Drug Detail:Yescarta (Axicabtagene ciloleucel [ aks-ee-kab-ta-jeen-sye-loe-loo-sel ])
Drug Class: Miscellaneous antineoplastics
Highlights of Prescribing Information
YESCARTA® (axicabtagene ciloleucel) suspension for intravenous infusion
Initial U.S. Approval: 2017
WARNING: CYTOKINE RELEASE SYNDROME and NEUROLOGIC TOXICITIES
See full prescribing information for complete boxed warning.
- Cytokine Release Syndrome (CRS), including fatal or life-threatening reactions, occurred in patients receiving YESCARTA. Do not administer YESCARTA to patients with active infection or inflammatory disorders. Treat severe or life-threatening CRS with tocilizumab or tocilizumab and corticosteroids (2.2, 2.3, 5.1).
- Neurologic toxicities, including fatal or life-threatening reactions, occurred in patients receiving YESCARTA, including concurrently with CRS or after CRS resolution. Monitor for neurologic toxicities after treatment with YESCARTA. Provide supportive care and/or corticosteroids, as needed (2.2, 2.3, 5.2).
- YESCARTA is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS) called the YESCARTA and TECARTUS REMS Program (5.3).
Recent Major Changes
| Indications and Usage, Large B-cell Lymphoma (1.1) | 4/2022 |
| Dosage and Administration (2.2) | 1/2022 |
| Dosage and Administration (2.3) | 4/2021 |
| Warnings and Precautions (5) | 1/2022, 4/2022 |
Indications and Usage for Yescarta
YESCARTA is a CD19-directed genetically modified autologous T cell immunotherapy indicated for the treatment of:
- Adult patients with large B-cell lymphoma that is refractory to first-line chemoimmunotherapy or that relapses within 12 months of first-line chemoimmunotherapy. (1.1)
- Adult patients with relapsed or refractory large B-cell lymphoma after two or more lines of systemic therapy, including diffuse large B-cell lymphoma (DLBCL) not otherwise specified, primary mediastinal large B-cell lymphoma, high grade B-cell lymphoma, and DLBCL arising from follicular lymphoma. (1.1)
Limitations of Use: YESCARTA is not indicated for the treatment of patients with primary central nervous system lymphoma. (1.1)
- Adult patients with relapsed or refractory follicular lymphoma (FL) after two or more lines of systemic therapy. This indication is approved under accelerated approval based on response rate. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trial(s). (1.2)
Yescarta Dosage and Administration
For autologous use only. For intravenous use only.
- Do NOT use a leukodepleting filter. (2.2)
- Administer a lymphodepleting regimen of cyclophosphamide and fludarabine before infusion of YESCARTA. (2.2)
- Verify the patient's identity prior to infusion. (2.2)
- Premedicate with acetaminophen and an H1-antihistamine. (2.2)
- Confirm availability of tocilizumab prior to infusion. (2.1, 5.1)
- Dosing of YESCARTA is based on the number of chimeric antigen receptor (CAR)-positive viable T cells. (2.1)
- The target YESCARTA dose is 2 × 106 CAR-positive viable T cells per kg body weight, with a maximum of 2 × 108 CAR-positive viable T cells. (2.1)
- Administer YESCARTA in a certified healthcare facility. (2.2, 5.1, 5.2, 5.3)
Dosage Forms and Strengths
- YESCARTA is available as a cell suspension for infusion. (3)
- YESCARTA comprises a suspension of 2 × 106 CAR-positive viable T cells per kg of body weight, with a maximum of 2 × 108 CAR-positive viable T cells in approximately 68 mL. (3)
Contraindications
- None. (4)
Warnings and Precautions
- Hypersensitivity Reactions: Monitor for hypersensitivity reactions during infusion. (5.4)
- Serious Infections: Monitor patients for signs and symptoms of infection; treat appropriately. (5.5)
- Prolonged Cytopenias: Patients may exhibit Grade 3 or higher cytopenias for several weeks following YESCARTA infusion. Monitor complete blood counts. (5.6)
- Hypogammaglobulinemia: Monitor and provide replacement therapy. (5.7)
- Secondary Malignancies: In the event that a secondary malignancy occurs after treatment with YESCARTA, contact Kite at 1-844-454-KITE (5483). (5.8)
- Effects on Ability to Drive and Use Machines: Advise patients to refrain from driving and engaging in hazardous occupations or activities, such as operating heavy or potentially dangerous machinery, for at least 8 weeks after receiving YESCARTA. (5.9)
Adverse Reactions/Side Effects
The most common adverse reactions (incidence ≥ 30%), excluding laboratory abnormalities, in patients with non-Hodgkin lymphoma are CRS, fever, hypotension, encephalopathy, fatigue, tachycardia, headache, nausea, febrile neutropenia, diarrhea, musculoskeletal pain, infections with pathogen unspecified, chills and decreased appetite. (6.1)
The most common Grade 3-4 laboratory abnormalities (≥ 30%) are leukopenia, lymphopenia, neutropenia, anemia, thrombocytopenia, and hypophosphatemia. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Kite at 1-844-454-KITE (5483) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 11/2022
Related/similar drugs
prednisone, rituximab, cyclophosphamide, Revlimid, Rituxan, lenalidomide, doxorubicinFull Prescribing Information
WARNING: CYTOKINE RELEASE SYNDROME and NEUROLOGIC TOXICITIES
- Cytokine Release Syndrome (CRS), including fatal or life-threatening reactions, occurred in patients receiving YESCARTA. Do not administer YESCARTA to patients with active infection or inflammatory disorders. Treat severe or life-threatening CRS with tocilizumab or tocilizumab and corticosteroids [see Dosage and Administration (2.2, 2.3), Warnings and Precautions (5.1)].
- Neurologic toxicities, including fatal or life-threatening reactions, occurred in patients receiving YESCARTA, including concurrently with CRS or after CRS resolution. Monitor for neurologic toxicities after treatment with YESCARTA. Provide supportive care and/or corticosteroids as needed [see Dosage and Administration (2.2, 2.3), Warnings and Precautions (5.2)].
- YESCARTA is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS) called the YESCARTA and TECARTUS REMS Program [see Warnings and Precautions (5.3)].
1. Indications and Usage for Yescarta
1.1 Large B-cell Lymphoma
YESCARTA is indicated for the treatment of:
- Adult patients with large B-cell lymphoma that is refractory to first-line chemoimmunotherapy or that relapses within 12 months of first-line chemoimmunotherapy.
- Adult patients with relapsed or refractory large B-cell lymphoma after two or more lines of systemic therapy, including diffuse large B-cell lymphoma (DLBCL) not otherwise specified, primary mediastinal large B-cell lymphoma, high grade B-cell lymphoma, and DLBCL arising from follicular lymphoma.
1.2 Follicular Lymphoma
YESCARTA is indicated for the treatment of adult patients with relapsed or refractory follicular lymphoma (FL) after two or more lines of systemic therapy.
This indication is approved under accelerated approval based on response rate [see Clinical Studies (14.2)]. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trial(s).
2. Yescarta Dosage and Administration
For autologous use only. For intravenous use only.
2.1 Dose
Each single infusion bag of YESCARTA contains a suspension of chimeric antigen receptor (CAR)-positive T cells in approximately 68 mL. The target dose is 2 × 106 CAR-positive viable T cells per kg body weight, with a maximum of 2 × 108 CAR-positive viable T cells.
2.2 Administration
YESCARTA is for autologous use only. The patient's identity must match the patient identifiers on the YESCARTA cassette and infusion bag. Do not infuse YESCARTA if the information on the patient-specific label does not match the intended patient.
