroamphetamine) transdermal system is a translucent product with a printed backing on one side and a release liner on the other packaged in an individual pouch supplied as:
-
4.5 mg/9 hours transdermal system (system size: 4.76 cm2)
Carton of 30 transdermal systems, each transdermal system is packaged in an individual pouch
NDC 68968-0205-3
-
9 mg/9 hours transdermal system (system size: 9.52 cm2)
Carton of 30 transdermal systems, each transdermal system is packaged in an individual pouch
NDC 68968-0210-3
-
13.5 mg/9 hours transdermal system (system size: 14.29 cm2)
Carton of 30 transdermal systems, each transdermal system is packaged in an individual pouch
NDC 68968-0215-3
-
18 mg/9 hours transdermal system (system size: 19.05 cm2)
Carton of 30 transdermal systems, each transdermal system is packaged in an individual pouch
NDC 68968-0220-3
Storage and Handling
Store at 68°F to 77° F (20°C to 25° C); excursions permitted between 15°C to 30° C (59 to 86° F) [see USP Controlled Room Temperature]. Protect from light.
Store XELSTRYM in the individual sealed pouch until use. Apply immediately upon removal from the protective pouch.
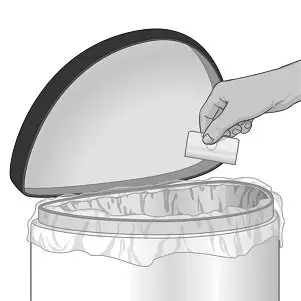
Disposal
Comply with local laws and regulations on drug disposal of CNS stimulants. Dispose of remaining, unused, or expired XELSTRYM by a medicine take-back program or at authorized collector registered with the Drug Enforcement Administration. If no take-back program or authorized collector is available, each unused system should be removed from its individual pouch, separated from the protective liner, folded in half, and disposed of in the same manner as used systems.
17. Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Medication Guide and Instructions for Use).
Controlled Substance Status/Potential for Abuse and Dependence
Advise patients that XELSTRYM is a federally controlled substance and it can be abused and lead to dependence [see DRUG ABUSE AND DEPENDENCE (9.1, 9.2, and 9.3)]. Instruct patients that they should not give XELSTRYM to anyone else. Advise patients to store XELSTRYM in a safe place, preferably locked, to prevent abuse. Advise patients to comply with laws and regulations on drug disposal. Advise patients to dispose of remaining, unused or expired XELSTRYM through a medicine take-back program if available. If no take-back program or authorized collector is available, each unused transdermal system should be removed from its individual pouch, separated from the protective liner, folded in half, and disposed of in the same manner as used transdermal systems [see WARNINGS AND PRECAUTIONS (5.1), DRUG ABUSE AND DEPENDENCE (9.2, 9.3), and HOW SUPPLIED/STORAGE AND HANDLING (16)].
Serious Cardiovascular Risks
Advise patients that there is a potential serious cardiovascular risk including sudden death, myocardial infarction, stroke, and hypertension with XELSTRYM. Instruct patients to contact a healthcare provider immediately if they develop symptoms such as exertional chest pain, unexplained syncope, or other symptoms suggestive of cardiac disease [see WARNINGS AND PRECAUTIONS (5.2).
Blood Pressure and Heart Rate Increases
Advise patients that XELSTRYM can elevate blood pressure and heart rate [WARNINGS AND PRECAUTIONS (5.3)].
Psychiatric Risks
Advise patients that XELSTRYM, at recommended doses, can cause psychotic or manic symptoms, even in patients without prior history of psychotic symptoms or mania [WARNINGS AND PRECAUTIONS (5.4)].
Suppression of Growth
Advise patients that XELSTRYM may cause slowing of growth including weight loss [WARNINGS AND PRECAUTIONS (5.5)].
Circulation problems in fingers and toes [Peripheral vasculopathy, including Raynaud's phenomenon]
Instruct patients beginning treatment with XELSTRYM about the risk of peripheral vasculopathy, including Raynaud's Phenomenon, and associated signs and symptoms: fingers or toes may feel numb, cool, painful, and/or may change from pale, to blue, to red. Instruct patients to report to their physician any new numbness, pain, skin color change, or sensitivity to temperature in fingers or toes. Instruct patients to call their physician immediately with any signs of unexplained wounds appearing on fingers or toes while taking XELSTRYM. Further clinical evaluation (e.g. rheumatology referral) may be appropriate for certain patients [see WARNINGS AND PRECAUTIONS (5.6)].
