Drug Detail:Yonsa (Abiraterone [ a-bir-a-te-rone ])
Drug Class: Miscellaneous antineoplastics
Highlights of Prescribing Information
YONSA® (abiraterone acetate) tablets, for oral use
Initial U.S. Approval: 2011
Indications and Usage for Yonsa
YONSA is a CYP17 inhibitor indicated in combination with methylprednisolone for the treatment of patients with metastatic castration-resistant prostate cancer (CRPC). (1)
Yonsa Dosage and Administration
To avoid medication errors and overdose, be aware that YONSA tablets may have different dosing and food effects than other abiraterone acetate products.
Recommended dose: YONSA 500 mg (four 125 mg tablets) administered orally once daily in combination with methylprednisolone 4 mg administered orally twice daily. (2.1)
Patients receiving YONSA should also receive a gonadotropin-releasing hormone (GnRH) analog concurrently or should have had bilateral orchiectomy. (2.2)
YONSA tablets must be taken as a single dose once daily with or without food. The tablets should be swallowed whole with water. Do not crush or chew tablets. (2.1)
Dose Modification:
- For patients with baseline moderate hepatic impairment (Child-Pugh Class B), reduce the YONSA starting dose to 125 mg once daily. (2.3)
- For patients who develop hepatotoxicity during treatment, hold YONSA until recovery. Retreatment may be initiated at a reduced dose. YONSA should be discontinued if patients develop severe hepatotoxicity. (2.3)
Dosage Forms and Strengths
Tablets: 125 mg (3)
Contraindications
None (4)
Warnings and Precautions
- Mineralocorticoid excess: Closely monitor patients with cardiovascular disease. Control hypertension and correct hypokalemia before treatment. Monitor blood pressure, serum potassium and symptoms of fluid retention at least monthly. (5.1)
- Adrenocortical insufficiency: Monitor for symptoms and signs of adrenocortical insufficiency. Increased dosage of corticosteroids may be indicated before, during and after stressful situations. (5.2)
- Hepatotoxicity: Can be severe and fatal. Monitor liver function and modify, interrupt, or discontinue YONSA dosing as recommended. (5.3)
- Increased fractures and mortality in combination with radium Ra 223 dichloride: Use of YONSA plus methylprednisolone in combination with radium Ra 223 dichloride is not recommended. (5.4)
- Embryo-Fetal Toxicity: YONSA can cause fetal harm. Advise males with female partners of reproductive potential to use effective contraception. (5.5, 8.1, 8.3)
- Hypoglycemia: Severe hypoglycemia has been reported in patients with pre-existing diabetes who are taking medications containing thiazolidinediones (including pioglitazone) or repaglinide. Monitor blood glucose in patients with diabetes and assess if antidiabetic agent dose modifications are required. (5.6)
Adverse Reactions/Side Effects
The most common adverse reactions (≥ 10%) are fatigue, joint swelling or discomfort, edema, hot flush, diarrhea, vomiting, cough, hypertension, dyspnea, urinary tract infection and contusion. (6.1)
The most common laboratory abnormalities (> 20%) are anemia, elevated alkaline phosphatase, hypertriglyceridemia, lymphopenia, hypercholesterolemia, hyperglycemia, elevated AST, hypophosphatemia, elevated ALT and hypokalemia. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Sun Pharmaceutical Industries, Inc. at 1-800-818-4555 or FDA at 1-800-FDA-1088 or www.FDA.gov/medwatch
Drug Interactions
- CYP3A4 Inducers: Avoid concomitant strong CYP3A4 inducers during YONSA treatment. If a strong CYP3A4 inducer must be co-administered, increase the YONSA dosing frequency (2.4, 7.1)
- CYP2D6 Substrates: Avoid co-administration of YONSA with CYP2D6 substrates that have a narrow therapeutic index. If an alternative treatment cannot be used, exercise caution and consider a dose reduction of the concomitant CYP2D6 substrate (7.2)
Use In Specific Populations
- Do not use YONSA in patients with baseline severe hepatic impairment (Child-Pugh Class C). (8.6)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 7/2022
Full Prescribing Information
1. Indications and Usage for Yonsa
YONSA is indicated in combination with methylprednisolone for the treatment of patients with metastatic castration-resistant prostate cancer.
2. Yonsa Dosage and Administration
2.1 Recommended Dosage
The recommended dose of YONSA is 500 mg (four 125 mg tablets) administered orally once daily in combination with methylprednisolone 4 mg administered orally twice daily.
2.2 Important Administration Instructions
To avoid medication errors and overdose, be aware that YONSA (abiraterone acetate) tablets may have different dosing and food effects than other abiraterone acetate products. Patients receiving YONSA should also receive a gonadotropin-releasing hormone (GnRH) analog concurrently or should have had bilateral orchiectomy.
YONSA tablets must be taken as a single dose once daily with or without food [see Clinical Pharmacology (12.3)]. The tablets should be swallowed whole with water. Do not crush or chew tablets.
2.3 Dose Modification Guidelines in Hepatic Impairment and Hepatotoxicity
Hepatic Impairment
In patients with baseline moderate hepatic impairment (Child-Pugh Class B), reduce the recommended dose of YONSA to 125 mg once daily. In patients with moderate hepatic impairment monitor ALT, AST, and bilirubin prior to the start of treatment, every week for the first month, every two weeks for the following two months of treatment and monthly thereafter. If elevations in ALT and/or AST greater than 5X upper limit of normal (ULN) or total bilirubin greater than 3X ULN occur in patients with baseline moderate hepatic impairment, discontinue YONSA and do not re-treat patients with abiraterone acetate [see Use in Specific Populations (8.6) and Clinical Pharmacology (12.3)].
Do not use YONSA in patients with baseline severe hepatic impairment (Child-Pugh Class C).
Hepatotoxicity
For patients who develop hepatotoxicity during treatment with YONSA (ALT and/or AST greater than 5X ULN or total bilirubin greater than 3X ULN), interrupt treatment with YONSA [see Warnings and Precautions (5.3)]. Treatment may be restarted at a reduced dose of 375 mg once daily following return of liver function tests to the patient’s baseline or to AST and ALT less than or equal to 2.5X ULN and total bilirubin less than or equal to 1.5X ULN. For patients who resume treatment, monitor serum transaminases and bilirubin at a minimum of every two weeks for three months and monthly thereafter.
If hepatotoxicity recurs at the dose of 375 mg once daily, re-treatment may be restarted at a reduced dose of 250 mg once daily following return of liver function tests to the patient’s baseline or to AST and ALT less than or equal to 2.5X ULN and total bilirubin less than or equal to 1.5X ULN.
If hepatotoxicity recurs at the reduced dose of 250 mg once daily, discontinue treatment with YONSA.
Permanently discontinue YONSA for patients who develop a concurrent elevation of ALT greater than 3 x ULN and total bilirubin greater than 2 x ULN in the absence of biliary obstruction or other causes responsible for the concurrent elevation [see Warnings and Precautions (5.3)].
2.4 Dose Modification Guidelines for Strong CYP3A4 Inducers
Avoid concomitant strong CYP3A4 inducers (e.g., phenytoin, carbamazepine, rifampin, rifabutin, rifapentine, phenobarbital) during YONSA treatment.
If a strong CYP3A4 inducer must be co-administered, increase the YONSA dosing frequency to twice a day only during the co-administration period (e.g., from 500 mg once daily to 500 mg twice a day). Reduce the dose back to the previous dose and frequency, if the concomitant strong CYP3A4 inducer is discontinued [see Drug Interactions (7.1) and Clinical Pharmacology (12.3)].
3. Dosage Forms and Strengths
YONSA (abiraterone acetate) tablets, 125 mg, are white to off-white, oval-shaped tablets debossed with “125 FP” on one side.
5. Warnings and Precautions
5.1 Hypokalemia, Fluid Retention, and Cardiovascular Adverse Reactions due to Mineralocorticoid Excess
YONSA may cause hypertension, hypokalemia, and fluid retention as a consequence of increased mineralocorticoid levels resulting from CYP17 inhibition [see Clinical Pharmacology (12.1)]. Monitor patients for hypertension, hypokalemia, and fluid retention at least once a month. Control hypertension and correct hypokalemia before and during treatment with YONSA.