2.3 Management of Severe Adverse Reactions
Cytokine Release Syndrome
Identify CRS based on clinical presentation [see Warnings and Precautions (5.1)]. Evaluate for and treat other causes of fever, hypoxia, and hypotension. If CRS is suspected, manage according to the recommendations in Table 1. Patients who experience Grade 2 or higher CRS (e.g., hypotension not responsive to fluids, or hypoxia requiring supplemental oxygenation) should be monitored with continuous cardiac telemetry and pulse oximetry. For patients experiencing severe CRS, consider performing an echocardiogram to assess cardiac function. For severe or life-threatening CRS, consider intensive-care supportive therapy.
| CRS Grade * | Tocilizumab | Corticosteroids |
|---|---|---|
|
||
| Grade 1
Symptoms require symptomatic treatment only (e.g., fever, nausea, fatigue, headache, myalgia, malaise). | If symptoms (e.g., fever) not improving after 24 hours, consider managing as Grade 2. | If not improving after 3 days, administer one dose of dexamethasone 10 mg intravenously. |
| Grade 2
Symptoms require and respond to moderate intervention. Oxygen requirement less than 40% FiO2 or hypotension responsive to fluids or low-dose of one vasopressor or Grade 2 organ toxicity.† | Administer tocilizumab‡ 8 mg/kg intravenously over 1 hour (not to exceed 800 mg). If no clinical improvement in the signs and symptoms of CRS after the first dose, repeat tocilizumab every 8 hours as needed. Limit to a maximum of 3 doses in a 24-hour period; maximum total of 4 doses. If improving, discontinue tocilizumab. | Administer dexamethasone 10 mg intravenously once daily. If improving, manage as Grade 1 above and continue corticosteroids until the severity is Grade 1 or less, then quickly taper as clinically appropriate. If not improving, manage as appropriate grade below. |
| Grade 3
Symptoms require and respond to aggressive intervention. Oxygen requirement greater than or equal to 40% FiO2 or hypotension requiring high-dose or multiple vasopressors or Grade 3 organ toxicity or Grade 4 transaminitis. | Per Grade 2. If improving, manage as appropriate grade above. | Dexamethasone 10 mg intravenously three times a day. If improving, manage as appropriate grade above and continue corticosteroids until the severity is Grade 1 or less, then quickly taper as clinically appropriate. If not improving, manage as Grade 4. |
| Grade 4
Life-threatening symptoms. Requirements for ventilator support, continuous veno-venous hemodialysis (CVVHD) or Grade 4 organ toxicity (excluding transaminitis). | Per Grade 2. If improving, manage as appropriate grade above. | Administer methylprednisolone 1000 mg intravenously once per day for 3 days. If improving, manage as appropriate grade above and continue corticosteroids until the severity is Grade 1 or less, then taper as clinically appropriate. If not improving, consider methylprednisolone 1000 mg 2-3 times a day or alternate therapy.§ |
Neurologic Toxicity
Monitor patients for signs and symptoms of neurologic toxicity/immune effector cell-associated neurotoxicity syndrome (ICANS) (Table 2). Rule out other causes of neurologic symptoms. Patients who experience Grade 2 or higher neurologic toxicities/ICANS should be monitored with continuous cardiac telemetry and pulse oximetry. Provide intensive-care supportive therapy for severe or life-threatening neurologic toxicities. Consider levetiracetam for seizure prophylaxis for any grade of neurologic toxicities.
| Grading Assessment* | Concurrent CRS | No Concurrent CRS |
|---|---|---|
|
||
| Grade 1 | Administer tocilizumab per Table 1 for management of Grade 1 CRS. In addition, administer one dose of dexamethasone 10 mg intravenously. If not improving after 2 days, repeat dexamethasone 10 mg intravenously. | Administer one dose of dexamethasone 10 mg intravenously. If not improving after 2 days, repeat dexamethasone 10 mg intravenously. |
| Consider levetiracetam for seizure prophylaxis. | ||
| Grade 2 | Administer tocilizumab per Table 1 for management of Grade 2 CRS. In addition, administer dexamethasone 10 mg intravenously four times a day. If improving, continue corticosteroids until the severity is Grade 1 or less, then quickly taper as clinically appropriate. If not improving, manage as appropriate grade below. | Administer dexamethasone 10 mg intravenously four times a day. If improving, continue corticosteroids until the severity is Grade 1 or less, then quickly taper as clinically appropriate. If not improving, manage as appropriate grade below. |
| Consider levetiracetam for seizure prophylaxis. | ||
| Grade 3 | Administer tocilizumab per Table 1 for management of Grade 2 CRS. In addition, administer methylprednisolone 1000 mg intravenously once daily. If improving, manage as appropriate grade above and continue corticosteroids until the severity is Grade 1 or less, then taper as clinically appropriate. If not improving, manage as Grade 4. | Administer methylprednisolone 1000 mg intravenously once daily. If improving, manage as appropriate grade above and continue corticosteroids until the severity is Grade 1 or less, then taper as clinically appropriate. If not improving, manage as Grade 4. |
| Consider levetiracetam for seizure prophylaxis. | ||
| Grade 4 | Administer tocilizumab per Table 1 for management of Grade 2 CRS. In addition, administer methylprednisolone 1000 mg intravenously twice per day. If improving, manage as appropriate grade above and continue corticosteroids until the severity is Grade 1 or less, then taper as clinically appropriate. If not improving, consider 1000 mg of methylprednisolone intravenously 3 times a day or alternate therapy.† | Administer methylprednisolone 1000 mg intravenously twice per day. If improving, manage as appropriate grade above and continue corticosteroids until the severity is Grade 1 or less, then taper as clinically appropriate. If not improving, consider 1000 mg of methylprednisolone intravenously 3 times a day or alternate therapy.† |
| Consider levetiracetam for seizure prophylaxis. | ||
3. Dosage Forms and Strengths
YESCARTA is available as a cell suspension for infusion.
A single dose of YESCARTA contains 2 × 106 CAR-positive viable T cells per kg of body weight (or maximum of 2 × 108 CAR-positive viable T cells for patients 100 kg and above) in approximately 68 mL suspension in an infusion bag [see How Supplied/Storage and Handling (16)].
5. Warnings and Precautions
5.1 Cytokine Release Syndrome
CRS, including fatal or life-threatening reactions, occurred following treatment with YESCARTA. CRS occurred in 90% (379/422) of patients with non-Hodgkin lymphoma (NHL) receiving YESCARTA, including ≥ Grade 3 (Lee grading system1) CRS in 9%. CRS occurred in 93% (256/276) of patients with large B-cell lymphoma (LBCL), including ≥ Grade 3 CRS in 9% [see Adverse Reactions (6)]. Among patients with LBCL who died after receiving YESCARTA, four had ongoing CRS events at the time of death. For patients with LBCL in ZUMA-1, the median time to onset of CRS was 2 days following infusion (range: 1 to12 days) and the median duration of CRS was 7 days (range: 2 to 58 days). For patients with LBCL in ZUMA-7, the median time to onset of CRS was 3 days following infusion (range: 1 to 10 days) and the median duration was 7 days (range: 2 to 43 days).
CRS occurred in 84% (123/146) of patients with indolent non-Hodgkin lymphoma (iNHL) in ZUMA-5, including ≥ Grade 3 CRS in 8% [see Adverse Reactions (6)]. Among patients with iNHL who died after receiving YESCARTA, one patient had an ongoing CRS event at the time of death. The median time to onset of CRS was 4 days (range: 1 to 20 days) and the median duration was 6 days (range: 1 to 27 days) for patients with iNHL.
Key manifestations of CRS (≥ 10%) in all patients combined included fever (85%), hypotension (40%), tachycardia (32%), chills (22%), hypoxia (20%), headache (15%), and fatigue (12%). Serious events that may be associated with CRS include, cardiac arrhythmias (including atrial fibrillation and ventricular tachycardia), renal insufficiency, cardiac failure, respiratory failure, cardiac arrest, capillary leak syndrome, multi-organ failure, and hemophagocytic lymphohistiocytosis/macrophage activation syndrome (HLH/MAS) [see Adverse Reactions (6)].