Serotonin Syndrome
Caution patients about the risk of serotonin syndrome with concomitant use of XELSTRYM and other serotonergic drugs including SSRIs, SNRIs, triptans, tricyclic antidepressants, fentanyl, lithium, tramadol, tryptophan, buspirone, St. John's Wort, and with drugs that impair metabolism of serotonin (in particular MAOIs, both those intended to treat psychiatric disorders and also others such as linezolid [see CONTRAINDICATIONS (4), [see WARNINGS AND PRECAUTIONS (5.7)] and DRUG INTERACTIONS (7.1)]. Advise patients to contact their healthcare provider or report to the emergency room if they experience signs or symptoms of serotonin syndrome.
Application Site Reactions
Inform patients that application site reactions, including pain, pruritus, burning sensation, erythema, discomfort, edema, or swelling have been reported with the use of XELSTRYM. Inform patients that increased skin irritation, discomfort or pain may occur if the same application site is used repeatedly. Instruct patients to select a different application site each day to minimize skin reactions. Patients should monitor for these reactions while wearing or immediately after removal of XELSTRYM. Discontinue XELSTRYM if contact sensitization is suspected [see WARNINGS AND PRECAUTIONS (5.9)].
External Heat
Inform patients to avoid exposing XELSTRYM to external heat sources, such as hair dryers, heating pads, electric blankets, heated water beds, etc., [see WARNINGS AND PRECAUTIONS (5.10)].
Concomitant Medications
Advise patients to notify their physicians if they are taking, or plan to take, any prescription or over-the-counter drugs because there is a potential for interactions [see DRUG INTERACTIONS (7.1)].
Pregnancy Registry
Advise patients that there is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to XELSTRYM during pregnancy [see USE IN SPECIFIC POPULATIONS (8.1)].
Pregnancy
Advise patients of the potential fetal effects from the use of XELSTRYM during pregnancy. Advise patients to notify their healthcare provider if they become pregnant or intend to become pregnant during treatment with XELSTRYM [see USE IN SPECIFIC POPULATIONS (8.1)].
Lactation
Advise women not to breastfeed if they are taking XELSTRYM [see USE IN SPECIFIC POPULATIONS (8.2)].
Impairment in Ability to Operate Machinery or Vehicles
XELSTRYM may impair the ability of the patient to engage in potentially hazardous activities such as operating machinery or vehicles. Instruct patients to find out how XELSTRYM will affect them before engaging in potentially hazardous activities [see ADVERSE REACTIONS (6.1, 6.2)].
Administration Instructions
Inform patients/caregivers:
- to apply one XELSTRYM transdermal system at a time for not more than 9 hours and use only one XELSTRYM per 24 hours.
- of the application sites: hip, upper arm, chest, upper back, or flank. Advise them to select a different application site each time a new XELSTRYM transdermal system is applied.
- to apply XELSTRYM to clean, dry, and intact skin.
- to wash their hands immediately if they touch the adhesive side of the transdermal system to avoid amphetamine absorption and to check XELSTRYM periodically throughout the wear time [see DOSAGE AND ADMINISTRATION (2.3)].
Advise patients/caregivers to follow recommendations for discarding used and unused XELSTRYM [see DOSAGE AND ADMINISTRATION (2.4)].
Manufactured by: Noven Pharmaceuticals, Inc., Miami, FL 33186
For more information, go to www.xelstrym.com or call 1-800-455-8070.
XelstrymTM is a trademark of Noven Therapeutics, LLC.
© 2022 Noven Pharmaceuticals, Inc.
102668-1
| This Medication Guide has been approved by the U.S. Food and Drug Administration. | Issued: 8/2022 |
|
Medication Guide
XELSTRYM™ (Zel’ Strim)
|
|
|
What is the most important information I should know about XELSTRYM? XELSTRYM may cause serious side effects, including:
Your healthcare provider should check you or your child carefully for heart problems before starting treatment with XELSTRYM. Tell your healthcare provider if you or your child have any heart problems, heart defects, high blood pressure, or a family history of these problems. Your healthcare provider should check your or your child’s blood pressure and heart rate regularly during treatment with XELSTRYM. Call your healthcare provider or go to the nearest hospital emergency room right away if you or your child have any signs of heart problems such as chest pain, shortness of breath, or fainting during treatment with XELSTRYM.