In the two randomized clinical trials, grade 3 to 4 hypertension occurred in 2% of patients, grade 3 to 4 hypokalemia in 4% of patients, and grade 3 to 4 edema in 1% of patients treated with abiraterone acetate [see Adverse Reactions (6)].
Closely monitor patients whose underlying medical conditions might be compromised by increases in blood pressure, hypokalemia or fluid retention, such as those with heart failure, recent myocardial infarction, cardiovascular disease, or ventricular arrhythmia. In postmarketing experience, QT prolongation and Torsades de Pointes have been observed in patients who develop hypokalemia while taking abiraterone acetate.
The safety of YONSA in patients with left ventricular ejection fraction < 50% or New York Heart Association (NYHA) Class III or IV heart failure (in Study 1) or NYHA Class II to IV heart failure (in Study 2) has not been established because these patients were excluded from these randomized clinical trials [see Clinical Studies (14)].
5.2 Adrenocortical Insufficiency
Adrenal insufficiency occurred in the two randomized clinical studies in 0.5% of patients taking abiraterone acetate and in 0.2% of patients taking placebo. Adrenocortical insufficiency was reported in patients receiving abiraterone acetate in combination with a corticosteroid, following interruption of daily steroids and/or with concurrent infection or stress.
Monitor patients for symptoms and signs of adrenocortical insufficiency, particularly if patients are withdrawn from corticosteroids, have corticosteroid dose reductions, or experience unusual stress. Symptoms and signs of adrenocortical insufficiency may be masked by adverse reactions associated with mineralocorticoid excess seen in patients treated with YONSA. If clinically indicated, perform appropriate tests to confirm the diagnosis of adrenocortical insufficiency. Increased dosage of corticosteroids may be indicated before, during and after stressful situations [see Warnings and Precautions (5.1)].
5.3 Hepatotoxicity
In postmarketing experience, there have been abiraterone acetate-associated severe hepatic toxicity, including fulminant hepatitis, acute liver failure and deaths [see Adverse Reactions (6.2)].
In the two randomized clinical trials, grade 3 or 4 ALT or AST increases (at least 5X ULN) were reported in 4% of patients who received abiraterone acetate, typically during the first 3 months after starting treatment. Patients whose baseline ALT or AST were elevated were more likely to experience liver test elevation than those beginning with normal values. Treatment discontinuation due to ALT and AST increases occurred in 1% of patients taking abiraterone acetate. In these clinical trials, no deaths clearly related to abiraterone acetate were reported due to hepatotoxicity events.
Measure serum transaminases (ALT and AST) and bilirubin levels prior to starting treatment with YONSA, every two weeks for the first three months of treatment and monthly thereafter. In patients with baseline moderate hepatic impairment receiving a reduced YONSA dose of 125 mg, measure ALT, AST, and bilirubin prior to the start of treatment, every week for the first month, every two weeks for the following two months of treatment and monthly thereafter. Promptly measure serum total bilirubin, AST, and ALT if clinical symptoms or signs suggestive of hepatotoxicity develop. Elevations of AST, ALT, or bilirubin from the patient's baseline should prompt more frequent monitoring. If at any time AST or ALT rise above five times the ULN, or the bilirubin rises above three times the ULN, interrupt YONSA treatment and closely monitor liver function.
Re-treatment with YONSA at a reduced dose level may take place only after return of liver function tests to the patient’s baseline or to AST and ALT less than or equal to 2.5X ULN and total bilirubin less than or equal to 1.5X ULN [see Dosage and Administration (2.3)].
Permanently discontinue treatment with YONSA for patients who develop a concurrent elevation of ALT greater than 3 x ULN and total bilirubin greater than 2 x ULN in the absence of biliary obstruction or other causes responsible for the concurrent elevation [see Dosage and Administration (2.3)].
The safety of YONSA re-treatment of patients who develop AST or ALT greater than or equal to 20X ULN and/or bilirubin greater than or equal to 10X ULN is unknown.
5.4 Increased Fractures and Mortality in Combination with Radium Ra 223 Dichloride
YONSA plus methylprednisolone is not recommended for use in combination with radium Ra 223 dichloride outside of clinical trials.
The clinical efficacy and safety of concurrent initiation of abiraterone acetate plus a corticosteroid and radium Ra 223 dichloride was assessed in a randomized, placebo-controlled multicenter study in 806 patients with asymptomatic or mildly symptomatic castration-resistant prostate cancer with bone metastases. The study was unblinded early based on an Independent Data Monitoring Committee recommendation.
At the primary analysis, increased incidences of fractures (28.6% vs 11.4%) and deaths (38.5% vs 35.5%) have been observed in patients who received abiraterone acetate plus a corticosteroid in combination with radium Ra 223 dichloride compared to patients who received placebo in combination with abiraterone acetate plus a corticosteroid.
5.5 Embryo-Fetal Toxicity
The safety and efficacy of YONSA have not been established in females. Based on animal reproductive studies and mechanism of action, YONSA can cause fetal harm and loss of pregnancy when administered to a pregnant female. In animal reproduction studies, oral administration of abiraterone acetate to pregnant rats during organogenesis caused adverse developmental effects at maternal exposures approximately ≥ 0.03 times the human exposure (AUC) at the recommended dose. Advise males with female partners of reproductive potential to use effective contraception during treatment with YONSA and for 3 weeks after the last dose of YONSA [see Use in Specific Populations (8.1, 8.3)]. YONSA should not be handled by females who are or may become pregnant [see How Supplied/Storage and Handling (16)].
5.6 Hypoglycemia
Severe hypoglycemia has been reported when abiraterone acetate was administered to patients with pre-existing diabetes receiving medications containing thiazolidinediones (including pioglitazone) or repaglinide [see Drug Interactions (7.2)]. Monitor blood glucose in patients with diabetes during and after discontinuation of treatment with YONSA. Assess if antidiabetic drug dosage needs to be adjusted to minimize the risk of hypoglycemia.
6. Adverse Reactions/Side Effects
The following are discussed in more detail in other sections of the labeling:
- Hypokalemia, Fluid Retention, and Cardiovascular Adverse Reactions due to Mineralocorticoid Excess [see Warnings and Precautions (5.1)].
- Adrenocortical Insufficiency [see Warnings and Precautions (5.2)].
- Hepatotoxicity [see Warnings and Precautions (5.3)].
- Increased Fractures and Mortality in Combination with Radium Ra 223 Dichloride [see Warnings and Precautions (5.4)].
6.1 Clinical Trial Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
Two randomized placebo-controlled, multicenter clinical trials (Study 1 and Study 2) enrolled patients who had metastatic castration-resistant prostate cancer who were using a gonadotropin-releasing hormone (GnRH) agonist or were previously treated with orchiectomy. In both Study 1 and Study 2 abiraterone acetate was administered at a dose equivalent to 500 mg of YONSA daily in combination with a different corticosteroid twice daily in the active treatment arms. Placebo plus corticosteroid was given to control patients.
The most common adverse drug reactions (>10%) reported in the two randomized clinical trials that occurred more commonly (>2%) in the abiraterone acetate arm were fatigue, joint swelling or discomfort, edema, hot flush, diarrhea, vomiting, cough, hypertension, dyspnea, urinary tract infection and contusion.
The most common laboratory abnormalities (>20%) reported in the two randomized clinical trials that occurred more commonly (≥2%) in the abiraterone acetate arm were anemia, elevated alkaline phosphatase, hypertriglyceridemia, lymphopenia, hypercholesterolemia, hyperglycemia, elevated AST, hypophosphatemia, elevated ALT and hypokalemia.
Study 1: Metastatic CRPC Following Chemotherapy
Study 1 enrolled 1195 patients with metastatic CRPC who had received prior docetaxel chemotherapy. Patients were not eligible if AST and/or ALT ≥ 2.5X ULN in the absence of liver metastases. Patients with liver metastases were excluded if AST and/or ALT > 5X ULN.