The impact of tocilizumab and/or corticosteroids on the incidence and severity of CRS was assessed in two subsequent cohorts of LBCL patients in ZUMA-1. Among patients who received tocilizumab and/or corticosteroids for ongoing Grade 1 events (see Table 1) [see Clinical Trials Experience (6.1)], CRS occurred in 93% (38/41), including 2% (1/41) with Grade 3 CRS; no patients experienced a Grade 4 or 5 event. The median time to onset of CRS was 2 days (range: 1 to 8 days) and the median duration of CRS was 7 days (range: 2 to 16 days).
Prophylactic treatment with corticosteroids was administered to a cohort of 39 patients for 3 days beginning on the day of infusion of YESCARTA [see Clinical Trials Experience (6.1)]. Thirty-one of the 39 patients (79%) developed CRS at which point the patients were managed with tocilizumab and/or therapeutic doses of corticosteroids with no patients developing Grade 3 or higher CRS. The median time to onset of CRS was 5 days (range: 1 to 15 days) and the median duration of CRS was 4 days (range: 1 to 10 days). Although there is no known mechanistic explanation, consider the risk and benefits of prophylactic corticosteroids in the context of pre-existing comorbidities for the individual patient and the potential for the risk of Grade 4 and prolonged neurologic toxicities [See Neurologic Toxicities (5.2)].
Ensure that 2 doses of tocilizumab are available prior to infusion of YESCARTA. Monitor patients at least daily for 7 days at the certified healthcare facility following infusion for signs and symptoms of CRS. Monitor patients for signs or symptoms of CRS for 4 weeks after infusion. Counsel patients to seek immediate medical attention should signs or symptoms of CRS occur at any time [see Patient Counseling Information (17)]. At the first sign of CRS, institute treatment with supportive care, tocilizumab, or tocilizumab and corticosteroids as indicated [see Dosage and Administration (2.3)].
5.2 Neurologic Toxicities
Neurologic toxicities (including ICANS) that were fatal or life-threatening occurred following treatment with YESCARTA. Neurologic toxicities occurred in 78% (330/422) of patients with NHL receiving YESCARTA, including ≥ Grade 3 cases in 25%.
Neurologic toxicities occurred in 87% (94/108) of patients with LBCL in ZUMA-1, including ≥ Grade 3 cases in 31% and in 74% (124/168) of patients in ZUMA-7 including ≥ Grade 3 cases in 25%. The median time to onset was 4 days (range: 1 to 43 days) and the median duration was 17 days in patients with LBCL in ZUMA-1. The median time to onset for neurologic toxicity was 5 days (range:1 to 133 days) and median duration was 15 days in patients with LBCL in ZUMA-7. Neurologic toxicities occurred in 77% (112/146) of patients with iNHL, including ≥ Grade 3 in 21%. The median time to onset was 6 days (range: 1 to 79 days) and the median duration was 16 days. Ninety-eight percent of all neurologic toxicities in patients with LBCL and 99% of all neurologic toxicities in patients with iNHL occurred within the first 8 weeks of YESCARTA infusion. Neurologic toxicities occurred within the first 7 days of YESCARTA infusion in 87% of affected patients with LBCL and 74% of affected patients with iNHL.
The most common neurologic toxicities (≥ 10%) in all patients combined included encephalopathy (50%), headache (43%), tremor (29%), dizziness (21%), aphasia (17%), delirium (15%), and insomnia (10%). Prolonged encephalopathy lasting up to 173 days was noted. Serious events including aphasia, leukoencephalopathy, dysarthria, lethargy, and seizures occurred with YESCARTA. Fatal and serious cases of cerebral edema and encephalopathy, including late-onset encephalopathy, have occurred in patients treated with YESCARTA.
The impact of tocilizumab and/or corticosteroids on the incidence and severity of neurologic toxicities was assessed in two subsequent cohorts of LBCL patients in ZUMA-1. Among patients who received corticosteroids at the onset of Grade 1 toxicities (see Table 2), neurologic toxicities occurred in 78% (32/41) and 20% (8/41) had Grade 3 neurologic toxicities; no patients experienced a Grade 4 or 5 event. The median time to onset of neurologic toxicities was 6 days (range: 1 to 93 days) with a median duration of 8 days (range: 1 to 144 days). Prophylactic treatment with corticosteroids was administered to a cohort of 39 patients for 3 days beginning on the day of infusion of YESCARTA [see Clinical Trials Experience (6.1)]. Of these 39 patients, 85% (33/39) developed neurologic toxicities; 8% (3/39) developed Grade 3 and 5% (2/39) developed Grade 4 neurologic toxicities. The median time to onset of neurological toxicities was 6 days (range: 1 to 274 days) with a median duration of 12 days (range: 1 to 107 days). Prophylactic corticosteroids for management of CRS and neurologic toxicities may result in higher grade of neurologic toxicities or prolongation of neurologic toxicities, delay the onset and decrease the duration of CRS [See Cytokine Release Syndrome (5.1)].
Monitor patients at least daily for 7 days at the certified healthcare facility following infusion for signs and symptoms of neurologic toxicities. Monitor patients for signs or symptoms of neurologic toxicities for 4 weeks after infusion and treat promptly [see Dosage and Administration (2.3)].
5.3 YESCARTA and TECARTUS REMS Program
Because of the risk of CRS and neurologic toxicities, YESCARTA is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS) called the YESCARTA and TECARTUS REMS Program [see Boxed Warning and Warnings and Precautions (5.1 and 5.2)]. The required components of the YESCARTA and TECARTUS REMS Program are:
- Healthcare facilities that dispense and administer YESCARTA must be enrolled and comply with the REMS requirements. Certified healthcare facilities must have on-site, immediate access to tocilizumab, and ensure that a minimum of 2 doses of tocilizumab are available for each patient for infusion within 2 hours after YESCARTA infusion, if needed for treatment of CRS.
- Certified healthcare facilities must ensure that healthcare providers who prescribe, dispense, or administer YESCARTA are trained about the management of CRS and neurologic toxicities.
Further information is available at www.YescartaTecartusREMS.com or 1-844-454-KITE (5483).
5.4 Hypersensitivity Reactions
Allergic reactions may occur with the infusion of YESCARTA. Serious hypersensitivity reactions, including anaphylaxis, may be due to dimethyl sulfoxide (DMSO) or residual gentamicin in YESCARTA.
5.5 Serious Infections
Severe or life-threatening infections occurred in patients after YESCARTA infusion. Infections (all grades) occurred in 45% of patients with NHL. Grade 3 or higher infections occurred in 17% of patients, including Grade 3 or higher infections with an unspecified pathogen in 12%, bacterial infections in 5%, viral infections in 3%, and fungal infections in 1%. YESCARTA should not be administered to patients with clinically significant active systemic infections. Monitor patients for signs and symptoms of infection before and after YESCARTA infusion and treat appropriately. Administer prophylactic antimicrobials according to local guidelines.
Febrile neutropenia was observed in 36% of patients with NHL after YESCARTA infusion and may be concurrent with CRS. In the event of febrile neutropenia, evaluate for infection and manage with broad-spectrum antibiotics, fluids, and other supportive care as medically indicated.
In immunosuppressed patients, including those who have received YESCARTA, life-threatening and fatal opportunistic infections including disseminated fungal infections (e.g., candida sepsis and aspergillus infections) and viral reactivation (e.g., human herpes virus-6 [HHV-6] encephalitis and JC virus progressive multifocal leukoencephalopathy [PML]) have been reported. The possibility of HHV-6 encephalitis and PML should be considered in immunosuppressed patients with neurologic events and appropriate diagnostic evaluations should be performed.
5.6 Prolonged Cytopenias
Patients may exhibit cytopenias for several weeks following lymphodepleting chemotherapy and YESCARTA infusion. Grade 3 or higher cytopenias not resolved by Day 30 following YESCARTA infusion occurred in 39% of all patients with NHL and included neutropenia (33%), thrombocytopenia (13%), and anemia (8%). Monitor blood counts after YESCARTA infusion.
