Drug Detail:Xofigo (Radium 223 dichloride)
Drug Class: Therapeutic radiopharmaceuticals
Highlights of Prescribing Information
XOFIGO (radium Ra 223 dichloride) injection, for intravenous use
Initial U.S. Approval: 2013
Recent Major Changes
- Warnings and Precautions, Embryo-Fetal Toxicity (5.3) 12/2019
Indications and Usage for Xofigo
Xofigo is an alpha particle-emitting radioactive therapeutic agent indicated for the treatment of patients with castration-resistant prostate cancer, symptomatic bone metastases and no known visceral metastatic disease. (1)
Xofigo Dosage and Administration
The dose regimen of Xofigo is 55 kBq (1.49 microcurie) per kg body weight, given at 4 week intervals for 6 injections. (2.1)
Dosage Forms and Strengths
Single-dose vial at a concentration of 1,100 kBq/mL (30 microcurie/mL) at the reference date with a total radioactivity of 6,600 kBq/vial (178 microcurie/vial) at the reference date. (3)
Contraindications
None. (4)
Warnings and Precautions
- •
- Bone Marrow Suppression: Measure blood counts prior to treatment initiation and before every dose of Xofigo. Discontinue Xofigo if hematologic values do not recover within 6 to 8 weeks after treatment. Monitor patients with compromised bone marrow reserve closely. Discontinue Xofigo in patients who experience life-threatening complications despite supportive care measures. (5.1)
- •
- Increased Fractures and Mortality in Combination with Abiraterone plus Prednisone/Prednisolone: Xofigo is not recommended in combination with abiraterone acetate plus prednisone/prednisolone. (5.2)
- •
- Embryo-Fetal Toxicity: Xofigo can cause fetal harm. Advise males with female partners of reproductive potential to use effective contraception. (5.3, 8.1, 8.3)
Adverse Reactions/Side Effects
The most common adverse drug reactions (≥ 10%) in patients receiving Xofigo were nausea, diarrhea, vomiting, and peripheral edema.
The most common hematologic laboratory abnormalities (≥ 10%) were anemia, lymphocytopenia, leukopenia, thrombocytopenia, and neutropenia. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Bayer HealthCare Pharmaceuticals Inc. at 1-888-842-2937 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 12/2019
Related/similar drugs
estradiol, Premarin, Xtandi, Zytiga, Casodex, LynparzaFull Prescribing Information
1. Indications and Usage for Xofigo
Xofigo is indicated for the treatment of patients with castration-resistant prostate cancer, symptomatic bone metastases and no known visceral metastatic disease.
2. Xofigo Dosage and Administration
2.1 Recommended Dosage
The dose regimen of Xofigo is 55 kBq (1.49 microcurie) per kg body weight, given at 4 week intervals for 6 injections. Safety and efficacy beyond 6 injections with Xofigo have not been studied.
The volume to be administered to a given patient should be calculated using the:
- •
- Patient’s body weight (kg)
- •
- Dosage level 55 kBq/kg body weight or 1.49 microcurie/kg body weight
- •
- Radioactivity concentration of the product (1,100 kBq/mL; 30 microcurie/mL) at the reference date
- •
- Decay correction factor to correct for physical decay of radium-223.
The total volume to be administered to a patient is calculated as follows:
|
Volume to be administered (mL) |
= |
Body weight in kg × 55 kBq/kg body weight |
|
Decay factor × 1,100 kBq/mL |
or
|
Volume to be administered (mL) |
= |
Body weight in kg × 1.49 microcurie/kg body weight |
|
Decay factor × 30 microcurie/mL |
|
Days from Reference Date |
Decay Factor |
Days from Reference Date |
Decay Factor |
|
-14 |
2.296 |
0 |
0.982 |
|
-13 |
2.161 |
1 |
0.925 |
|
-12 |
2.034 |
2 |
0.870 |
|
-11 |
1.914 |
3 |
0.819 |
|
-10 |
1.802 |
4 |
0.771 |
|
-9 |
1.696 |
5 |
0.725 |
|
-8 |
1.596 |
6 |
0.683 |
|
-7 |
1.502 |
7 |
0.643 |
|
-6 |
1.414 |
8 |
0.605 |
|
-5 |
1.330 |
9 |
0.569 |
|
-4 |
1.252 |
10 |
0.536 |
|
-3 |
1.178 |
11 |
0.504 |
|
-2 |
1.109 |
12 |
0.475 |
|
-1 |
1.044 |
13 |
0.447 |
|
14 |
0.420 |
||
|
The Decay Correction Factor Table is corrected to 12 noon Central Standard Time (CST). To determine the decay correction factor, count the number of days before or after the reference date. The Decay Correction Factor Table includes a correction to account for the 7 hour time difference between 12 noon Central European Time (CET) at the site of manufacture and 12 noon US CST, which is 7 hours earlier than CET. |
|||
Immediately before and after administration, the net patient dose of administered Xofigo should be determined by measurement in an appropriate radioisotope dose calibrator that has been calibrated with a National Institute of Standards and Technology (NIST) traceable radium-223 standard (available upon request from Bayer) and corrected for decay using the date and time of calibration. The dose calibrator must be calibrated with nationally recognized standards, carried out at the time of commissioning, after any maintenance procedure that could affect the dosimetry and at intervals not to exceed one year.
