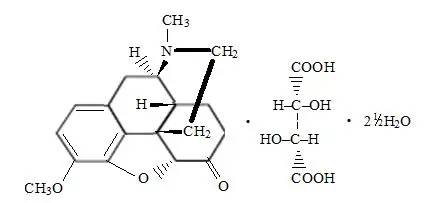
Drug Detail:Zamicet (Acetaminophen and hydrocodone [ a-seet-a-min-oh-fen-and-hye-droe-koe-done ])
Drug Class: Narcotic analgesic combinations
Hepatotoxicity
Acetaminophen has been associated with cases of acute liver failure, at times resulting in liver transplant and death. Most of the cases of liver injury are associated with the use of acetaminophen at doses that exceed 4000 milligrams per day, and often involve more than one acetaminophen-containing product.
Zamicet - Clinical Pharmacology
Hydrocodone is a semisynthetic narcotic analgesic and antitussive with multiple actions qualitatively similar to those of codeine. Most of these involve the central nervous system and smooth muscle. The precise mechanism of action of hydrocodone and other opiates is not known, although it is believed to relate to the existence of opiate receptors in the central nervous system. In addition to analgesia, narcotics may produce drowsiness, changes in mood and mental clouding.
The analgesic action of acetaminophen involves peripheral influences, but the specific mechanism is as yet undetermined. Antipyretic activity is mediated through hypothalamic heat regulating centers. Acetaminophen inhibits prostaglandin synthetase. Therapeutic doses of acetaminophen have negligible effects on the cardiovascular or respiratory systems; however, toxic doses may cause circulatory failure and rapid, shallow breathing.
Precautions
Information for Patients/Caregivers
- Do not take Zamicet® if you are allergic to any of its ingredients.
- If you develop signs of allergy such as a rash or difficulty breathing stop taking Zamicet® and contact your healthcare provider immediately.
- Do not take more than 4000 milligrams of acetaminophen per day. Call your doctor if you took more than the recommended dose.
Hydrocodone, like all narcotics, may impair mental and/or physical abilities required for the performance of potentially hazardous tasks such as driving a car or operating machinery. Such tasks should be avoided while taking this product.
Alcohol and other CNS depressants may produce an additive CNS depression, when taken with this combination product, and should be avoided.
Hydrocodone may be habit-forming. Patients should take the drug only for as long as it is prescribed, in the amounts prescribed, and no more frequently than prescribed.
Physicians should instruct patients and caregivers to read the patient information leaflet, which appears as the last section of the labeling.
Pediatric Use
Safety and effectiveness in the pediatric population below the age of two years have not been established. Use of hydrocodone bitartrate and acetaminophen in the pediatric population is supported by the evidence from adequate and well controlled studies of hydrocodone and acetaminophen combination products in adults with additional data which support the development of metabolic pathways in children two years of age and over (see DOSAGE AND ADMINISTRATION for pediatric dosage information).
Overdosage
Following an acute overdosage, toxicity may result from hydrocodone or acetaminophen.
Zamicet Dosage and Administration
Dosage should be adjusted according to the severity of the pain and the response of the patient. However, it should be kept in mind that tolerance to hydrocodone can develop with continued use and that the incidence of untoward effects is dose related.
The usual adult dosage is one tablespoonful (15 mL) every four to six hours as needed for pain. The total daily dosage should not exceed 6 tablespoonfuls.
The usual dosages for children are given by the table below, and are to be given every 4 to 6 hours as needed for pain. These dosages correspond to an average individual dose of 0.20 mL/kg of Zamicet® (providing 0.135 mg/kg of hydrocodone bitartrate and 4.38 mg/kg of acetaminophen). Dosing should be based on weight whenever possible.
| BODY WEIGHT | APPROXIMATE AGE | DOSE every 4 to 6 hours | MAXIMUM TOTAL DAILY DOSE (6 doses per day) |
|---|---|---|---|
| 12 to 15 kg (27 to 34 lbs) | 2 to 3 years | 2.8 mL (approx. ½ teaspoonful) | 16.8 mL (approx. 3¼ teaspoonfuls) |
| 16 to 22 kg (35 to 50 lbs) | 4 to 6 years | 3.75 mL (approx. ¾ teaspoonful) | 22.5 mL (approx. 4½ teaspoonfuls) |
| 23 to 31 kg (51 to 69 lbs) | 7 to 9 years | 5.6 mL (approx. 1 teaspoonful) | 33.6 mL (approx. 6½ teaspoonfuls) |
| 32 to 45 kg (70 to 100 lbs) | 10 to 13 years | 7.5 mL (approx. 1½ teaspoonfuls) | 45 mL (approx. 9 teaspoonfuls) |
| 46 kg and up (101 lbs and up) | 14 years to adult | 11.25 mL (approx. 2¼ teaspoonfuls) | 67.5 mL (approx. 13½ teaspoonfuls) |
| — | adult | 15 mL (1 Tablespoonful) | 90 mL (6 Tablespoonfuls) |
The total daily dosage for children should not exceed 6 doses per day. It is of utmost importance that the dose of Zamicet® be administered accurately. A household teaspoon or tablespoon is not an adequate measuring device, especially when one-half or three-fourths of a teaspoonful is to be measured. Given the inexactitude of the household spoon measure and the possibility of using a tablespoon instead of a teaspoon, which could lead to overdosage, it is strongly recommended that caregivers obtain and use a calibrated measuring device. Health care providers should recommend a dropper that can measure and deliver the prescribed dose accurately, and instruct caregivers to use extreme caution in measuring the dosage.
| ZAMICET
hydrocodone bitartrate and acetaminophen solution |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| ZAMICET
hydrocodone bitartrate and acetaminophen solution |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - Pharmaceutical Associates, Inc. (044940096) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Pharmaceutical Associates, Inc. | 097630693 | MANUFACTURE(0121-1542, 0121-2313) | |