5.7 Hypogammaglobulinemia
B-cell aplasia and hypogammaglobulinemia can occur in patients receiving treatment with YESCARTA. Hypogammaglobulinemia was reported as an adverse reaction in 14% of all patients with NHL. Monitor immunoglobulin levels after treatment with YESCARTA and manage using infection precautions, antibiotic prophylaxis, and immunoglobulin replacement.
The safety of immunization with live viral vaccines during or following YESCARTA treatment has not been studied. Vaccination with live virus vaccines is not recommended for at least 6 weeks prior to the start of lymphodepleting chemotherapy, during YESCARTA treatment, and until immune recovery following treatment with YESCARTA.
5.8 Secondary Malignancies
Patients treated with YESCARTA may develop secondary malignancies. Monitor life-long for secondary malignancies. In the event that a secondary malignancy occurs, contact Kite at 1-844-454-KITE (5483) to obtain instructions on patient samples to collect for testing.
5.9 Effects on Ability to Drive and Use Machines
Due to the potential for neurologic events, including altered mental status or seizures, patients receiving YESCARTA are at risk for altered or decreased consciousness or coordination in the 8 weeks following YESCARTA infusion. Advise patients to refrain from driving and engaging in hazardous occupations or activities, such as operating heavy or potentially dangerous machinery, during this initial period.
6. Adverse Reactions/Side Effects
The following adverse reactions are described elsewhere in the labeling:
- Cytokine Release Syndrome [see Warnings and Precautions (5.1, 5.3)]
- Neurologic Toxicities [see Warnings and Precautions (5.2, 5.3)]
- Hypersensitivity Reactions [see Warnings and Precautions (5.4)]
- Serious Infections [see Warnings and Precautions (5.5)]
- Prolonged Cytopenias [see Warnings and Precautions (5.6)]
- Hypogammaglobulinemia [see Warnings and Precautions (5.7)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The data described in the WARNINGS AND PRECAUTIONS reflect exposure to a single dose of YESCARTA in one randomized, open-label study with 168 patients with relapsed or refractory LBCL (ZUMA-7) and two open-label, single-arm studies with 108 patients with relapsed or refractory LBCL (ZUMA-1 study) and 146 patients with relapsed or refractory iNHL (including 124 with FL; ZUMA-5 study).
Relapsed or Refractory Large B-cell Lymphoma
ZUMA-7
The safety of YESCARTA was evaluated in ZUMA-7, a randomized, open-label, multicenter study in which patients with primary refractory LBCL or first relapse of LBCL received YESCARTA (N = 168) or standard therapy (N = 168) [see Clinical Studies (14)]. Patients had not yet received treatment for relapsed or refractory lymphoma and were potential candidates for autologous HSCT. The trial excluded patients who were not deemed candidates for transplant or who had a history of central nervous system (CNS) disorders (such as seizures or cerebrovascular ischemia), serious or uncontrolled infection, or autoimmune disease requiring systemic immunosuppression. The study required ANC ≥ 1000/mm3, platelet count ≥ 75,000/mm3, creatinine clearance ≥ 60 ml/min, AST/ALT ≤ 2.5 × ULN, and total bilirubin ≤ 1.5mg/dL.
The median age of the YESCARTA-treated safety population was 59 years (range: 21 to 80 years); 62% were male. The baseline Eastern Cooperative Oncology Group (ECOG) performance status was 0 in 54% of patients and 1 in 46%.
The most common non-laboratory adverse reactions to YESCARTA (incidence ≥ 20%) included fever, CRS, fatigue, hypotension, encephalopathy, tachycardia, diarrhea, headache, musculoskeletal pain, nausea, febrile neutropenia, chills, cough, infection with unspecified pathogen, dizziness, tremor, decreased appetite, edema, hypoxia, abdominal pain, aphasia, constipation, and vomiting. Serious adverse reactions occurred in 50% of patients. The most common serious adverse reactions (> 5%) included CRS, fever, encephalopathy, hypotension, infection with unspecified pathogen, and pneumonia. Fatal adverse reactions occurred in 2% of patients.
The most common (≥ 10%) Grade 3 or higher non-laboratory adverse reactions included febrile neutropenia, encephalopathy, and hypotension.
Sixty-seven percent (112/168) of patients received tocilizumab after infusion of YESCARTA.
Table 3 summarizes selected non-laboratory adverse reactions in patients treated with YESCARTA, and Table 4 summarizes selected new or worsening Grade 3 or 4 laboratory abnormalities.
| Adverse Reaction | YESCARTA N = 168 |
|
|---|---|---|
| Any Grade (%) | Grade 3 or Higher (%) | |
| The following events were also counted in the incidence of CRS: coagulopathy, tachycardia, arrhythmia, cardiac failure, diarrhea, nausea, vomiting, fever, fatigue, chills, edema, decreased appetite, musculoskeletal pain, headache, tremor, dizziness, renal insufficiency, cough, hypoxia, dyspnea, pleural effusion, respiratory failure, rash, hypotension, and hypertension. | ||
|
||
| Febrile neutropenia | 31 | 31 |
| Cardiac Disorders | ||
| Tachycardia * | 43 | 2 |
| Arrhythmia † | 14 | 3 |
| Gastrointestinal Disorders | ||
| Diarrhea ‡ | 42 | 3 |
| Nausea | 40 | 2 |
| Abdominal pain § | 20 | 4 |
| Constipation | 20 | 0 |
| Vomiting | 20 | 0 |
| Dry Mouth | 10 | 0 |
| General Disorders and Administration Site Conditions | ||
| Fever ¶ | 93 | 9 |
| Fatigue # | 52 | 7 |
| Chills | 28 | 1 |
| Edema Þ | 23 | 1 |
| Immune System Disorders | ||
| Cytokine release syndrome | 92 | 7 |
| Hypogammaglobulinemia | 11 | 0 |
| Infections and Infestations | ||
| Infections with pathogen unspecified | 25 | 8 |
| Viral infections | 15 | 4 |
| Bacterial infections | 10 | 5 |
| Fungal infections | 10 | 1 |
| Metabolism and Nutrition Disorders | ||
| Decreased appetite | 24 | 4 |
| Musculoskeletal and Connective Tissue Disorders | ||
| Musculoskeletal pain ß | 40 | 1 |
| Motor dysfunction à | 15 | 4 |
| Nervous System Disorders | ||
| Encephalopathy è | 46 | 18 |
| Headache ð | 41 | 3 |
| Tremor | 25 | 1 |
| Dizziness ø | 25 | 4 |
| Aphasia | 20 | 7 |
| Neuropathy peripheral ý | 11 | 2 |
| Psychiatric Disorders | ||
| Insomnia £ | 13 | 0 |
| Delirium ¥ | 12 | 4 |
| Renal and Urinary Disorders | ||
| Renal insufficiency Π| 11 | 2 |
| Respiratory, Thoracic and Mediastinal Disorders | ||
| Cough œ | 27 | 1 |
| Hypoxia | 21 | 9 |
| Skin and Subcutaneous Tissue Disorders | ||
| Rash Ɖ | 17 | 1 |
| Vascular Disorders | ||
| Hypotension A | 47 | 11 |
Other clinically important adverse reactions that occurred in less than 10% of patients treated with YESCARTA include the following:
- Blood and lymphatic system disorders: Coagulopathy (9%)
- Cardiac disorders: Cardiac failure (1%)
- Eye Disorders: Visual impairment (7%)
- Infections and infestations: Pneumonia (8%), Sepsis (4%)
- Nervous system disorders: Ataxia (6%), seizure (3%), myoclonus (2%), facial paralysis (2%), paresis (2%)
- Respiratory, thoracic and mediastinal disorders: Dyspnea (8%), pleural effusion (6%), respiratory failure (2%)
- Vascular disorders: Hypertension (9%), thrombosis (7%)
Laboratory abnormalities:
| Laboratory Abnormality | YESCARTA |
|---|---|
| Grades 3 or 4 (%) | |
|
|
| Leukocyte decrease | 95 |
| Neutrophil decrease | 94 |
| Lymphocyte decrease | 94 |
| Hemoglobin decrease | 40 |
| Platelet decrease | 26 |
| Sodium decrease | 12 |
| Glucose increase | 11 |
ZUMA-1
The safety of YESCARTA was evaluated in ZUMA-1, a study in which 108 patients with relapsed or refractory LBCL received CD19-positive CAR T cells based on a recommended dose which was weight-based [see Clinical Studies (14)]. Patients with a history of CNS disorders (such as seizures or cerebrovascular ischemia) or autoimmune disease requiring systemic immunosuppression were ineligible. The median age of the study population was 58 years (range: 23 to 76 years); 68% were male. The baseline Eastern Cooperative Oncology Group (ECOG) performance status was 0 in 43% of patients and 1 in 57% of patients.