Table 1 shows adverse reactions on the abiraterone acetate arm in Study 1 that occurred with a ≥2% absolute increase in frequency compared to placebo or were events of special interest. The median duration of treatment with abiraterone acetate with a corticosteroid was 8 months.
Table 1: Adverse Reactions due to Abiraterone Acetate in Study 1
| Abiraterone Acetate with Corticosteroid(N=791) | Placebo with Corticosteroid (N=394) | |||
|---|---|---|---|---|
|
System Organ Class Adverse Reaction |
All Grades1 % |
Grade 3-4 % |
All Grades % |
Grade 3-4 % |
|
Musculoskeletal and connective tissue disorders |
||||
|
Joint swelling/ discomfort2 |
30 |
4.2 |
23 |
4.1 |
|
Muscle discomfort3 |
26 |
3.0 |
23 |
2.3 |
|
General Disorders |
||||
|
Edema4 |
27 |
1.9 |
18 |
0.8 |
|
Vascular Disorders |
||||
|
Hot Flush |
19 |
0.3 |
17 |
0.3 |
|
Hypertension |
8.5 |
1.3 |
6.9 |
0.3 |
|
Gastrointestinal Disorders |
||||
|
Diarrhea |
18 |
0.6 |
14 |
1.3 |
|
Dyspepsia |
6.1 |
0 |
3.3 |
0 |
|
Infections and infestations |
||||
|
Urinary tract infection |
12 |
2.1 |
7.1 |
0.5 |
|
Upper respiratory tract infection |
5.4 |
0 |
2.5 |
0 |
|
Respiratory, thoracic and mediastinal disorders |
||||
|
Cough |
11 |
0 |
7.6 |
0 |
|
Renal and urinary disorders |
||||
|
Urinary frequency |
7.2 |
0.3 |
5.1 |
0.3 |
|
Nocturia |
6.2 |
0 |
4.1 |
0 |
|
Injury, poisoning and procedural complications |
||||
|
Fractures5 |
5.9 |
1.4 |
2.3 |
0 |
|
Cardiac disorders |
||||
|
Arrhythmia6 |
7.2 |
1.1 |
4.6 |
1.0 |
|
Chest pain or chest discomfort7 |
3.8 |
0.5 |
2.8 |
0 |
|
Cardiac failure8 |
2.3 |
1.9 |
1.0 |
0.3 |
|
1 Adverse events graded according to CTCAE version 3.0 |
||||
|
2 Includes terms Arthritis, Arthralgia, Joint swelling, and Joint stiffness |
||||
|
3 Includes terms Muscle spasms, Musculoskeletal pain, Myalgia, Musculoskeletal discomfort, and Musculoskeletal stiffness |
||||
|
4 Includes terms Edema, Edema peripheral, Pitting edema, and Generalized edema |
||||
|
5 Includes all fractures with the exception of pathological fracture |
||||
|
6 Includes terms Arrhythmia, Tachycardia, Atrial fibrillation, Supraventricular tachycardia, Atrial tachycardia, Ventricular tachycardia, Atrial flutter, Bradycardia, Atrioventricular block complete, Conduction disorder, and Bradyarrhythmia |
||||
|
7 Includes terms Angina pectoris, Chest pain, and Angina unstable. Myocardial infarction or ischemia occurred more commonly in the placebo arm than in the abiraterone acetate arm (1.3% vs. 1.1% respectively). |
||||
|
8 Includes terms Cardiac failure, Cardiac failure congestive, Left ventricular dysfunction, Cardiogenic shock, Cardiomegaly, Cardiomyopathy, and Ejection fraction decreased |
||||
Table 2 shows laboratory abnormalities of interest from Study 1. Grade 3-4 low serum phosphorus (7%) and low potassium (5%) occurred at a greater than or equal to 5% rate in the abiraterone acetate with a corticosteroid arm.
Table 2: Laboratory Abnormalities of Interest in Study 1
| Abiraterone Acetate with Corticosteroid(N=791) | Placebo with Corticosteroid(N=394) | |||
|---|---|---|---|---|
|
Laboratory Abnormality |
All Grades % |
Grade 3-4 % |
All Grades % |
Grade 3-4 % |
|
Hypertriglyceridemia |
63 |
0.4 |
53 |
0 |
|
High AST |
31 |
2.1 |
36 |
1.5 |
|
Hypokalemia |
28 |
5.3 |
20 |
1.0 |
|
Hypophosphatemia |
24 |
7.2 |
16 |
5.8 |
|
High ALT |
11 |
1.4 |
10 |
0.8 |
|
High Total Bilirubin |
6.6 |
0.1 |
4.6 |
0 |
Study 2: Metastatic CRPC Prior to Chemotherapy
Study 2 enrolled 1088 patients with metastatic CRPC who had not received prior cytotoxic chemotherapy. Patients were ineligible if AST and/or ALT ≥ 2.5X ULN and patients were excluded if they had liver metastases.
Table 3 shows adverse reactions on the abiraterone acetate arm in Study 2 that occurred in ≥ 5% of patients with a ≥ 2% absolute increase in frequency compared to placebo. The median duration of treatment with abiraterone acetate with a corticosteroid was 13.8 months.
Table 3: Adverse Reactions in ≥5% of Patients on the Abiraterone Acetate Arm in Study 2
| Abiraterone Acetate with Corticosteroid(N=542) | Placebo with Corticosteroid(N=540) | |||
|---|---|---|---|---|
|
System Organ Class Adverse Reaction |
All Grades1 % |
Grade 3-4 % |
All Grades % |
Grade 3-4 % |
|
General Disorders |
||||
|
Fatigue |
39 |
2.2 |
34 |
1.7 |
|
Edema2 |
25 |
0.4 |
21 |
1.1 |
|
Pyrexia |
8.7 |
0.6 |
5.9 |
0.2 |
|
Musculoskeletal and connective tissue disorders |
||||
|
Joint swelling/ discomfort3 |
30 |
2.0 |
25 |
2.0 |
|
Groin Pain |
6.6 |
0.4 |
4.1 |
0.7 |
|
Gastrointestinal Disorders |
||||
|
Constipation |
23 |
0.4 |
19 |
0.6 |
|
Diarrhea |
22 |
0.9 |
18 |
0.9 |
|
Dyspepsia |
11 |
0.0 |
5.0 |
0.2 |
|
Vascular Disorders |
||||
|
Hot Flush |
22 |
0.2 |
18 |
0.0 |
|
Hypertension |
22 |
3.9 |
13 |
3.0 |
|
Respiratory, thoracic and mediastinal disorders |
||||
|
Cough |
17 |
0.0 |
14 |
0.2 |
|
Dyspnea |
12 |
2.4 |
9.6 |
0.9 |
|
Psychiatric Disorders |
||||
|
Insomnia |
14 |
0.2 |
11 |
0.0 |
|
Injury, poisoning and procedural complications |
||||
|
Contusion |
13 |
0.0 |
9.1 |
0.0 |
|
Falls |
5.9 |
0.0 |
3.3 |
0.0 |
|
Infections and infestations |
||||
|
Upper respiratory tract infection |
13 |
0.0 |
8.0 |
0.0 |
|
Nasopharyngitis |
11 |
0.0 |
8.1 |
0.0 |
|
Renal and urinary disorders |
||||
|
Hematuria |
10 |
1.3 |
5.6 |
0.6 |
|
Skin and subcutaneous tissue disorders |
||||
|
Rash |
8.1 |
0.0 |
3.7 |
0.0 |
|
1 Adverse events graded according to CTCAE version 3.0 |
||||
|
2 Includes terms Edema peripheral, Pitting edema, and Generalized edema |
||||
|
3 Includes terms Arthritis, Arthralgia, Joint swelling, and Joint stiffness |
||||
Table 4 shows laboratory abnormalities that occurred in greater than 15% of patients, and more frequently (>5%) in the abiraterone acetate arm compared to placebo in Study 2.