2.2 Administration
Administer Xofigo by slow intravenous injection over 1 minute.
Flush the intravenous access line or cannula with isotonic saline before and after injection of Xofigo.
Discard any unused portion, if applicable [see Dosage and Administration (2.3)].
2.3 Instructions for Use/Handling
General warning
Xofigo (an alpha particle-emitting pharmaceutical) should be received, used and administered only by authorized persons in designated clinical settings. The receipt, storage, use, transfer and disposal of Xofigo are subject to the regulations and/or appropriate licenses of the competent official organization.
Xofigo should be handled by the user in a manner which satisfies both radiation safety and pharmaceutical quality requirements. Appropriate aseptic precautions should be taken.
Radiation protection
The administration of Xofigo is associated with potential risks to other persons (e.g., medical staff, caregivers and patient’s household members) from radiation or contamination from spills of bodily fluids such as urine, feces, or vomit. Therefore, radiation protection precautions must be taken in accordance with national and local regulations.1
For drug handling
Follow the normal working procedures for the handling of radiopharmaceuticals and use universal precautions for handling and administration such as gloves and barrier gowns when handling blood and bodily fluids to avoid contamination. In case of contact with skin or eyes, the affected area should be flushed immediately with water. In the event of spillage of Xofigo, the local radiation safety officer should be contacted immediately to initiate the necessary measurements and required procedures to decontaminate the area. A complexing agent such as 0.01 M ethylene-diamine-tetraacetic acid (EDTA) solution is recommended to remove contamination.
For patient care
Whenever possible, patients should use a toilet and the toilet should be flushed several times after each use. When handling bodily fluids, simply wearing gloves and hand washing will protect caregivers. Clothing soiled with Xofigo or patient fecal matter or urine should be washed promptly and separately from other clothing.
Radium-223 is primarily an alpha emitter, with a 95.3% fraction of energy emitted as alpha-particles. The fraction emitted as beta-particles is 3.6%, and the fraction emitted as gamma-radiation is 1.1%. The external radiation exposure associated with handling of patient doses is expected to be low, because the typical treatment activity will be below 8,000 kBq (216 microcurie). In keeping with the As Low As Reasonably Achievable (ALARA) principle for minimization of radiation exposure, it is recommended to minimize the time spent in radiation areas, to maximize the distance to radiation sources, and to use adequate shielding. Any unused product or materials used in connection with the preparation or administration are to be treated as radioactive waste and should be disposed of in accordance with local regulations.
The gamma radiation associated with the decay of radium-223 and its daughters allows for the radioactivity measurement of Xofigo and the detection of contamination with standard instruments.
Instructions for preparation
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
Xofigo is a ready-to-use solution and should not be diluted or mixed with any solutions. Each vial is for single use only.
Dosimetry
The absorbed radiation doses in major organs were calculated based on clinical biodistribution data in five patients with castration-resistant prostate cancer. Calculations of absorbed radiation doses were performed using OLINDA/EXM (Organ Level INternal Dose Assessment/EXponential Modeling), a software program based on the Medical Internal Radiation Dose (MIRD) algorithm, which is widely used for established beta and gamma emitting radionuclides. For radium-223, which is primarily an alpha particle-emitter, assumptions were made for intestine, red marrow and bone/osteogenic cells to provide the best possible absorbed radiation dose calculations for Xofigo, considering its observed biodistribution and specific characteristics. Additional particular modeling was applied for the lungs. The absorbed dose to the lungs is estimated as the dose contribution from 223Ra and daughter decays in the blood-containing fraction of the lung mass and also the dose contribution from 219Rn and daughter decays in the respiratory tract.
The calculated absorbed radiation doses to different organs per administered activity are listed in Table 2. The organs with highest absorbed radiation doses are bone (osteogenic cells), red marrow, and large intestine walls. The absorbed doses to other organs are lower.
|
|||
|
|
|
|
|
1152 |
4263 |
|
|
139 |
514 |
|
|
46 |
172 |
|
|
38 |
142 |
|
|
32 |
120 |
|
|
7.3 |
27 |
|
|
4.0 |
15 |
|
|
3.2 |
12 |
|
|
3.0 |
11 |
|
|
1.7 |
6.4 |
|
|
1.2 |
4.5 |
|
|
|
1.8 |
|
|
|
0.94 |
|
|
|
0.85 |
|
|
|
0.51 |
|
|
|
0.44 |
|
|
|
0.44 |
|
|
|
0.41 |
|
|
|
0.37 |
|
|
|
0.33 |
|
|
|
0.31 |
|
|
|
0.27 |
|
|
|
0.26 |
|
|
|
0.21 |
|
|
|
0.18 |
|
|
|
86 |
|
3. Dosage Forms and Strengths
Xofigo (radium Ra 223 dichloride injection) is available in single-dose vials containing 6 mL of clear, colorless solution at a concentration of 1,100 kBq/mL (30 microcurie/mL) at the reference date with a total radioactivity of 6,600 kBq/vial (178 microcurie/vial) at the referencedate.