The most common adverse reactions (incidence ≥ 20%) included CRS, fever, hypotension, encephalopathy, tachycardia, fatigue, headache, decreased appetite, chills, diarrhea, febrile neutropenia, infections with pathogen unspecified, nausea, hypoxia, tremor, cough, vomiting, dizziness, constipation, and cardiac arrhythmias. Serious adverse reactions occurred in 52% of patients. The most common serious adverse reactions (> 2%) included encephalopathy, fever, lung infection, febrile neutropenia, cardiac arrhythmia, cardiac failure, urinary tract infection, renal insufficiency, aphasia, cardiac arrest, Clostridium difficile infection, delirium, hypotension, and hypoxia.
The most common (≥ 10%) Grade 3 or higher reactions included febrile neutropenia, fever, CRS, encephalopathy, infections with pathogen unspecified, hypotension, hypoxia, and lung infections.
Forty-five percent (49/108) of patients received tocilizumab after infusion of YESCARTA.
Table 5 summarizes non-laboratory adverse reactions that occurred in ≥ 10% of patients treated with YESCARTA, and Table 6 describes the laboratory abnormalities of Grade 3 or 4 that occurred in ≥ 10% of patients.
| Adverse Reaction | Any Grade (%) | Grade 3 or Higher (%) |
|---|---|---|
| The following events were also counted in the incidence of CRS: tachycardia, arrhythmia, fever, chills, hypoxia, renal insufficiency, and hypotension. | ||
|
||
| Blood and Lymphatic System Disorders | ||
| Febrile neutropenia | 34 | 31 |
| Cardiac Disorders | ||
| Tachycardia * | 57 | 2 |
| Arrhythmia † | 23 | 7 |
| Gastrointestinal Disorders | ||
| Diarrhea | 38 | 4 |
| Nausea | 34 | 0 |
| Vomiting | 26 | 1 |
| Constipation | 23 | 0 |
| Abdominal pain ‡ | 14 | 1 |
| Dry mouth | 11 | 0 |
| General Disorders and Administration Site Conditions | ||
| Fever § | 86 | 16 |
| Fatigue ¶ | 46 | 3 |
| Chills | 40 | 0 |
| Edema # | 19 | 1 |
| Immune System Disorders | ||
| Cytokine release syndrome | 94 | 13 |
| Hypogammaglobulinemia Þ | 15 | 0 |
| Infections and Infestations | ||
| Infections with pathogen unspecified | 26 | 16 |
| Viral infections | 16 | 4 |
| Bacterial infections | 13 | 9 |
| Investigations | ||
| Decreased appetite | 44 | 2 |
| Weight decreased | 16 | 0 |
| Dehydration | 11 | 3 |
| Musculoskeletal and Connective Tissue Disorders | ||
| Motor dysfunction ß | 19 | 1 |
| Pain in extremity à | 17 | 2 |
| Back pain | 15 | 1 |
| Muscle pain | 14 | 1 |
| Arthralgia | 10 | 0 |
| Nervous System Disorders | ||
| Encephalopathy è | 57 | 29 |
| Headache ð | 45 | 1 |
| Tremor | 31 | 2 |
| Dizziness ø | 21 | 1 |
| Aphasia ý | 18 | 6 |
| Psychiatric Disorders | ||
| Delirium £ | 17 | 6 |
| Respiratory, Thoracic and Mediastinal Disorders | ||
| Hypoxia ¥ | 32 | 11 |
| Cough Π| 30 | 0 |
| Dyspnea œ | 19 | 3 |
| Pleural effusion | 13 | 2 |
| Renal and Urinary Disorders | ||
| Renal insufficiency | 12 | 5 |
| Vascular Disorders | ||
| Hypotension Ɖ | 57 | 15 |
| Hypertension | 15 | 6 |
| Thrombosis A | 10 | 1 |
Other clinically important adverse reactions that occurred in less than 10% of patients treated with YESCARTA include the following:
- Blood and lymphatic system disorders: Coagulopathy (2%)
- Cardiac disorders: Cardiac failure (6%), cardiac arrest (4%)
- Immune system disorders: Hemophagocytic lymphohistiocytosis/macrophage activation syndrome (HLH/MAS) (1%), hypersensitivity (1%)
- Infections and infestations disorders: Fungal infections (5%)
- Nervous system disorders: Ataxia (6%), seizure (4%), dyscalculia (2%), myoclonus (2%)
- Respiratory, thoracic and mediastinal disorders: Pulmonary edema (9%)
- Skin and subcutaneous tissue disorders: Rash (9%)
- Vascular disorders: Capillary leak syndrome (3%)
Laboratory abnormalities:
| Laboratory Abnormality | Grades 3 or 4 (%) |
|---|---|
|
|
| Lymphocyte decrease | 96 |
| Leukocyte decrease | 96 |
| Neutrophil decrease | 92 |
| Hemoglobin decrease | 60 |
| Platelet decrease | 56 |
| Phosphate decrease | 52 |
| Sodium decrease | 19 |
| Albumin decrease | 19 |
| Direct bilirubin increased | 14 |
| Uric acid increased | 13 |
| Potassium decrease | 11 |
The safety and efficacy of YESCARTA was evaluated in two subsequent cohorts of LBCL patients. The first subsequent, open label, safety management cohort in ZUMA-1 evaluated the safety and efficacy of YESCARTA with the use of tocilizumab and/or corticosteroid and prophylactic levetiracetam (750mg PO or IV twice daily) for Grade 1 CRS or neurologic events (see Tables 1 and 2). A total of 46 patients with relapsed or refractory LBCL were enrolled and 41 patients were treated with YESCARTA. Of the remaining 5 patients who were not treated, 2 patients died prior to receiving YESCARTA and 3 patients were ineligible due to disease progression. Twenty-eight patients (68%) treated with YESCARTA received bridging therapy between leukapheresis and lymphodepleting chemotherapy. Thirty-two patients (78%) treated with YESCARTA received tocilizumab and/or corticosteroid for CRS and/or neurologic events. Fifteen of 36 with Grade 1 CRS and 21 of 24 patients with Grade 2 CRS received tocilizumab and/or corticosteroids. Among patients who received treatment for Grade 1 or Grade 2 CRS, most patients (13 of 15 and 19 of 21 patients, respectively) received both tocilizumab and corticosteroids. Most patients received 1 or 2 doses of each drug. Ten of 27 patients with Grade 1 and 7 of 15 patients with Grade 2 neurologic events received corticosteroids alone or in combination with tocilizumab.
The second subsequent, open label, safety management cohort in ZUMA-1 evaluated the safety and efficacy of YESCARTA with the use of prophylactic corticosteroids (oral dexamethasone 10 mg once daily for 3 days, starting prior to YESCARTA infusion on Day 0) and prophylactic levetiracetam (750 mg PO or IV) [see Warnings and Precautions (5.1 and 5.2)].