Table 4: Laboratory Abnormalities in > 15% of Patients in the Abiraterone Acetate Arm of Study 2
| Abiraterone Acetate with Corticosteroid(N=542) | Placebo with Corticosteroid(N=540) | |||
|---|---|---|---|---|
|
Laboratory Abnormality |
Grade 1-4 % |
Grade 3-4 % |
Grade 1-4 % |
Grade 3-4 % |
|
Hematology |
||||
|
Lymphopenia |
38 |
8.7 |
32 |
7.4 |
|
Chemistry |
||||
|
Hyperglycemia1 |
57 |
6.5 |
51 |
5.2 |
|
High ALT |
42 |
6.1 |
29 |
0.7 |
|
High AST |
37 |
3.1 |
29 |
1.1 |
|
Hypernatremia |
33 |
0.4 |
25 |
0.2 |
|
Hypokalemia |
17 |
2.8 |
10 |
1.7 |
|
1 Based on non-fasting blood draws |
||||
Cardiovascular Adverse Reactions
In the combined data for studies 1 and 2, cardiac failure occurred more commonly in patients treated with abiraterone acetate compared to patients on the placebo arm (2.1% versus 0.7%). Grade 3-4 cardiac failure occurred in 1.6% of patients taking abiraterone acetate and led to 5 treatment discontinuations and 2 deaths. Grade 3-4 cardiac failure occurred in 0.2% of patients taking placebo. There were no treatment discontinuations and one death due to cardiac failure in the placebo group.
In Study 1 and 2, the majority of arrhythmias were grade 1 or 2. There was one death associated with arrhythmia and one patient with sudden death in the abiraterone acetate arms and no deaths in the placebo arms. There were 7 (0.5 %) deaths due to cardiorespiratory arrest in the abiraterone acetate arms and 3 (0.3 %) deaths in the placebo arms. Myocardial ischemia or myocardial infarction led to death in 3 patients in the placebo arms and 2 deaths in the abiraterone acetate arms.
6.2 Postmarketing Experience
The following additional adverse reactions have been identified during post approval use of abiraterone acetate with a different corticosteroid. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Respiratory, Thoracic and Mediastinal Disorders: non-infectious pneumonitis.
Musculoskeletal and Connective Tissue Disorders: myopathy, including rhabdomyolysis.
Hepatobiliary Disorders: fulminant hepatitis, including acute hepatic failure and death.
Cardiac Disorders: QT prolongation and Torsades de Pointes (observed in patients who developed hypokalemia or had underlying cardiovascular conditions).
Immune System Disorders – Hypersensitivity: anaphylactic reactions (severe allergic reactions that include, but are not limited to difficulty swallowing or breathing, swollen face, lips, tongue or throat, or an itchy rash (urticaria)).
7. Drug Interactions
7.1 Effect of Other Drugs on YONSA
Strong CYP3A4 Inducers
The co-administration of rifampin, a strong CYP3A4 inducer, decreased exposure of abiraterone by 55%. Avoid concomitant strong CYP3A4 inducers during YONSA treatment. If a strong CYP3A4 inducer must be co-administered with YONSA, increase the YONSA dosing frequency [see Dosage and Administration (2.4) and Clinical Pharmacology (12.3)].
7.2 Effect of YONSA on Other Drugs
CYP2D6 Substrates
Abiraterone is an inhibitor of the hepatic drug-metabolizing enzymes CYP2D6. The co-administration of YONSA with CYP2D6 substrates increases the concentration of the CYP2D6 substrate, which may increase the frequency and/or severity of adverse reactions of these substrates. Avoid co-administration of abiraterone acetate with substrates of CYP2D6 with a narrow therapeutic index. If alternative treatments cannot be used, consider a dose reduction of the concomitant CYP2D6 substrate drug in accordance with its Prescribing Information [see Clinical Pharmacology (12.3)].
CYP2C8 Substrates
Abiraterone is an inhibitor of the hepatic drug-metabolizing enzymes CYP2D6 and CYP2C8. The co-administration of YONSA with CYP2C8 substrates increases the concentration of the CYP2C8 substrate, which may increase the frequency and/or severity of adverse reactions of these substrates. Therefore, patients should be monitored closely for signs of toxicity related to a CYP2C8 substrate with a narrow therapeutic index if used concomitantly with abiraterone acetate [see Clinical Pharmacology (12.3)].
8. Use In Specific Populations
8.1 Pregnancy
Risk Summary
The safety and efficacy of YONSA have not been established in females. Based on findings from animal studies and the mechanism of action, YONSA can cause fetal harm and potential loss of pregnancy.
There are no human data on the use of YONSA in pregnant women. In animal reproduction studies, oral administration of abiraterone acetate to pregnant rats during organogenesis, caused adverse developmental effects at maternal exposures of approximately ≥ 0.03 times the human exposure (AUC) at the recommended dose (see Data).
Data
Animal Data
In an embryo-fetal developmental toxicity study in rats, abiraterone acetate caused developmental toxicity when administered at oral doses of 10, 30 or 100 mg/kg/day throughout the period of organogenesis (gestational days 6-17). Findings included embryo-fetal lethality (increased post implantation loss and resorptions and decreased number of live fetuses), fetal developmental delay (skeletal effects) and urogenital effects (bilateral ureter dilation) at doses >10 mg/kg/day, decreased fetal ano-genital distance at >30 mg/kg/day, and decreased fetal body weight at 100 mg/kg/day. Doses >10 mg/kg/day caused maternal toxicity. The doses tested in rats resulted in systemic exposures (AUC) approximately 0.03, 0.1 and 0.3 times, respectively, the AUC in patients.
8.2 Lactation
Risk Summary
The safety and efficacy of YONSA have not been established in females. There is no information available on the presence of abiraterone acetate in human milk, or on the effects on the breastfed child or milk production.
8.3 Females and Males of Reproductive Potential
Contraception
Males
Based on findings in animal reproduction studies and its mechanism of action, advise male patients with female partners of reproductive potential to use effective contraception during treatment and for 3 weeks after the last dose of YONSA [see Use in Specific Populations (8.1)].
Infertility
Based on animal studies, abiraterone acetate may impair reproductive function and fertility in males of reproductive potential [see Nonclinical Toxicology (13.1)].
8.5 Geriatric Use
Of the total number of patients receiving abiraterone acetate in the two randomized trials, 73% of patients were 65 years and over and 30% were 75 years and over. No overall differences in safety or effectiveness were observed between these elderly patients and younger patients. Other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
8.6 Hepatic Impairment
The pharmacokinetics of abiraterone were examined in subjects with baseline mild (N=8) or moderate (N=8) hepatic impairment (Child-Pugh Class A and B, respectively) and in 8 healthy control subjects with normal hepatic function. The systemic exposure (AUC) of abiraterone after a single oral dose equivalent to 500 mg of YONSA increased by approximately 1.1-fold and 3.6-fold in subjects with mild and moderate baseline hepatic impairment, respectively compared to subjects with normal hepatic function.
In another trial, the pharmacokinetics of abiraterone were examined in subjects with baseline severe (N=8) hepatic impairment (Child-Pugh Class C) and in 8 healthy control subjects with normal hepatic function. The systemic exposure (AUC) of abiraterone increased by approximately 7-fold and the fraction of free drug increased 2-fold in subjects with severe baseline hepatic impairment compared to subjects with normal hepatic function.
No dosage modification is recommended for patients with baseline mild hepatic impairment (Child-Pugh Class A). In patients with baseline moderate hepatic impairment (Child-Pugh Class B), reduce the recommended dose of YONSA to 125 mg once daily. Do not use YONSA in patients with baseline severe hepatic impairment (Child-Pugh Class C). If elevations in ALT or AST >5X ULN or total bilirubin >3X ULN occur in patients with baseline moderate hepatic impairment, discontinue abiraterone acetate treatment [see Dosage and Administration (2.3) and Clinical Pharmacology (12.3)].
For patients who develop hepatotoxicity during treatment, interruption of treatment and dosage adjustment may be required [see Dosage and Administration (2.3), Warnings and Precautions (5.3), and Clinical Pharmacology (12.3)].
10. Overdosage
Human experience of overdose with YONSA is limited.
There is no specific antidote. In the event of an overdose, stop YONSA, undertake general supportive measures, including monitoring for arrhythmias and cardiac failure and assess liver function.