5. Warnings and Precautions
5.1 Bone Marrow Suppression
In the randomized trial, 2% of patients on the Xofigo arm experienced bone marrow failure or ongoing pancytopenia compared to no patients treated with placebo. There were two deaths due to bone marrow failure and for 7 of 13 patients treated with Xofigo, bone marrow failure was ongoing at the time of death. Among the 13 patients who experienced bone marrow failure, 54% required blood transfusions. Four percent (4%) of patients on the Xofigo arm and 2% on the placebo arm permanently discontinued therapy due to bone marrow suppression.
In the randomized trial, deaths related to vascular hemorrhage in association with myelosuppression were observed in 1% of Xofigo-treated patients compared to 0.3% of patients treated with placebo. The incidence of infection-related deaths (2%), serious infections (10%), and febrile neutropenia (<1%) were similar for patients treated with Xofigo and placebo. Myelosuppression; notably thrombocytopenia, neutropenia, pancytopenia, and leukopenia; has been reported in patients treated with Xofigo. In the randomized trial, complete blood counts (CBCs) were obtained every 4 weeks prior to each dose and the nadir CBCs and times of recovery were not well characterized. In a separate single-dose phase 1 study of Xofigo, neutrophil and platelet count nadirs occurred 2 to 3 weeks after Xofigo administration at doses that were up to 1 to 5 times the recommended dose, and most patients recovered approximately 6 to 8 weeks after administration [see Adverse Reactions (6)].
Hematologic evaluation of patients must be performed at baseline and prior to every dose of Xofigo. Before the first administration of Xofigo, the absolute neutrophil count (ANC) should be ≥ 1.5 x 109/L, the platelet count ≥ 100 x 109/L and hemoglobin ≥ 10 g/dL. Before subsequent administrations of Xofigo, the ANC should be ≥ 1 x 109/L and the platelet count ≥ 50 x 109/L. If there is no recovery to these values within 6 to 8 weeks after the last administration of Xofigo, despite receiving supportive care, further treatment with Xofigo should be discontinued. Patients with evidence of compromised bone marrow reserve should be monitored closely and provided with supportive care measures when clinically indicated. Discontinue Xofigo in patients who experience life-threatening complications despite supportive care for bone marrow failure.
The safety and efficacy of concomitant chemotherapy with Xofigo have not been established. Outside of a clinical trial, concomitant use with chemotherapy is not recommended due to the potential for additive myelosuppression. If chemotherapy, other systemic radioisotopes or hemibody external radiotherapy are administered during the treatment period, Xofigo should be discontinued.
5.2 Increased Fractures and Mortality in Combination with Abiraterone plus Prednisone/Prednisolone
Xofigo is not recommended for use in combination with abiraterone acetate plus prednisone/prednisolone outside of clinical trials.
The clinical efficacy and safety of concurrent initiation of Xofigo treatment and abiraterone acetate plus prednisone/prednisolone treatment was assessed in a randomized, placebo-controlled multicenter phase 3 study (ERA-223 trial) in 806 patients with asymptomatic or mildly symptomatic castration resistant prostate cancer with bone metastases. The study was unblinded early based on an Independent Data Monitoring Committee recommendation.
At the primary analysis, an increased incidence of fractures (28.6% vs 11.4%) and deaths (38.5% vs 35.5%) have been observed in patients who received Xofigo in combination with abiraterone acetate plus prednisone/prednisolone compared to patients who received placebo in combination with abiraterone acetate plus prednisone/prednisolone. Safety and efficacy with the combination of Xofigo and agents other than gonadotropin-releasing hormone analogues have not been established.
-167640120650005.3 Embryo-Fetal Toxicity
The safety and efficacy of Xofigo have not been established in females. Based on its mechanism of action, Xofigo can cause fetal harm when administered to a pregnant female. Advise pregnant females and females of reproductive potential of the potential risk to a fetus. Advise male patients to use condoms and their female partners of reproductive potential to use effective contraception during and for 6 months after completing treatment with Xofigo [see Use in Specific Populations (8.1, 8.3) and Clinical Pharmacology (12.1)].