Relapsed or Refractory Follicular Lymphoma
The safety of YESCARTA was evaluated in ZUMA-5, a study that included 146 patients with relapsed or refractory iNHL (124 patients with FL and 22 with marginal zone lymphoma) who received CD19-positive CAR T cells [see Clinical Studies (14)]. Patients with a history of CNS disorders or autoimmune disease requiring systemic immunosuppression were ineligible. The median age was 61 years (range: 34 to 79 years), 43% were female, 93% were white, 3% were black, and 1% were Asian.
The most common non-laboratory adverse reactions (incidence ≥ 20%) included fever, CRS, hypotension, encephalopathy, fatigue, headache, infections with pathogen unspecified, tachycardia, febrile neutropenia, musculoskeletal pain, nausea, tremor, chills, diarrhea, constipation, decreased appetite, cough, vomiting, hypoxia, arrhythmia, and dizziness. Serious adverse reactions occurred in 48% of patients. Serious adverse reactions in > 2% of patients included febrile neutropenia, encephalopathy, fever, CRS, infections with pathogen unspecified, pneumonia, hypoxia, and hypotension.
The most common (≥ 10%) Grade 3 or higher reactions included febrile neutropenia, encephalopathy, and infections with pathogen unspecified. Fatal adverse reactions occurred in 1% of patients and included CRS and fungal infection.
Fifty-one percent (75/146) of patients received tocilizumab after infusion of YESCARTA.
Table 7 summarizes the adverse reactions, excluding laboratory terms, that occurred in at least 10% of patients treated with YESCARTA and Table 8 describes Grade 3 or 4 laboratory abnormalities that developed or worsened in at least 10% of patients.
| Adverse Reaction | Any Grade (%) | Grade 3 or Higher (%) |
|---|---|---|
|
||
| Blood and lymphatic system disorders | ||
| Febrile neutropenia * | 41 | 41 |
| Cardiac Disorders | ||
| Tachycardia † | 44 | 1 |
| Arrhythmia ‡ | 21 | 2 |
| Gastrointestinal Disorders | ||
| Nausea | 40 | 0 |
| Diarrhea § | 29 | 1 |
| Constipation | 28 | 0 |
| Vomiting | 24 | 1 |
| Abdominal pain ¶ | 16 | 0 |
| General Disorders and Administration Site Conditions | ||
| Fever | 85 | 8 |
| Fatigue # | 49 | 1 |
| Chills | 29 | 0 |
| Edema Þ | 13 | 1 |
| Immune System Disorders | ||
| Cytokine release syndrome | 84 | 8 |
| Immunoglobulins decreased ß | 18 | 1 |
| Infections and Infestations | ||
| Infections with pathogen unspecified | 45 | 14 |
| Pneumonia à | 13 | 8 |
| Fungal infections | 12 | 2 |
| Viral Infections | 13 | 2 |
| Metabolism and Nutrition Disorders | ||
| Decreased appetite è | 26 | 1 |
| Musculoskeletal and Connective Tissue Disorders | ||
| Musculoskeletal pain ð | 40 | 1 |
| Motor dysfunction ø | 18 | 2 |
| Nervous System Disorders | ||
| Encephalopathy ý | 49 | 16 |
| Headache | 45 | 1 |
| Tremor | 31 | 1 |
| Dizziness £ | 20 | 0 |
| Aphasia | 14 | 4 |
| Neuropathy peripheral ¥ | 12 | 0 |
| Ataxia Π| 10 | 0 |
| Psychiatric Disorders | ||
| Delirium œ | 16 | 5 |
| Insomnia | 16 | 0 |
| Affective disorder Ɖ | 10 | 1 |
| Respiratory, Thoracic and Mediastinal Disorders | ||
| Cough A | 25 | 0 |
| Hypoxia | 23 | 8 |
| Dyspnea B | 12 | 1 |
| Nasal congestion | 10 | 0 |
| Skin and Subcutaneous Tissue Disorders | ||
| Rash C | 19 | 3 |
| Vascular Disorders | ||
| Hypotension D | 51 | 4 |
| Hypertension | 13 | 6 |
| Thrombosis E | 12 | 4 |
Other clinically important adverse reactions that occurred in less than 10% of patients treated with YESCARTA include the following:
- Blood and lymphatic system disorders: Coagulopathy (6%)
- Cardiac disorders: Cardiac failure (2%)
- Eye disorders: Visual impairment (5%), blindness (1%)
- Gastrointestinal disorders: Dysphagia (6%)
- General disorders and administration site conditions: Multiple organ dysfunction syndrome (1%)
- Infections and infestations: Bacterial infections (8%), sepsis (2%), herpesvirus infection (4%)
- Musculoskeletal and connective tissue disorders: Muscle injury (1%)
- Nervous system disorders: Seizure (2%), hemiparesis (2%), ischemic stroke (1%)
- Renal and urinary disorders: Renal insufficiency (8%)
- Respiratory, thoracic and mediastinal disorders: Respiratory failure (1%)
- Vascular disorders: Hemorrhage (8%)
Laboratory abnormalities:
| Laboratory Abnormality | Grades 3 or 4 (%) |
|---|---|
|
|
| Lymphocyte decrease | 96 |
| Leukocyte decrease | 94 |
| Neutrophil decrease | 92 |
| Platelet decrease | 35 |
| Hemoglobin decrease | 32 |
| Phosphate decrease | 25 |
| Sodium decrease | 10 |
| Glucose increase | 10 |
| Calcium decrease | 10 |
6.2 Immunogenicity
YESCARTA has the potential to induce anti-product antibodies. The immunogenicity of YESCARTA has been evaluated using an enzyme-linked immunosorbent assay (ELISA) for the detection of binding antibodies against FMC63, the originating antibody of the anti-CD19 CAR. Eleven patients (4%) tested positive for pre-dose anti-FMC63 antibodies at baseline in ZUMA-7 and ZUMA-1, and one patient (1%) who had a negative test result at baseline had a positive test result post administration of YESCARTA in the screening ELISA in ZUMA-7. In ZUMA-5, 19 patients (13%) were antibody-positive at baseline, and 3 patients (2%) who had negative test results at baseline had positive test results post administration of YESCARTA in the screening ELISA. Results of a confirmatory cell-based assay, leveraging a properly folded and expressed extracellular portion of the CAR (ScFv, hinge and linker) demonstrated that all patients treated with YESCARTA that had a positive result in the screening ELISA were antibody negative at all time points tested. There is no evidence that the kinetics of initial expansion and persistence of YESCARTA, or the safety or effectiveness of YESCARTA, was altered in these patients.
6.3 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of YESCARTA. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
8. Use In Specific Populations
11. Yescarta Description
YESCARTA (axicabtagene ciloleucel) is a CD19-directed genetically modified autologous T cell immunotherapy. To prepare YESCARTA, a patient's own T cells are harvested and genetically modified ex vivo by retroviral transduction to express a chimeric antigen receptor (CAR) comprising a murine anti-CD19 single chain variable fragment (scFv) linked to CD28 and CD3-zeta co-stimulatory domains. The anti-CD19 CAR T cells are expanded and infused back into the patient, where they can recognize and eliminate CD19-expressing target cells.
YESCARTA is prepared from the patient's peripheral blood mononuclear cells, which are obtained via a standard leukapheresis procedure. The mononuclear cells are enriched for T cells and activated with anti-CD3 antibody in the presence of IL-2, then transduced with the replication incompetent retroviral vector containing the anti-CD19 CAR transgene. The transduced T cells are expanded in cell culture, washed, formulated into a suspension, and cryopreserved. The product must pass a sterility test before release for shipping as a frozen suspension in a patient-specific infusion bag. The product is thawed prior to infusion [see Dosage and Administration (2.2), How Supplied/Storage and Handling (16)].
In addition to T cells, YESCARTA may contain NK and NK-T cells. The formulation contains 5% dimethyl sulfoxide (DMSO) and 2.5% albumin (human).