11. Yonsa Description
Abiraterone acetate, the active ingredient of YONSA tablet is the acetyl ester of abiraterone. Abiraterone is an inhibitor of CYP17 (17α-hydroxylase/C17,20-lyase). Each YONSA Tablet contains 125 mg of abiraterone acetate. Abiraterone acetate is designated chemically as (3β)-17-(3-pyridinyl) androsta-5,16-dien-3-yl acetate and its structure is:
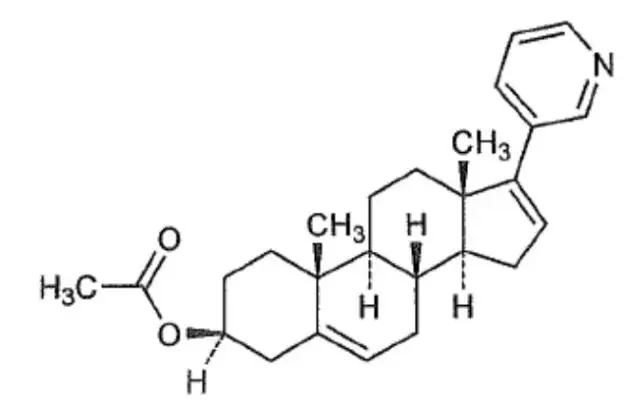
Abiraterone acetate is micronized (smaller particle size) white to off-white, non-hygroscopic, crystalline powder. Its molecular formula is C26H33NO2 and it has a molecular weight of 391.55. Abiraterone acetate is a lipophilic compound with an octanol-water partition coefficient of 5.12 (Log P) and is practically insoluble in water. The pKa of the aromatic nitrogen is 5.19.
Inactive ingredients in the tablets are lactose monohydrate, microcrystalline cellulose, croscarmellose sodium, sodium lauryl sulfate, sodium stearyl fumarate, butylated hydroxyanisole, butylated hydroxytoluene.
12. Yonsa - Clinical Pharmacology
12.1 Mechanism of Action
Abiraterone acetate (YONSA) is converted in vivo to abiraterone, an androgen biosynthesis inhibitor, that inhibits 17 α-hydroxylase/C17,20-lyase (CYP17). This enzyme is expressed in testicular, adrenal, and prostatic tumor tissues and is required for androgen biosynthesis.
CYP17 catalyzes two sequential reactions: 1) the conversion of pregnenolone and progesterone to their 17α-hydroxy derivatives by 17α-hydroxylase activity and 2) the subsequent formation of dehydroepiandrosterone (DHEA) and androstenedione, respectively, by C17,20-lyase activity. DHEA and androstenedione are androgens and are precursors of testosterone. Inhibition of CYP17 by abiraterone can also result in increased mineralocorticoid production by the adrenals [see Warnings and Precautions (5.1)].
Androgen sensitive prostatic carcinoma responds to treatment that decreases androgen levels. Androgen deprivation therapies, such as treatment with GnRH agonists or orchiectomy, decrease androgen production in the testes but do not affect androgen production by the adrenals or in the tumor.
Abiraterone acetate decreased serum testosterone and other androgens in patients in the placebo-controlled clinical trial. It is not necessary to monitor the effect of YONSA on serum testosterone levels.
Changes in serum prostate specific antigen (PSA) levels may be observed but have not been shown to correlate with clinical benefit in individual patients.
12.2 Pharmacodynamics
In a clinical study in patients with metastatic CRPC who were treated with YONSA 500 mg once daily and methylprednisolone 4 mg twice daily for 84 days, the average serum testosterone level ± standard deviation (SD) on days 9 and 10 of treatment was 0.33 ± 0.09 ng/dL.
Cardiac Electrophysiology
In a multi-center, open-label, single-arm trial, 33 patients with metastatic CRPC received a dose of 1,000 mg once daily orally of another abiraterone acetate product at least 1 hour before or 2 hours after a meal in combination with a different corticosteroid orally twice daily. Assessments up to Cycle 2 Day 2 showed no large changes in the QTc interval (i.e., >20 ms) from baseline. However, small increases in the QTc interval (i.e., <10 ms) due to abiraterone acetate cannot be excluded due to study design limitations.
12.3 Pharmacokinetics
Following administration of abiraterone acetate, the pharmacokinetics of abiraterone have been studied in healthy subjects and in patients with metastatic CRPC. In vivo, abiraterone acetate is converted to abiraterone. In clinical studies of other abiraterone acetate formulations, abiraterone acetate plasma concentrations were below detectable levels (< 0.2 ng/mL) in > 99% of the analyzed samples.
Geometric mean ±SD abiraterone Cmax was 73 ± 44 ng/mL and AUC0-∞ was 373 ± 249 ng·hr/mL following a single dose of YONSA 500 mg in overnight fasted healthy subjects. Dose proportionality was observed in single doses of YONSA in a range of 125 mg to 625 mg.
Absorption
Following oral administration of YONSA to patients with metastatic CRPC, the median time to reach maximum plasma abiraterone concentrations is 2 hours.
Effect of Food
Abiraterone Cmax was approximately 6.5-fold higher and AUC0-∞ was 4.4-fold higher when a single dose of YONSA 500 mg was administered with a high-fat meal (56-60% fat, 900-1,000 calories) compared to overnight fasting in healthy subjects.
YONSA can be taken with or without food.
Distribution
Abiraterone is highly bound (>99%) to the human plasma proteins, albumin and alpha-1 acid glycoprotein. The apparent steady-state volume of distribution (mean ± SD) is 19,669 ± 13,358 L.
Elimination
In patients with metastatic CRPC, the mean terminal half-life of abiraterone in plasma (mean ± SD) is 12 ± 5 hours.
Metabolism
Following oral administration of 14C-abiraterone acetate as capsules, abiraterone acetate is hydrolyzed to abiraterone (active metabolite). The conversion is likely through esterase activity (the esterases have not been identified) and is not CYP mediated. The two main circulating metabolites of abiraterone in human plasma are abiraterone sulphate (inactive) and N-oxide abiraterone sulphate (inactive), which account for about 43% of exposure each. CYP3A4 and SULT2A1 are the enzymes involved in the formation of N-oxide abiraterone sulphate and SULT2A1 is involved in the formation of abiraterone sulphate.
Excretion
Following oral administration of 14C-abiraterone acetate, approximately 88% of the radioactive dose is recovered in feces and approximately 5% in urine. The major compounds present in feces are unchanged abiraterone acetate and abiraterone (approximately 55% and 22% of the administered dose, respectively).
Specific Populations
Patients with Hepatic Impairment
The pharmacokinetics of abiraterone was examined in subjects with baseline mild (N=8) or moderate (N=8) hepatic impairment (Child-Pugh Class A and B, respectively) and in 8 healthy control subjects with normal hepatic function. Systemic exposure to abiraterone after a single oral 1,000 mg dose of another abiraterone acetate product given under fasting conditions increased approximately 1.1-fold and 3.6-fold in subjects with mild and moderate baseline hepatic impairment, respectively. The mean half-life of abiraterone is prolonged to approximately 18 hours in subjects with mild hepatic impairment and to approximately 19 hours in subjects with moderate hepatic impairment.
In another trial, the pharmacokinetics of abiraterone were examined in subjects with baseline severe (N=8) hepatic impairment (Child-Pugh Class C) and in 8 healthy control subjects with normal hepatic function. The systemic exposure (AUC) of abiraterone increased by approximately 7-fold in subjects with severe baseline hepatic impairment compared to subjects with normal hepatic function. In addition, the mean protein binding was found to be lower in the severe hepatic impairment group compared to the normal hepatic function group, which resulted in a two-fold increase in the fraction of free drug in patients with severe hepatic impairment.
Patients with Renal Impairment
The pharmacokinetics of abiraterone were examined in patients with end-stage renal disease (ESRD) on a stable hemodialysis schedule (N=8) and in matched control subjects with normal renal function (N=8). In the ESRD cohort of the trial, a single 1,000 mg dose of another abiraterone acetate product was given under fasting conditions 1 hour after dialysis, and samples for pharmacokinetic analysis were collected up to 96 hours post dose. Systemic exposure to abiraterone after a single oral 1,000 mg dose of another abiraterone acetate product did not increase in subjects with end-stage renal disease on dialysis, compared to subjects with normal renal function.