6. Adverse Reactions/Side Effects
The following serious adverse reactions are discussed in greater detail in another section of the label:
- •
- Bone Marrow Suppression [see Warnings and Precautions (5.1)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In the randomized clinical trial in patients with metastatic castration-resistant prostate cancer with bone metastases, 600 patients received intravenous injections of 55 kBq/kg (1.49 microcurie/kg) of Xofigo and best standard of care and 301 patients received placebo and best standard of care once every 4 weeks for up to 6 injections. Prior to randomization, 58% and 57% of patients had received docetaxel in the Xofigo and placebo arms, respectively. The median duration of treatment was 20 weeks (6 cycles) for Xofigo and 18 weeks (5 cycles) for placebo.
The most common adverse reactions (≥ 10%) in patients receiving Xofigo were nausea, diarrhea, vomiting, and peripheral edema (Table 3). Grade 3 and 4 adverse events were reported among 57% of Xofigo-treated patients and 63% of placebo-treated patients. The most common hematologic laboratory abnormalities in Xofigo-treated patients (≥ 10%) were anemia, lymphocytopenia, leukopenia, thrombocytopenia, and neutropenia (Table 4).
Treatment discontinuations due to adverse events occurred in 17% of patients who received Xofigo and 21% of patients who received placebo. The most common hematologic laboratory abnormalities leading to discontinuation for Xofigo were anemia (2%) and thrombocytopenia (2%).
Table 3 shows adverse reactions occurring in ≥ 2% of patients and for which the incidence for Xofigo exceeds the incidence for placebo.
|
System/Organ Class
|
Xofigo (n=600) |
Placebo (n=301) |
||
|
Grades 1-4
|
Grades 3-4
|
Grades 1-4
|
Grades 3-4
|
|
|
Blood and lymphatic system disorders |
||||
|
Pancytopenia |
2 |
1 |
0 |
0 |
|
Gastrointestinal disorders |
||||
|
Nausea |
36 |
2 |
35 |
2 |
|
Diarrhea |
25 |
2 |
15 |
2 |
|
Vomiting |
19 |
2 |
14 |
2 |
|
General disorders and administration site conditions |
||||
|
Peripheral edema |
13 |
2 |
10 |
1 |
|
Renal and urinary disorders |
||||
|
Renal failure and impairment |
3 |
1 |
1 |
1 |
Laboratory Abnormalities
Table 4 shows hematologic laboratory abnormalities occurring in > 10% of patients and for which the incidence for Xofigo exceeds the incidence for placebo.
|
Hematologic Laboratory Abnormalities |
Xofigo (n=600) |
Placebo (n=301) |
||
|
Grades 1-4
|
Grades 3-4
|
Grades 1-4
|
Grades 3-4
|
|
|
Anemia |
93 |
6 |
88 |
6 |
|
Lymphocytopenia |
72 |
20 |
53 |
7 |
|
Leukopenia |
35 |
3 |
10 |
<1 |
|
Thrombocytopenia |
31 |
3 |
22 |
<1 |
|
Neutropenia |
18 |
2 |
5 |
<1 |
|
Laboratory values were obtained at baseline and prior to each 4-week cycle. |
||||
As an adverse reaction, grade 3-4 thrombocytopenia was reported in 6% of patients on Xofigo and in 2% of patients on placebo. Among patients who received Xofigo, the laboratory abnormality grade 3-4 thrombocytopenia occurred in 1% of docetaxel naïve patients and in 4% of patients who had received prior docetaxel. Grade 3-4 neutropenia occurred in 1% of docetaxel naïve patients and in 3% of patients who have received prior docetaxel.
Fluid Status
Dehydration occurred in 3% of patients on Xofigo and 1% of patients on placebo. Xofigo increases adverse reactions such as diarrhea, nausea, and vomiting which may result in dehydration. Monitor patients’ oral intake and fluid status carefully and promptly treat patients who display signs or symptoms of dehydration or hypovolemia.
Injection Site Reactions
Erythema, pain, and edema at the injection site were reported in 1% of patients on Xofigo.
Secondary Malignant Neoplasms
Xofigo contributes to a patient’s overall long-term cumulative radiation exposure. Long-term cumulative radiation exposure may be associated with an increased risk of cancer and hereditary defects. Due to its mechanism of action and neoplastic changes, including osteosarcomas, in rats following administration of radium-223 dichloride, Xofigo may increase the risk of osteosarcoma or other secondary malignant neoplasms [see Nonclinical Toxicology (13.1)]. However, the overall incidence of new malignancies in the randomized trial was lower on the Xofigo arm compared to placebo (<1% vs. 2%; respectively), but the expected latency period for the development of secondary malignancies exceeds the duration of follow up for patients on the trial.
Subsequent Treatment with Cytotoxic Chemotherapy
In the randomized clinical trial, 16% patients in the Xofigo group and 18% patients in the placebo group received cytotoxic chemotherapy after completion of study treatments. Adequate safety monitoring and laboratory testing was not performed to assess how patients treated with Xofigo will tolerate subsequent cytotoxic chemotherapy.