12. Yescarta - Clinical Pharmacology
12.1 Mechanism of Action
YESCARTA, a CD19-directed genetically modified autologous T cell immunotherapy, binds to CD19-expressing cancer cells and normal B cells. Studies demonstrated that following anti-CD19 CAR T cell engagement with CD19-expressing target cells, the CD28 and CD3-zeta co-stimulatory domains activate downstream signaling cascades that lead to T cell activation, proliferation, acquisition of effector functions and secretion of inflammatory cytokines and chemokines. This sequence of events leads to killing of CD19-expressing cells.
12.2 Pharmacodynamics
After YESCARTA infusion, pharmacodynamic responses were evaluated over a 4-week interval by measuring transient elevation of cytokines, chemokines and other molecules in blood. Levels of cytokines and chemokines such as IL-6, IL-8, IL-10, IL-15, TNF-α, IFN-γ, and sIL2Rα were analyzed. Peak elevation was observed within the first 14 days after infusion, and levels generally returned to baseline within 28 days.
Due to the on-target effect of YESCARTA, a period of B-cell aplasia is expected.
12.3 Pharmacokinetics
Following infusion of YESCARTA, anti-CD19 CAR T cells exhibited an initial rapid expansion followed by a decline to near baseline levels by 3 months. Peak levels of anti-CD19 CAR T cells occurred within the first 7 - 14 days after YESCARTA infusion.
Age (range: 21 to 80 years) and gender had no significant impact on AUC Day 0 - 28 and Cmax of YESCARTA.
14. Clinical Studies
14.1 Relapsed or Refractory Large B-Cell Lymphoma
ZUMA-7
A randomized, open-label, multicenter trial evaluated the efficacy of YESCARTA in adult patients with relapsed or refractory LBCL after first-line chemoimmunotherapy that included rituximab and anthracycline (ZUMA-7; NCT03391466). Patients had not yet received treatment for relapsed or refractory lymphoma and were potential candidates for autologous HSCT. Patients were required to have primary refractory disease or relapse within 12 months following completion of first-line therapy. The study excluded patients with primary mediastinal B-cell lymphoma, any history of central nervous system lymphoma, need for urgent therapy due to tumor mass effect, active or serious infections, and ECOG performance status of 2 or greater.
In total, 359 patients were randomized in a 1:1 ratio to receive a single infusion of YESCARTA or to receive second-line standard therapy, consisting of 2 or 3 cycles of chemoimmunotherapy followed by high-dose therapy and autologous HSCT in patients who attained CR or PR. Randomization was stratified by response to first-line therapy and second-line age-adjusted International Prognostic Index.
Following lymphodepleting chemotherapy, YESCARTA was administered as a single intravenous infusion at a target dose of 2 × 106 CAR-positive viable T cells/kg (maximum permitted dose: 2 × 108 cells). The lymphodepleting regimen consisted of cyclophosphamide 500 mg/m2 intravenously and fludarabine 30 mg/m2 intravenously, both given on the fifth, fourth, and third day before YESCARTA. All patients who received YESCARTA were monitored at a healthcare facility for a minimum of 7 days. Bridging therapy, administered between leukapheresis and lymphodepleting chemotherapy, was limited to corticosteroids and was permitted for patients with high disease burden.
In the overall study population, the median age was 59 years (range: 21 to 81 years), 66% were male, 83% were white, 6% were Asian, and 5% were Black. The diagnoses included de novo DLBCL NOS (63%), HGBL with or without MYC and BCL-2 and/or BCL-6 rearrangements (19%), and large cell transformation of follicular lymphoma (13%). In total, 74% of patients had primary refractory LBCL, and 26% had relapsed disease within 12 months of first-line therapy.
Of the 180 patients randomized to receive YESCARTA, 178 underwent leukapheresis and 170 were treated with YESCARTA, of whom 60 (33%) received bridging corticosteroid therapy. Eight patients (4%) were not treated following leukapheresis, primarily due to progressive disease, serious adverse events, or death. The median time from leukapheresis to product delivery was 18 days (range: 13 to 49 days), and from leukapheresis to YESCARTA infusion was 26 days (range: 16 to 52 days). The median dose was 2.0 × 106 CAR-positive viable T cells/kg (range: 1.0 to 2.1 × 106 cells/kg).
Of the 179 patients randomized to receive standard therapy, 168 patients received any study treatment, and 62 (35%) received high-dose therapy and on-protocol HSCT. The most common reason for not receiving HSCT was lack of response to salvage chemotherapy.
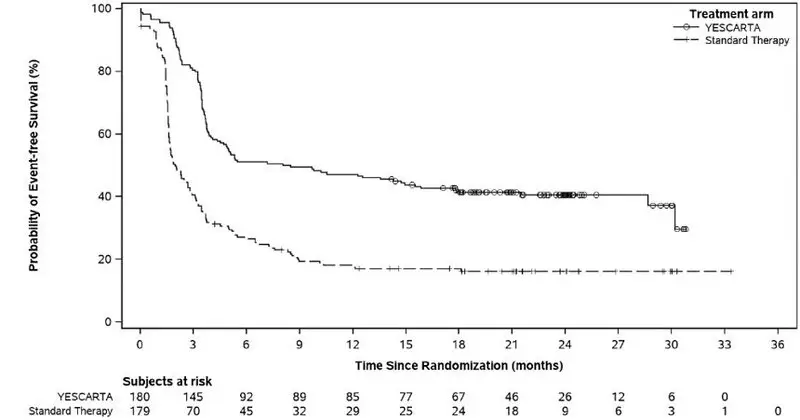
The primary efficacy measure was event-free survival (EFS) as determined by an independent review committee. Efficacy is summarized in Table 9 and Figure 1. The estimated EFS rate at 18 months was 41.5% [95% CI: 34.2, 48.6] in the YESCARTA arm and 17.0% [95% CI: 11.8, 23.0] in the standard therapy arm.
In the YESCARTA arm, the estimated median DOR was 28.4 months (95% CI: 26.9, NE) in patients who achieved CR and 1.6 months (95% CI: 1.4, 1.9) in patients who achieved a best response of PR.
An interim analysis of overall survival was conducted at the time of the primary EFS analysis. The interim analysis of overall survival has not met the criteria for statistical significance. Fifty-five percent of patients randomized to the standard therapy arm subsequently received CD19-directed CAR T therapy off protocol.
| Outcome* | YESCARTA (N = 180)† | Standard Therapy (N = 179) |
|---|---|---|
| CI, confidence interval; NE, not estimable. | ||
|
||
| Event-Free Survival‡ | ||
| Number of events, n (%) | 108 (60) | 144 (80) |
| Median, months [95% CI]§ | 8.3 [4.5, 15.8] | 2.0 [1.6, 2.8] |
| Stratified hazard ratio [95% CI] | 0.40 [0.31, 0.51] | |
| Stratified log-rank p-value | <0.0001 | |
| Best Objective Response Rate, % [95% CI] | 83 [77, 88] | 50 [43, 58] |
| Difference in ORR, % [95% CI] | 33 [23, 42] | |
| Stratified p-value¶ | <0.0001 | |
| Complete remission rate, % [95% CI] | 65 [58, 72] | 32 [26, 40] |
| Partial remission rate, % [95% CI] | 18 [13, 25] | 18 [13, 24] |
| Progression-Free Survival | ||
| Number of events, n (%) | 93 (52) | 81 (45) |
| Median, months [95% CI] § | 14.9 [7.2, NE] | 5.0 [3.4, 8.5] |
| Stratified hazard ratio [95% CI] | 0.56 [0.41, 0.76] | |
Figure 1. Kaplan-Meier Curve of Event-Free Survival in ZUMA-7
14.2 Relapsed or Refractory Follicular Lymphoma
Efficacy in FL is based on a single-arm, open-label, multicenter trial (ZUMA-5; NCT03105336) that evaluated a single infusion of YESCARTA in adult patients with relapsed or refractory FL after two or more lines of systemic therapy, including the combination of an anti-CD20 monoclonal antibody and an alkylating agent. The study excluded patients with active or serious infections, transformed lymphoma or other aggressive lymphomas, prior allogeneic HSCT, or any history of CNS lymphoma or CNS disorders. Following lymphodepleting chemotherapy, YESCARTA was administered as a single intravenous infusion with a target dose of 2 × 106 anti-CD19 CAR T cells/kg (maximum permitted dose: 2 × 108 cells). The lymphodepleting regimen consisted of cyclophosphamide 500 mg/m2 intravenously and fludarabine 30 mg/m2 intravenously, both given on the fifth, fourth, and third day before YESCARTA.