Tell your healthcare provider about any mental problems you or your child have or about a family history of suicide, bipolar illness, or depression. Call your healthcare provider right away if you or your child have any new or worsening mental symptoms or problems during treatment with XELSTRYM, especially hearing voices, seeing or believing things that are not real, or new manic symptoms. |
|
|
What is XELSTRYM? XELSTRYM is a central nervous system (CNS) stimulant prescription medicine used for the treatment of Attention-Deficit/ Hyperactivity Disorder (ADHD) in adults and children 6 years of age and older. XELSTRYM may help increase attention and decrease impulsiveness and hyperactivity in adults and children 6 years of age and older with ADHD. It is not known if XELSTRYM is safe and effective in children under 6 years of age. XELSTRYM is a federally controlled substance (CII) because it contains dextroamphetamine that can be a target for people who abuse prescription medicines or street drugs. Keep XELSTRYM in a safe place to protect it from theft. Never give your XELSTRYM to anyone else, because it may cause death or harm them. Selling or giving away XELSTRYM may harm others and is against the law. |
|
|
Do not use XELSTRYM if you or your child are:
|
|
Tell your healthcare provider about all the medicines that you or your child takes, including prescription and over-the-counter medicines, vitamins, and herbal supplements. XELSTRYM may affect the way other medicines work and other medicines may affect how XELSTRYM works. Using XELSTRYM with other medicines may cause serious side effects. Sometimes the doses of other medicines will need to be changed while using XELSTRYM. |
|
|
Especially tell your healthcare provider if you or your child take: |
|
|
|
|
Your healthcare provider will decide whether XELSTRYM can be used with other medicines. Do not start any new medicine during treatment with XELSTRYM without first talking to your healthcare provider. |
|
|
How should XELSTRYM be used?
If you or your child use too much XELSTRYM, call your healthcare provider or poison control center at 1-800-222-1222 or go to the nearest hospital emergency room right away. |
|
|
What should I avoid while using XELSTRYM?
|
|
|
What are possible side effects of XELSTRYM? XELSTRYM may cause serious side effects, including:
|
|
|
|
|
|
|
|
|
These are not all the possible side effects of XELSTRYM. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
|
|
How should I store XELSTRYM?
Keep XELSTRYM and all medicines out of the reach of children. |
|
|
General information about the safe and effective use of XELSTRYM. Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use XELSTRYM for a condition for which it was not prescribed. Do not give XELSTRYM to other people, even if they have the same symptoms that you have. It may harm them and it is against the law. You can ask your healthcare provider or pharmacist for information about XELSTRYM that is written for healthcare professionals. |
|
|
What are the ingredients in XELSTRYM? Active ingredient: dextroamphetamine Inactive ingredients: acrylic adhesives, green ink, polyester/polyurethane backing, and polyester release liner Manufactured by: Noven Pharmaceuticals, Inc., Miami, FL 33186 Xelstrym™ is a trademark of Noven Therapeutics, LLC. For more information, go to www.xelstrym.com or call 1-800-455-8070. |
|
102668-1
|
INSTRUCTIONS FOR USE
XELSTRYM™ (Zel’ Strim)
|
This Instructions for Use contains information on how to apply, remove and dispose of XELSTRYM transdermal system (patch).
XELSTRYM transdermal system (patch) is for use on skin only.
Read this Instructions for Use before you start using XELSTRYM and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your or your child’s medical condition or treatment.
XELSTRYM is supplied in cartons containing 30 patches. Each patch is sealed in a pouch protecting it until you are ready to apply it.
Important information you need to know before applying XELSTRYM:
- Wear only 1 patch at a time.
- A new patch must be applied every day.
- Do not wear XELSTRYM for more than 9 hours. Use only 1 patch per day (per 24 hours).
- When you apply a new patch, choose a different application site from the one used the previous day to reduce the chance of skin reactions such as irritation, discomfort or pain at the application site.
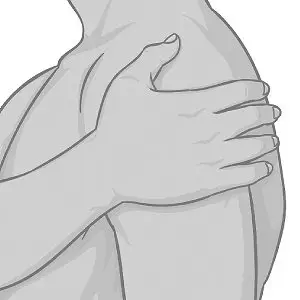
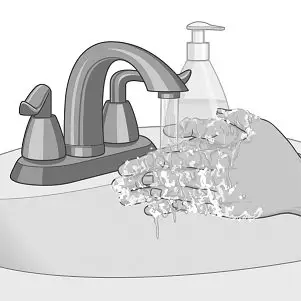
- Avoid touching the sticky side of XELSTRYM with your fingers. If you accidentally touch the sticky side of the patch, wash your hands right away with soap and water to remove any potential medicine that may have stuck to your fingers. Do not use hand sanitizer in place of soap and water.
- Avoid exposing the application site to direct external heat sources, such as heating pads or electric blankets, heat lamps, saunas, hot tubs, heated water beds, and hair dryers.
- Check to see if the patch has become loose after bathing, showering or swimming.
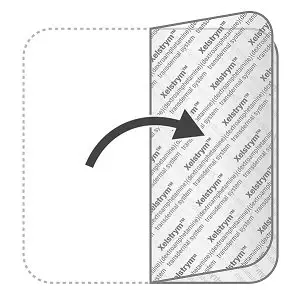
- If the patch falls off:
- Do not reapply the same patch. Fold the patch in half so that the sticky sides are together and throw away the patch as instructed below in Steps 5 to 7.