7. Drug Interactions
No formal clinical drug interaction studies have been performed.
Subgroup analyses indicated that the concurrent use of bisphosphonates or calcium channel blockers did not affect the safety and efficacy of Xofigo in the randomized clinical trial.
8. Use In Specific Populations
8.1 Pregnancy
Risk Summary
The safety and efficacy of Xofigo have not been established in females. Based on mechanism of action, Xofigo can cause fetal harm when administered to a pregnant female [see Clinical Pharmacology (12.1)]. While there are no human or animal data on the use of Xofigo in pregnancy, maternal use of a radioactive therapeutic agent could affect development of a fetus. Advise pregnant females and females of reproductive potential of the potential risk to a fetus.
8.2 Lactation
Risk Summary
The safety and efficacy of Xofigo have not been established in females. There is no data on the presence of radium-223 dichloride in human milk, the effects on the breastfed child, or the effects on milk production.
8.3 Females and Males of Reproductive Potential
Contraception
Males
Because of potential effects on spermatogenesis associated with radiation, advise male patients to use condoms and their female partners of reproductive potential to use effective contraception during and for 6 months after completing treatment with Xofigo [see Use in Specific Populations (8.1)].
8.4 Pediatric Use
The safety and efficacy of Xofigo in pediatric patients have not been established.
In single- and repeat-dose toxicity studies in rats, findings in the bones (depletion of osteocytes, osteoblasts, osteoclasts, fibro-osseous lesions, disruption/disorganization of the physis/growth line) and teeth (missing, irregular growth, fibro-osseous lesions in bone socket) correlated with a reduction of osteogenesis that occurred at clinically relevant doses beginning in the range of 22 – 88 kBq (0.59 - 2.38 microcurie) per kg body weight.
8.5 Geriatric Use
Of the 600 patients treated with Xofigo in the randomized trial, 75% were 65 years of age and over and while 33% were 75 years of age and over. No dosage adjustment is considered necessary in elderly patients. No overall differences in safety or effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
8.6 Patients with Hepatic Impairment
No dedicated hepatic impairment trial for Xofigo has been conducted. Since radium-223 is neither metabolized by the liver nor eliminated via the bile, hepatic impairment is unlikely to affect the pharmacokinetics of radium-223 dichloride [see Clinical Pharmacology (12.3)]. Based on subgroup analyses in the randomized clinical trial, dose adjustment is not needed in patients with mild hepatic impairment. No dose adjustments can be recommended for patients with moderate or severe hepatic impairment due to lack of clinical data.
8.7 Patients with Renal Impairment
No dedicated renal impairment trial for Xofigo has been conducted. Based on subgroup analyses in the randomized clinical trial, dose adjustment is not needed in patients with existing mild (creatinine clearance [CrCl] 60 to 89 mL/min) or moderate (CrCl 30 to 59 mL/min) renal impairment. No dose adjustment can be recommended for patients with severe renal impairment (CrCl less than 30 mL/min) due to limited data available (n = 2) [see Clinical Pharmacology (12.3)].
10. Overdosage
There have been no reports of inadvertent overdosing of Xofigo during clinical studies.
There is no specific antidote. In the event of an inadvertent overdose of Xofigo, utilize general supportive measures, including monitoring for potential hematological and gastrointestinal toxicity, and consider using medical countermeasures such as aluminum hydroxide, barium sulfate, calcium carbonate, calcium gluconate, calcium phosphate, or sodium alginate.1
Single Xofigo doses up to 276 kBq (7.46 microcurie) per kg body weight were evaluated in a phase 1 clinical trial and no dose-limiting toxicities were observed.
11. Xofigo Description
Radium Ra 223 dichloride, an alpha particle-emitting pharmaceutical, is a radiotherapeutic drug.
Xofigo is supplied as a clear, colorless, isotonic, and sterile solution to be administered intravenously with pH between 6 and 8.
Each milliliter of solution contains 1,100 kBq radium-223 dichloride (30 microcurie), corresponding to 0.58 ng radium-223, at the reference date. Radium is present in the solution as a free divalent cation.
Each vial contains 6 mL of solution (6,600 kBq (178 microcurie) radium-223 dichloride at the reference date). The inactive ingredients are 6.3 mg/mL sodium chloride USP (tonicity agent), 7.2 mg/mL sodium citrate USP (for pH adjustment), 0.2 mg/mL hydrochloric acid USP (for pH adjustment), and water for injection USP.
The molecular weight of radium-223 dichloride, 223RaCl2, is 293.9 g/mol.
Radium-223 has a half-life of 11.4 days. The specific activity of radium-223 is 1.9 MBq (51.4 microcurie)/ng.