Drug Interactions Studies
Clinical Studies
Effect of Other Drugs on Abiraterone
Strong CYP3A4 inducers: In a clinical pharmacokinetic interaction study of healthy subjects, pretreated with a strong CYP3A4 inducer (rifampin, 600 mg daily for 6 days) followed by a single dose of 1,000 mg of another abiraterone acetate product that is dose equivalent to a single YONSA 500 mg dose, the mean plasma AUC∞ of abiraterone was decreased by 55%.
Strong CYP3A4 inhibitors: Co-administration of ketoconazole, a strong inhibitor of CYP3A4, had no clinically meaningful effect on the pharmacokinetics of abiraterone.
Effect of Abiraterone on Other Drugs
CYP2D6 substrates: The Cmax and AUC of dextromethorphan (CYP2D6 substrate) were increased 2.8- and 2.9-fold, respectively when dextromethorphan 30 mg was given with another abiraterone acetate product of 1,000 mg daily that is dose equivalent to YONSA 500 mg daily. The AUC for dextrorphan, the active metabolite of dextromethorphan, increased approximately 1.3 fold.
CYP1A2 substrates: When another abiraterone acetate product of 1,000 mg daily that is dose equivalent to YONSA 500 mg daily was given with a single dose of 100 mg theophylline (CYP1A2 substrate), no increase in systemic exposure of theophylline was observed.
CYP2C8 substrates: The AUC of pioglitazone (CYP2C8 substrate) was increased by 46% when pioglitazone was given to healthy subjects with a single dose of 1,000 mg of another abiraterone acetate product that is dose equivalent to a single YONSA 500 mg dose.
In Vitro Studies
Cytochrome P450 (CYP) Enzymes: Abiraterone is a substrate of CYP3A4 and has the potential to inhibit CYP1A2, CYP2D6, CYP2C8 and to a lesser extent CYP2C9, CYP2C19 and CYP3A4/5.
Transporter Systems: In vitro studies show that at clinically relevant concentrations, abiraterone acetate and abiraterone are not substrates of P-glycoprotein (P-gp) and that abiraterone acetate is an inhibitor of P-gp. In vitro, abiraterone and its major metabolites were shown to inhibit the hepatic uptake transporter OATP1B1. There are no clinical data available to confirm transporter based interaction.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, and Impairment of Fertility
A two-year carcinogenicity study was conducted in rats at abiraterone acetate oral doses of 5, 15, and 50 mg/kg/day for males and 15, 50, and 150 mg/kg/day for females. Abiraterone acetate increased the combined incidence of interstitial cell adenomas and carcinomas in the testes at all dose levels tested. This finding is considered to be related to the pharmacological activity of abiraterone. Rats are regarded as more sensitive than humans to developing interstitial cell tumors in the testes. Abiraterone acetate was not carcinogenic in female rats at exposure levels up to 0.8 times the human clinical exposure based on AUC. Abiraterone acetate was not carcinogenic in a 6-month study in the transgenic (Tg.rasH2) mouse.
Abiraterone acetate and abiraterone was not mutagenic in an in vitro microbial mutagenesis (Ames) assay or clastogenic in an in vitro cytogenetic assay using primary human lymphocytes or an in vivo rat micronucleus assay.
In repeat-dose toxicity studies in male rats (13- and 26-weeks) and monkeys (39-weeks), atrophy, aspermia/hypospermia, and hyperplasia in the reproductive system were observed at ≥ 50 mg/kg/day in rats and ≥ 250 mg/kg/day in monkeys and were consistent with the antiandrogenic pharmacological activity of abiraterone. These effects were observed in rats at systemic exposures similar to humans and in monkeys at exposures approximately 0.6 times the AUC in humans.
In a fertility study in male rats, reduced organ weights of the reproductive system, sperm counts, sperm motility, altered sperm morphology and decreased fertility were observed in animals dosed for 4 weeks at ≥ 30 mg/kg/day orally. Mating of untreated females with males that received 30 mg/kg/day oral abiraterone acetate resulted in a reduced number of corpora lutea, implantations and live embryos and an increased incidence of pre-implantation loss. Effects on male rats were reversible after 16 weeks from the last abiraterone acetate administration.
In a fertility study in female rats, animals dosed orally for 2 weeks until day 7 of pregnancy at > 30 mg/kg/day had an increased incidence of irregular or extended estrous cycles and pre-implantation loss (300 mg/kg/day). There were no differences in mating, fertility, and litter parameters in female rats that received abiraterone acetate. Effects on female rats were reversible after 4 weeks from the last abiraterone acetate administration.
The dose of 30 mg/kg/day in rats is approximately 0.6 times the recommended dose of 500 mg of YONSA/day based on body surface area.
In 13- and 26-week studies in rats and 13- and 39-week studies in monkeys, a reduction in circulating testosterone levels occurred with abiraterone acetate at approximately one half the human clinical exposure based on AUC. As a result, decreases in organ weights and toxicities were observed in the male and female reproductive system, adrenal glands, liver, pituitary (rats only), and male mammary glands. The changes in the reproductive organs are consistent with the antiandrogenic pharmacological activity of abiraterone acetate.
13.2 Animal Toxicology and/or Pharmacology
A dose-dependent increase in cataracts was observed in rats after daily oral abiraterone acetate administration for 26 weeks starting at ≥ 50 mg/kg/day (similar to the human clinical exposure based on AUC). In a 39-week monkey study with daily oral abiraterone acetate administration, no cataracts were observed at higher doses (2 times greater than the clinical exposure based on AUC).
14. Clinical Studies
The efficacy and safety of abiraterone acetate with prednisone in patients with metastatic castration-resistant prostate cancer (CRPC) that has progressed on androgen deprivation therapy was demonstrated in two randomized, placebo-controlled, international clinical studies. Patients with prior ketoconazole treatment for prostate cancer and a history of adrenal gland or pituitary disorders were excluded from these trials. Concurrent use of spironolactone was not allowed during the study period.
Study 1: Patients with metastatic CRPC who had received prior docetaxel chemotherapy
A total of 1195 patients were randomized 2:1 to receive either abiraterone acetate orally at a dose equivalent to 500 mg of YONSA once daily in combination with a different corticosteroid orally twice daily (N=797) or placebo once daily plus a different corticosteroid orally twice daily (N=398). Patients randomized to either arm were to continue treatment until disease progression (defined as a 25% increase in PSA over the patient’s baseline/nadir together with protocol-defined radiographic progression and symptomatic or clinical progression), initiation of new treatment, unacceptable toxicity or withdrawal.
The following patient demographics and baseline disease characteristics were balanced between the treatment arms. The median age was 69 years (range 39-95) and the racial distribution was 93% Caucasian, 3.6% Black, 1.7% Asian, and 1.6% Other. Eighty-nine percent of patients enrolled had an ECOG performance status score of 0-1 and 45% had a Brief Pain Inventory-Short Form score of ≥ 4 (patient’s reported worst pain over the previous 24 hours). Ninety percent of patients had metastases in bone and 30% had visceral involvement. Seventy percent of patients had radiographic evidence of disease progression and 30% had PSA-only progression. Seventy percent of patients had previously received one cytotoxic chemotherapy regimen and 30% received two regimens.
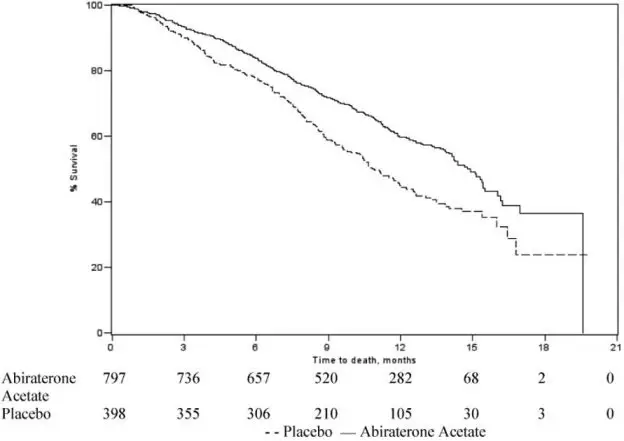
The protocol pre-specified interim analysis was conducted after 552 deaths and showed a statistically significant improvement in overall survival (OS) in patients treated with abiraterone acetate with a corticosteroid compared to patients in the placebo with a corticosteroid arm (Table 5 and Figure 1). An updated survival analysis was conducted when 775 deaths (97% of the planned number of deaths for final analysis) were observed. Results from this analysis were consistent with those from the interim analysis (Table 5).