- You should choose a different application site and apply a new patch.
- The total wear time for the first patch that fell off and the second replacement patch should not total more than 9 hours in 1 day (24 hours).
- Parents or caregivers of children prescribed XELSTRYM should instruct the child to tell an adult if the patch becomes loose or falls off.
Figure A (not to scale)
Applying XELSTRYM:
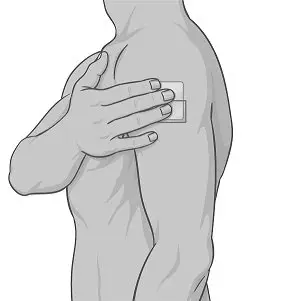
Step 1: Select the patch application site (see Figure B).
| ||
Step 2: Check the skin where XELSTRYM will be applied. Make sure it is:
If there is hair at the patch application site, do not shave the application site. Use scissors to clip hair as close to the skin as possible. |
||
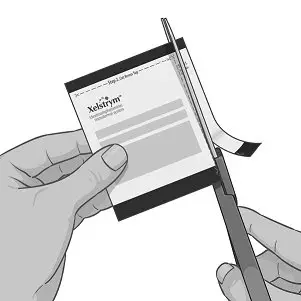
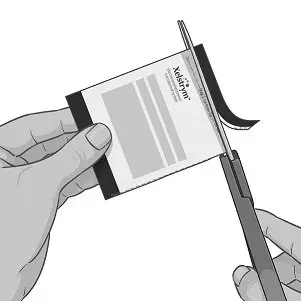
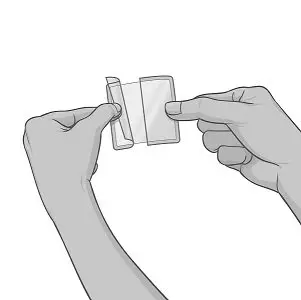
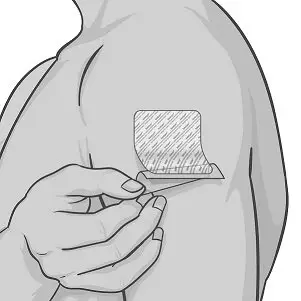
Step 3: Applying XELSTRYM
|
Figure C Figure D Figure E |
|
|
Figure F Figure G |
|
XELSTRYM patch should not be applied or reapplied with dressings, tape, or other adhesives. |
Figure H |
Figure I |
|
Figure J |
|
|
Step 4: Check XELSTRYM regularly during the day to make sure it is completely sticking to the application site. Also check the patch when: |
||
|
|
|
|
||
XELSTRYM Application Schedule for 9 Hour Wear Time:
| If you put the patch on at: | On the same day, remove the patch no later than: |
| 6:00 a.m | 3:00 p.m |
| 7:00 a.m | 4:00 p.m |
| 8:00 a.m | 5:00 p.m |
| 9:00 a.m | 6:00 p.m |
| 10:00 a.m | 7:00 p.m |
| 11:00 a.m | 8:00 p.m |
| 12:00 p.m | 9:00 p.m |
Removing and Disposing Used XELSTRYM:
|
This Instructions for Use has been approved by the U.S. Food and Drug Administration. | Revised: 11/2022 |
Step 5: After 9 hours, remove XELSTRYM and fold it in half so that the sticky sides stick together (see Figure K) to avoid accidental exposure to mnedicine.
|
Figure K |
| Step 6: Throw away the used patch in a container with a lid right away (see Figure L). |
Figure L |
| Step 7: Wash your hands with soap and water after throwing the patch away (see Figure M). Do not use hand sanitizer in place of soap and water. |
Figure M |
|
Storing XELSTRYM
|
|
|
Disposing of Unused XELSTRYM: Dispose of remaining unused or expired XELSTRYM by a medication take-back program at authorized collection sites such as retail pharmacies, hospital or clinic pharmacies, and law enforcement locations. If no take-back program or authorized collector is available, each unused patch should be removed from its individual pouch, separated from the protective liner, folded in half, and thrown away (disposed of) in the same manner as used patches. |
|
| XELSTRYM
dextroamphetamine patch, extended release |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| XELSTRYM
dextroamphetamine patch, extended release |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| XELSTRYM
dextroamphetamine patch, extended release |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| XELSTRYM
dextroamphetamine patch, extended release |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Noven Therapeutics, LLC (166888268) |
| Registrant - Noven Pharmaceuticals, Inc. (148585441) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Noven Pharmaceuticals, Inc. | 148585441 | MANUFACTURE(68968-0205, 68968-0210, 68968-0215, 68968-0220) | |