The six-stage-decay of radium-223 to stable lead-207 occurs via short-lived daughters, and is accompanied predominantly by alpha emissions. There are also beta and gamma emissions with different energies and emission probabilities. The fraction of energy emitted from radium-223 and its daughters as alpha-particles is 95.3% (energy range of 5 - 7.5 MeV). The fraction emitted as beta-particles is 3.6% (average energies are 0.445 MeV and 0.492 MeV), and the fraction emitted as gamma-radiation is 1.1% (energy range of 0.01 - 1.27 MeV).
12. Xofigo - Clinical Pharmacology
12.1 Mechanism of Action
The active moiety of Xofigo is the alpha particle-emitting isotope radium-223 (as radium Ra 223 dichloride), which mimics calcium and forms complexes with the bone mineral hydroxyapatite at areas of increased bone turnover, such as bone metastases (see Table 2). The high linear energy transfer of alpha particles (80 keV/micrometer) leads to a high frequency of double-strand DNA breaks in adjacent cells including tumor cells, osteoblasts and osteoclasts, resulting in an anti-tumor effect on bone metastases. The alpha particle range from radium-223 dichloride is less than 100 micrometers (less than 10 cell diameters) which limits damage to the surrounding normal tissue.
12.2 Pharmacodynamics
Compared with placebo, there was a difference in favor of Xofigo for all five serum biomarkers for bone turnover studied in a phase 2 randomized study (bone formation markers: bone alkaline phosphatase [ALP], total ALP and procollagen I N propeptide [PINP], bone resorption markers: C-terminal crosslinking telopeptide of type I collagen [S-CTX-I] and type I collagen crosslinked C-telopeptide [ICTP]).
12.3 Pharmacokinetics
The pharmacokinetics of radium-223 dichloride in blood was linear in terms of dose proportionality and time independence in the dose range investigated (51 to 276 kBq [1.38 to 7.46 microcurie] per kg body weight).
Distribution
After intravenous injection, radium-223 is rapidly cleared from the blood and is distributed primarily into bone or is excreted into intestine. Fifteen minutes post-injection, about 20% of the injected radioactivity remained in blood. At 4 hours, about 4% of the injected radioactivity remained in blood, decreasing to less than 1% at 24 hours after the injection. At 10 minutes post-injection, radioactivity was observed in bone and in intestine. At 4 hours post-injection, the percentage of the radioactive dose present in bone and intestine was approximately 61% and 49%, respectively. No significant uptake was seen in other organs such as heart, liver, kidneys, urinary bladder, and spleen at 4 hours post-injection [see Dosage and Administration (2.3)].
Elimination
The whole body measurements indicated that approximately 63% of the administered radioactivity was excreted from the body within 7 days after injection (after correcting for decay). Fecal excretion is the major route of elimination from the body. At 48 hours after injection, the cumulative fecal excretion was 13% (range 0 - 34%), and the cumulative urine excretion was 2% (range 1 - 5%). There was no evidence of hepato-biliary excretion based on imaging data.
The rate of elimination of radium-223 dichloride from the gastrointestinal tract is influenced by the high variability in intestinal transit rates across the population. Patients with a slower intestinal transit rate could potentially receive a higher intestinal radiation exposure. It is not known whether this will result in increased gastrointestinal toxicity.
Special Populations
Pediatric patients
Safety and effectiveness of Xofigo have not been established in children and adolescents below 18 years of age.
Patients with hepatic impairment
No dedicated pharmacokinetic study in patients with hepatic impairment has been conducted. However, since radium-223 is not metabolized and there is no evidence of hepato-biliary excretion based on imaging data, hepatic impairment is not expected to affect the pharmacokinetics of radium-223 dichloride.
Patients with renal impairment
No dedicated pharmacokinetic study in patients with renal impairment has been conducted. However, since excretion in urine is minimal and the major route of elimination is via the feces, renal impairment is not expected to affect the pharmacokinetics of radium-223 dichloride.
12.6 Cardiac Electrophysiology
The effect of a single dose of 55 kBq/kg of radium-223 dichloride on the QTc interval was evaluated in a subgroup of 29 patients (21 received Xofigo and 8 received placebo) in the randomized clinical trial. No large changes in the mean QTc interval (i.e., greater than 20 ms) were detected up to 6 hours post-dose. The potential for delayed effects on the QT interval after 6 hours was not evaluated.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Animal studies have not been conducted to evaluate the carcinogenic potential of radium-223 dichloride. However, in repeat-dose toxicity studies in rats, osteosarcomas, a known effect of bone-seeking radionuclides, were observed at clinically relevant doses 7 to 12 months after the start of treatment. The presence of other neoplastic changes, including lymphoma and mammary gland carcinoma, was also reported in 12- to 15-month repeat-dose toxicity studies in rats.
Genetic toxicology studies have not been conducted with radium-223 dichloride. However, the mechanism of action of radium-223 dichloride involves induction of double-strand DNA breaks, which is a known effect of radiation.