Table 5: Overall Survival of Patients Treated with Either Abiraterone Acetate or Placebo in Combination with Corticosteroid in Study 1 (Intent-to-Treat Analysis)
| Abiraterone Acetate with Corticosteroid(N=797) | Placebo with Corticosteroid(N=398) | ||
|---|---|---|---|
|
Primary Survival Analysis |
|||
|
Deaths (%) |
333 (42%) |
219 (55%) |
|
|
Median survival (months) |
14.8 (14.1, 15.4) |
10.9 (10.2, 12.0) |
|
|
(95% CI) p-value1 |
<0.0001 | ||
|
Hazard ratio (95% CI)2 |
0.646 (0.543, 0.768) | ||
|
Updated Survival Analysis |
|||
|
Deaths (%) |
501 (63%) |
274 (69%) |
|
|
Median survival (months) |
15.8 (14.8, 17.0) |
11.2 (10.4, 13.1) |
|
|
(95% CI) | |||
|
Hazard ratio (95% CI)2 |
0.740 (0.638, 0.859) | ||
|
1 p-value is derived from a log-rank test stratified by ECOG performance status score (0-1 vs. 2), pain score (absent vs. present), number of prior chemotherapy regimens (1 vs. 2), and type of disease progression (PSA only vs. radiographic) |
|||
|
2 Hazard Ratio is derived from a stratified proportional hazards model. Hazard ratio <1 favors abiraterone acetate with prednisone. |
|||
Figure 1: Kaplan-Meier Overall Survival Curves in Study 1 (Intent-to-Treat Analysis)
Study 2: Patients with metastatic CRPC who had not received prior cytotoxic chemotherapy
In Study 2, 1088 patients were randomized 1:1 to receive either abiraterone acetate at a dose equivalent to 500 mg of YONSA once daily (N=546) or Placebo orally once daily (N=542). Both arms were also given a different corticosteroid twice daily. Patients continued treatment until radiographic or clinical (cytotoxic chemotherapy, radiation or surgical treatment for cancer, pain requiring chronic opioids, or ECOG performance status decline to 3 or more) disease progression, unacceptable toxicity or withdrawal. Patients with moderate or severe pain, opiate use for cancer pain, or visceral organ metastases were excluded.
Patient demographics were balanced between the treatment arms. The median age was 70 years. The racial distribution of patients treated with abiraterone acetate was 95% Caucasian, 2.8% Black, 0.7% Asian and 1.1% Other. The ECOG performance status was 0 for 76% of patients, and 1 for 24% of patients. Co-primary efficacy endpoints were overall survival and radiographic progression-free survival (rPFS). Baseline pain assessment was 0-1 (asymptomatic) in 66% of patients and 2-3 (mildly symptomatic) in 26% of patients as defined by the Brief Pain Inventory-Short Form (worst pain over the last 24 hours).
Radiographic progression-free survival was assessed with the use of sequential imaging studies and was defined by bone scan identification of 2 or more new bone lesions with confirmation (Prostate Cancer Working Group 2 criteria) and/or modified Response Evaluation Criteria In Solid Tumors (RECIST) criteria for progression of soft tissue lesions. Analysis of rPFS utilized centrally-reviewed radiographic assessment of progression.
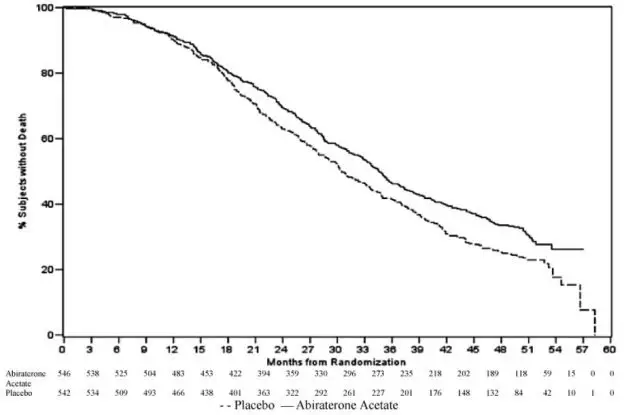
The planned final analysis for OS, conducted after 741 deaths (median follow up of 49 months) demonstrated a statistically significant OS improvement in patients treated with abiraterone acetate with a corticosteroid compared to those treated with placebo with a corticosteroid (Table 6 and Figure 2). Sixty-five percent of patients on the abiraterone acetate arm and 78% of patients on the placebo arm used subsequent therapies that may prolong OS in metastatic CRPC. Abiraterone acetate was used as a subsequent therapy in 13% of patients on the abiraterone acetate arm and 44% of patients on the placebo arm.
Table 6: Overall Survival of Patients Treated with Either Abiraterone Acetate or Placebo in Combination with Corticosteroid in Study 2 (Intent-to-Treat Analysis)
| Overall Survival | Abiraterone Acetate with Corticosteroid(N=546) | Placebo withCorticosteroid(N=542) |
|---|---|---|
|
Deaths |
354 (65%) |
387 (71%) |
|
Median survival (months) (95% CI) |
34.7 (3.7, 36.8) |
30.3 (28.7, 33.3) |
|
p-value1 |
0.0033 |
|
|
Hazard ratio2 (95% CI) |
0.81 (0.70, 0.93) |
|
|
1 p-value is derived from a log-rank test stratified by ECOG performance status score (0 vs. 1). 2 Hazard Ratio is derived from a stratified proportional hazards model. Hazard ratio <1 favors abiraterone acetate with prednisone. |
||
Figure 2: Kaplan Meier Overall Survival Curves in Study 2
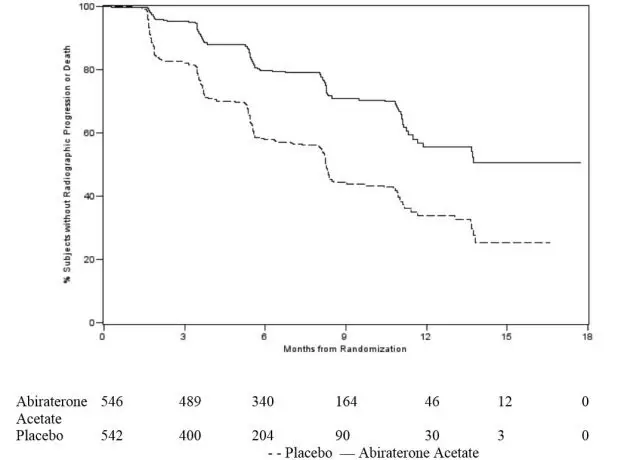
At the pre-specified rPFS analysis, 150 (28%) patients treated with abiraterone acetate with a corticosteroid and 251 (46%) patients treated with placebo with a corticosteroid had radiographic progression. A significant difference in rPFS between treatment groups was observed (Table 7 and Figure 3).
Table 7: Radiographic Progression-free Survival of Patients Treated with Either Abiraterone Acetate or Placebo in Combination with Corticosteroid in Study 2 (Intent-to-Treat Analysis)
| Radiographic Progression-free Survival | Abiraterone Acetate with Corticosteroid(N=546) | Placebo with Corticosteroid(N=542) |
|---|---|---|
|
Progression or death |
150 (28%) |
251 (46%) |
|
Median rPFS (months) (95% CI) |
NR (11.66, NR) |
8.28 (8.12, 8.54) |
|
p-value1 |
<0.0001 |
|
|
Hazard ratio2 (95% CI) |
0.425 (0.347, 0.522) |
|
|
NR= Not Reached |
||
|
1 p-value is derived from a log-rank test stratified by ECOG performance status score (0 vs. 1). |
||
|
2 Hazard Ratio is derived from a stratified proportional hazards model. Hazard ratio <1 favors abiraterone acetate with prednisone. |
||
Figure 3: Kaplan Meier Curves of Radiographic Progression-free Survival in Study 2 (Intent-to-Treat Analysis)
The primary efficacy analyses are supported by the following prospectively defined endpoints. The median time to initiation of cytotoxic chemotherapy was 25.2 months for patients in the abiraterone acetate arm and 16.8 months for patients in the placebo arm (HR=0.580; 95% CI: [0.487, 0.691], p<0.0001).