Of 123 patients with FL who underwent leukapheresis, 120 received YESCARTA. Of the remaining three patients (2%) who were not treated, one was ineligible due to thrombocytopenia, one went into remission prior to initiating lymphodepletion, and one died of cardiac arrest. There were no manufacturing failures. Of the 120 patients with FL infused with YESCARTA, the 81 consecutive patients included in the primary efficacy analysis had at least 9 months of potential follow-up from date of first response.
Among the 81 patients with FL included in the primary efficacy analysis, the median age was 62 years (range: 34 to 79 years), 46% were female, 93% were white, 4% were black, and 3% were Asian. The median number of prior systemic therapies was 3 (range: 2 to 9), with 32% having 2 prior lines, 22% having 3 prior lines, and 46% having ≥ 4 prior lines. Thirty-one percent had received a PI3K inhibitor, 72% had progression within 6 months of the most recent regimen, and 56% had progression within 24 months of initiating their first anti-CD20 combination therapy. Between leukapheresis and administration of YESCARTA, one patient (1%) in the primary efficacy analysis received bridging therapy.
Among the 81 patients included in the primary efficacy analysis, the median time from leukapheresis to product delivery was 17 days (range: 13 to 33 days) and leukapheresis to product infusion was 27 days (range: 19 to 250 days). The median dose of YESCARTA was 2.0 × 106 CAR T cells/kg (range 1.3 to 2.1 × 106 CAR T cells/kg). All treated patients received YESCARTA infusion on day 0 and were hospitalized until at least day 7.
Efficacy was established on the basis of objective response rate and DOR as determined by an independent review committee (Table 12 and Table 13). The median time to response in the primary efficacy population was 1.0 month (range: 0.8 to 3.1 months).
| Primary Efficacy Analysis (N = 81) | All Leukapheresed Patients (N = 123) |
|
|---|---|---|
| CI, confidence interval. | ||
|
||
| Objective Response Rate*, n (95% CI) | 74 (91%) (83, 96) | 110 (89%) (83, 94) |
| Complete Remission†, n (95% CI) | 49 (60%) (49, 71) | 76 (62%) (53, 70) |
| Partial Remission, n (95% CI) | 25 (31%) (21, 42) | 34 (28%) (20, 36) |
| From N of 81 | |
|---|---|
| CR, complete remission; DOR, duration of response; NE, not estimable; PR, partial remission. | |
|
|
| Number of Responders | 74 |
| DOR (Months)* | |
| Median†
(95% CI) | NE (20.8, NE) |
| Range‡ | 0.0, 25.0+ |
| Rate of Continued Remission*, †, § | |
| At 12 months (95% CI), % | 76.2 (63.9, 84.7) |
| At 18 months (95% CI), % | 74.2 (61.5, 83.2) |
| Median Follow-up for DOR (Months)*, † | 14.5 |
15. References
- Lee DW et al (2014). Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014 Jul 10; 124(2): 188–195.
16. How is Yescarta supplied
YESCARTA is supplied in an infusion bag (NDC 71287-119-01) containing approximately 68 mL of frozen suspension of genetically modified autologous T cells in 5% DMSO and 2.5% albumin (human).
17. Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Ensure that patients understand the risk of manufacturing failure (< 1% in clinical trials). In case of a manufacturing failure, a second manufacturing of YESCARTA may be attempted. In addition, while the patient awaits the product, additional chemotherapy (not the lymphodepletion) may be necessary and may increase the risk of adverse events during the pre-infusion period.
Advise patients to seek immediate attention for any of the following:
- Cytokine Release Syndrome (CRS) - Signs or symptoms associated with CRS, including fever, chills, fatigue, tachycardia, nausea, hypoxia, and hypotension [see Warnings and Precautions (5.1) and Adverse Reactions (6)].
- Neurologic Toxicities - Signs or symptoms associated with neurologic events, including encephalopathy, seizures, changes in level of consciousness, speech disorders, tremors, and confusion [see Warnings and Precautions (5.2) and Adverse Reactions (6)].
- Serious Infections - Signs or symptoms associated with infection [see Warnings and Precautions (5.5) and Adverse Reactions (6)].
- Prolonged Cytopenia - Signs or symptoms associated with bone marrow suppression, including neutropenia, anemia, thrombocytopenia, or febrile neutropenia [see Warnings and Precautions (5.6) and Adverse Reactions (6)].
Advise patients of the need to:
- Refrain from driving or operating heavy or potentially dangerous machinery after YESCARTA infusion for at least 8 weeks after infusion [see Warnings and Precautions (5.9)].
- Have periodic monitoring of blood counts.
- Contact Kite at 1-844-454-KITE (5483) if they are diagnosed with a secondary malignancy [see Warnings and Precautions (5.8)].
| MEDICATION GUIDE YESCARTA (pronounced yes-kar-ta) (axicabtagene ciloleucel) |
|
|---|---|
| This Medication Guide has been approved by the U.S. Food and Drug Administration. | Revised: April 2022 |
| Read this Medication Guide before you start your YESCARTA treatment. The more you know about your treatment, the more active you can be in your care. Talk with your healthcare provider if you have questions about your health condition or treatment. Reading this Medication Guide does not take the place of talking with your healthcare provider about your treatment. | |
| What is the most important information I should know about YESCARTA?
YESCARTA may cause side effects that are life-threatening and can lead to death. Call or see your healthcare provider or get emergency help right away if you get any of the following:
|
|
| What is YESCARTA?
YESCARTA is a prescription medicine used to treat two types of non-Hodgkin lymphoma:
|
|
Before getting YESCARTA, tell your healthcare provider about all your medical problems, including if you have or have had:
|
|
How will I receive YESCARTA?
|
|
| What are the possible or reasonably likely side effects of YESCARTA?
The most common side effects of YESCARTA include:
|
|
| General information about the safe and effective use of YESCARTA
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. If you would like more information about YESCARTA, talk with your healthcare provider. You can ask your healthcare provider for information about YESCARTA that is written for health professionals. You can get additional information by contacting Kite at 1-844-454-KITE (5483) or at www.Yescarta.com. |
|
| What are the ingredients in YESCARTA?
Active ingredients: axicabtagene ciloleucel. Inactive ingredients: albumin (human); DMSO. YESCARTA is a trademark of Kite Pharma, Inc. All other trademarks referenced herein are the property of their respective owners. © 2022 Kite Pharma, Inc. All Rights Reserved. |
|
Package/Label Principal Display Panel
axicabtagene ciloleucel
YESCARTA
STOP
Confirm patient ID prior to infusion
VERIFY PATIENT ID
Lot: 123456789-0X
Kite Patient ID: 123456789
Expiration Date: 31-Dec-2900
First Name M.I.: FIRST NAME W
Last Name: LAST NAME
DOB.: 31-Dec-1900
Hospital Patient ID: 1234567890123456
DIN:
AS-00829 W0123 45 678900
| YESCARTA
axicabtagene ciloleucel suspension |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Kite Pharma, Inc. (963353359) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Kite Pharma, Inc. | 963353359 | MANUFACTURE(71287-119) , LABEL(71287-119) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Kite Pharma, Inc. | 116931311 | ANALYSIS(71287-119) | |