Animal studies have not been conducted to evaluate the effects of radium-223 dichloride on male or female fertility or reproductive function. Xofigo may impair fertility and reproductive function in humans based on its mechanism of action.
14. Clinical Studies
The efficacy and safety of Xofigo were evaluated in a double-blind, randomized, placebo-controlled phase 3 clinical trial of patients with castration-resistant prostate cancer with symptomatic bone metastases. Patients with visceral metastases and malignant lymphadenopathy exceeding 3 cm were excluded. The primary efficacy endpoint was overall survival. A key secondary efficacy endpoint was time to first symptomatic skeletal event (SSE) defined as external beam radiation therapy (EBRT) to relieve skeletal symptoms, new symptomatic pathologic bone fracture, occurrence of spinal cord compression, or tumor-related orthopedic surgical intervention. There were no scheduled radiographic assessments performed on study. All patients were to continue androgen deprivation therapy. At the cut-off date of the pre-planned interim analysis, a total of 809 patients had been randomized 2:1 to receive Xofigo 55 kBq (1.49 microcurie)/kg intravenously every 4 weeks for 6 cycles (n = 541) plus best standard of care or matching placebo plus best standard of care (n = 268). Best standard of care included local EBRT, corticosteroids, antiandrogens, estrogens, estramustine or ketoconazole. Therapy was continued until unacceptable toxicity or initiation of cytotoxic chemotherapy, other systemic radioisotope, hemi-body EBRT or other investigational drug. Patients with Crohn’s disease, ulcerative colitis, prior hemibody radiation or untreated imminent spinal cord compression were excluded from the study. In patients with bone fractures, orthopedic stabilization was performed before starting or resuming treatment with Xofigo.
The following patient demographics and baseline disease characteristics were balanced between the arms. The median age was 71 (range 44-94) with a racial distribution of 94% Caucasian, 4% Asian, 2% Black and <1% Other. Patients were enrolled predominantly from Europe (85%) with 4% of patients enrolled from North America. ECOG performance status was 0-1 in 86% of patients. Eighty-five percent of patients had 6 or more bone scan lesions and of those 40% had > 20 lesions or a superscan. Opiate pain medications were used for cancer-related pain in 54% of patients, non-opiate pain medications in 44% of patients and no pain medications in 2% of patients. Patients were stratified by baseline ALP, bisphosphonate use, and prior docetaxel exposure. Prior bisphosphonates were used by 41% of patients and 58% had received prior docetaxel. During the treatment period, 83% of Xofigo patients and 82% of placebo patients received gonadotropin-releasing hormone agonists and 21% of Xofigo patients and 34% of placebo patients received concomitant antiandrogens. Use of systemic steroids (41%) and bisphosphonates (40%) was balanced between the arms.
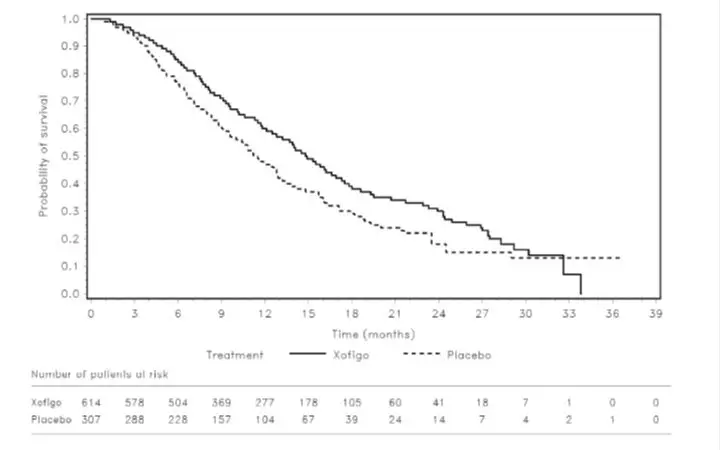
The pre-specified interim analysis of overall survival revealed a statistically significant improvement in patients receiving XOFIGO plus best standard of care compared with patients receiving placebo plus best standard of care. An exploratory updated overall survival analysis performed before patient crossover with an additional 214 events resulted in findings consistent with the interim analysis (Table 5).
|
||
|
Xofigo |
Placebo |
|
|
Interim Analysis | ||
|
Subjects randomized |
541 |
268 |
|
Number of deaths |
191 (35.3%) |
123 (45.9%) |
|
Censored |
350 (64.7%) |
145 (54.1%) |
|
Median survival (months)* (95% CI) |
14.0 (12.1, 15.8) |
11.2 (9.0, 13.2) |
|
p-value† |
0.00185 |
|
|
Hazard ratio (95% CI)‡ |
0.695 (0.552, 0.875) |
|
|
Updated Analysis | ||
|
Subjects randomized |
614 |
307 |
|
Number of deaths |
333 (54.2%) |
195 (63.5%) |
|
Censored |
281 (45.8%) |
112 (36.5%) |
|
Median survival (months)* (95% CI) |
14.9 (13.9, 16.1) |
11.3 (10.4, 12.8) |
|
Hazard ratio (95% CI)‡ |
0.695 (0.581, 0.832) |
|
The Kaplan-Meier curves for overall survival based on the updated survival results are shown in Figure 1.