The median time to opiate use for prostate cancer pain was not reached for patients receiving abiraterone acetate and was 23.7 months for patients receiving placebo (HR=0.686; 95% CI: [0.566, 0.833], p=0.0001). The time to opiate use result was supported by a delay in patient reported pain progression favoring the abiraterone acetate arm.
16. How is Yonsa supplied
YONSA (abiraterone acetate) tablets, 125 mg
White to off-white, oval-shaped tablets debossed with “125 FP” on one side
120 tablets available in high-density polyethylene bottles with child resistant closure
NDC Number 47335-401-81
Storage and Handling
Store at 20oC to 25oC (68oF to 77oF); excursions permitted in the range from 15oC to 30oC (59oF to 86°F) [see USP Controlled Room Temperature].
Keep out of reach of children.
Based on its mechanism of action, YONSA may harm a developing fetus. Women who are pregnant or women who may be pregnant should not handle YONSA tablets if broken, crushed, or damaged without protection, e.g., gloves [see Use in Specific Populations (8.1)].
17. Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Patient Information)
Hypokalemia, Fluid Retention, and Cardiovascular Adverse Reactions
- Inform patients that YONSA is associated with hypertension, hypokalemia, and peripheral edema that may lead to QT prolongation and Torsades de Pointes in patients who develop hypokalemia while taking YONSA. Advise patients that their blood pressure, serum potassium and signs and symptoms of fluid retention will be monitored clinically at least monthly. Advise patients to adhere to corticosteroids and to report symptoms of hypertension, hypokalemia, or edema to their healthcare provider [see Warnings and Precautions (5.1)].
Adrenocortical Insufficiency
- Inform patients that YONSA with methylprednisolone is associated with adrenal insufficiency. Advise patients to report symptoms of adrenocortical insufficiency to their healthcare provider [see Warnings and Precautions (5.2)].
Hepatotoxicity
- Inform patients that YONSA is associated with severe hepatotoxicity. Inform patients that their liver function will be monitored using blood tests. Advise patients to immediately report symptoms of hepatotoxicity to their healthcare provider [see Warnings and Precautions (5.3)].
Hypoglycemia
- Inform patients that severe hypoglycemia has been reported when abiraterone acetate was administered to patients with pre-existing diabetes who were receiving medications containing thiazolidinediones (including pioglitazone) or repaglinide – antidiabetic drugs. Advise patients with diabetes to monitor blood glucose levels during and after treatment with YONSA [see Warnings and Precautions (5.6) and Drug Interactions (7.2)].
Use in Combination with Radium Ra 223 Dichloride
- Advise patients that radium Ra 223 dichloride showed an increase in mortality and an increased rate of fracture when used in combination with abiraterone acetate plus a corticosteroid. Inform patients to speak with their healthcare provider about any other medications or treatment they are currently taking for prostate cancer [see Warnings and Precautions (5.4)].
Dosing and Administration
- Inform patients that YONSA tablets may not be interchangeable with other abiraterone acetate products due to different dosing and food effects [see Dosage and Administration (2.2)].
- Inform patients that YONSA is taken once daily with methylprednisolone twice daily and to not interrupt or stop either of these medications without consulting their healthcare provider [see Dosage and Administration (2.2)].
- Inform patients receiving GnRH therapy that they need to maintain this treatment during the course of treatment with YONSA [see Dosage and Administration (2.2)].
- Instruct patients to take YONSA tablets as a single dose once orally with or without food. Instruct patients to swallow tablets whole with water and not to crush or chew the tablets [see Dosage and Administration (2.2)].
- Inform patients that if they miss a dose of YONSA or methylprednisolone, they should take their normal dose the following day. If more than one daily dose is skipped, inform patients to contact their healthcare provider [see Dosage and Administration (2.2)].
Embryo-Fetal Toxicity
- Inform patients that YONSA may harm a developing fetus and can cause loss of pregnancy.
- Advise males with female partners of reproductive potential to use effective contraception during treatment and for 3 weeks after the last dose of YONSA [see Use in Specific Populations (8.1)].
- Advise females who are pregnant or women who may be pregnant not to handle YONSA tablets if broken, crushed, or damaged without protection, e.g., gloves [see Use in Specific Populations (8.1) and How Supplied/Storage and Handling (16)].
Infertility
- Advise male patients that YONSA may impair fertility [see Use in Specific Populations (8.3)].
Manufactured for:
Sun Pharmaceutical Industries Limited
Distributed by:
Sun Pharmaceutical Industries, Inc.
Cranbury, NJ 08512
U.S. Patent Nos. 8,808,751, 9,889,144 and 10,292,990
YONSA is a registered trademark of Sun Pharmaceutical Industries Limited
© 2022 Sun Pharmaceutical Industries Limited
All rights reserved.
Revised: 2022 July
uspi-YONSA-tab-00005
|
PATIENT INFORMATION YONSA® (Yon-suh) (abiraterone acetate) tablets |
|
|
What is YONSA? YONSA is a prescription medicine that is used along with methylprednisolone. YONSA is used to treat men with prostate cancer that has spread to other parts of the body and no longer responds to medical or surgical treatment that lowers testosterone. It is not known if YONSA is safe and effective in females or children. |
|
|
Before taking YONSA, tell your healthcare provider about all of your medical conditions, including if you:
Tell your healthcare provider about all the medicines you take or treatments you receive, including prescription and over-the-counter medicines, vitamins, and herbal supplements. YONSA can interact with many other medicines. You should not start or stop any medicine before you talk with the healthcare provider that prescribed YONSA. Know the medicines you take. Keep a list of them with you to show to your healthcare provider and pharmacist when you get a new medicine. |
|
|
How should I take YONSA?
|
|
|
What are the possible side effects of YONSA? YONSA may cause serious side effects, including:
Tell your healthcare provider if you get any of the following symptoms: |
|
|
|
|
|
|
|
|
The most common side effects of YONSA include: |
|
|
|
|
YONSA may cause fertility problems in males, which may affect the ability to father children. Talk to your healthcare provider if you have concerns about fertility. These are not all the possible side effects of YONSA. Call your healthcare provider for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
|
|
How should I store YONSA?
Keep YONSA and all medicines out of the reach of children. |
|
|
General information about the safe and effective use of YONSA. Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use YONSA for a condition for which it was not prescribed. Do not give YONSA to other people, even if they have the same symptoms that you have. It may harm them. You can ask your healthcare provider or pharmacist for information about YONSA that is written for health professionals. |
|
|
What are the ingredients in YONSA? Active ingredient: abiraterone acetate. Inactive ingredients: lactose monohydrate, microcrystalline cellulose, croscarmellose sodium, sodium lauryl sulfate, sodium stearyl fumarate, butylated hydroxyanisole, butylated hydroxytoluene. Manufactured for Sun Pharmaceutical Industries Limited Distributed by Sun Pharmaceutical Industries, Inc., Cranbury, NJ 08512 YONSA is a registered trademark of Sun Pharmaceutical Industries Limited © 2022 Sun Pharmaceutical Industries Limited All rights reserved. For more information, call Sun Pharmaceutical Industries, Inc at 1-800-818-4555 or go to www.yonsarx.com |
|
This Patient Information has been approved by the U.S. Food and Drug Administration Revised: 07/2022
| YONSA
abiraterone acetate tablet |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Sun Pharmaceutical Industries, Inc. (146974886) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Catalent Greenville, Inc. | 118812386 | manufacture(47335-401) , pack(47335-401) , analysis(47335-401) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Sterling Chemical Malta Ltd | 360251875 | api manufacture(47335-401) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Powdersize, LLC | 080120056 | particle size reduction(47335-401) | |