Figure 1: Kaplan-Meier Overall Survival Curves from the Phase 3 Clinical Trial
The survival results were supported by a delay in the time to first SSE favoring the Xofigo arm. The majority of events consisted of external beam radiotherapy to bone metastases.
15. References
- 1.
- Radiation Emergency Medical Management. [REMM/National Library of Medicine Website.] http://www.remm.nlm.gov/int_contamination.htm#blockingagents
- 2.
- International Commission on Radiological Protection, ICRP Publication 128, 2015. http://www.icrp.org/publication.asp?id=ICRP Publication 128
16. How is Xofigo supplied
Xofigo (radium Ra 223 dichloride injection) is supplied in single-dose vials containing 6 mL of clear, colorless solution at a concentration of 1,100 kBq/mL (30 microcurie/mL) with a total radioactivity of 6,600 kBq/vial (178 microcurie/vial) at the reference date (NDC 50419-208-01).
Store at room temperature, below 40° C (104° F). Store Xofigo in the original container or equivalent radiation shielding.
This preparation is approved for use by persons under license by the Nuclear Regulatory Commission or the relevant regulatory authority of an Agreement State.
Follow procedures for proper handling and disposal of radioactive pharmaceuticals [see Dosage and Administration (2.3)].
17. Patient Counseling Information
- Bone Marrow Suppression
- Increased Fractures and Mortality in Combination with Abiraterone plus Prednisone/Prednisolone
- Fluid Status
- Instructions for Use/Handling
- Embryo-Fetal Toxicity
- Infertility
- •
- Advise patients to be compliant with blood cell count monitoring appointments while receiving Xofigo. Explain the importance of routine blood cell counts. Instruct patients to report signs of bleeding or infections [see Warnings and Precautions (5.1)].
Increased Fractures and Mortality in Combination with Abiraterone plus Prednisone/Prednisolone
- •
- Advise patients that Xofigo had increased bone fractures and mortality when used in combination with abiraterone acetate and prednisone/prednisolone. Inform patients to speak with their healthcare provider about any other medications they are currently taking for prostate cancer [see Warnings and Precautions (5.2)].
Fluid Status
- •
- Advise patients to stay well hydrated and to monitor oral intake, fluid status, and urine output while being treated with Xofigo. Instruct patients to report signs of dehydration, hypovolemia, urinary retention, or renal failure / insufficiency [see Adverse Reactions (6.1)].
Instructions for Use/Handling
- •
- Inform patients that there are no restrictions regarding personal contact (visual or physical proximity) with other people after receiving Xofigo. Advise patients to follow good hygiene practices while receiving Xofigo and for at least 1 week after the last injection in order to minimize radiation exposure from bodily fluids to household members and caregivers. Whenever possible, patients should use a toilet and the toilet should be flushed several times after each use. Clothing soiled with patient fecal matter or urine should be washed promptly and separately from other clothing. Caregivers should use universal precautions for patient care such as gloves and barrier gowns when handling bodily fluids to avoid contamination. When handling bodily fluids, wearing gloves and hand washing will protect caregivers [see Dosage and Administration (2.3)].
Embryo-Fetal Toxicity
- •
- Advise male patients to use condoms and their female partners of reproductive potential to use effective contraception during and for 6 months following completion of Xofigo treatment [see Use in Specific Populations (8.1, 8.3)].
Infertility
- •
- Inform male patients that Xofigo may impair fertility [see Use in Specific Populations (8.3)].
Manufactured for:
Bayer HealthCare Pharmaceuticals Inc.
Whippany, NJ 07981
Xofigo is a trademark of Bayer Aktiengesellschaft.
© 2013, Bayer HealthCare Pharmaceuticals Inc.
All rights reserved.
NDC 50419-208-01
6 mL
Xofigo®
radium Ra 223 dichloride injection
1100 kBq/mL (30 microcurie/mL)
For Intravenous Administration
Sterile
Single-Dose Vial:
Discard Unused Portion
Manufactured for:
Bayer HealthCare
Whippany, NJ 07981
6600 kBq/vial (178 microcurie/vial) at 5AM CST (12 noon CET) on reference date:
LOT:
EXP:
| XOFIGO
radium ra 223 dichloride injection |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - Bayer HealthCare Pharmaceuticals Inc. (005436809) |
| Registrant - Bayer HealthCare Pharmaceuticals Inc. (005436809) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Institutt For Energiteknikk | 518771688 | MANUFACTURE(50419-208) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Cardinal Health 414, LLC | 079955366 | MANUFACTURE(50419-208) , ANALYSIS(50419-208) | |