Drug Detail:Zelnorm (Tegaserod [ te-gas-e-rod ])
Drug Class: Serotoninergic neuroenteric modulators
Highlights of Prescribing Information
ZELNORM™ (tegaserod) tablets, for oral use
Initial U.S. Approval: 2002
Recent Major Changes
| Indications and Usage (1) | 03/2019 |
| Contraindications (4) | 03/2019 |
| Warnings and Precautions (5.1, 5.4) | 03/2019 |
Indications and Usage for Zelnorm
ZELNORM is a serotonin-4 (5-HT4) receptor agonist indicated for the treatment of adult women less than 65 years of age with irritable bowel syndrome with constipation (IBS-C). (1)
Limitations of Use:
- The safety and effectiveness of ZELNORM in men with IBS-C have not been established. (1)
Zelnorm Dosage and Administration
The recommended dosage in adult women less than 65 years of age is 6 mg taken twice daily orally at least 30 minutes before meals. Discontinue ZELNORM in patients who have not had adequate control of symptoms after 4 to 6 weeks of treatment. (2)
Dosage Forms and Strengths
Tablets: 6 mg tegaserod. (3)
Contraindications
ZELNORM is contraindicated in patients with:
- A history of myocardial infarction, stroke, transient ischemic attack, or angina. (4, 5.1)
- A history of ischemic colitis or other forms of intestinal ischemia. (4, 5.2)
- Severe renal impairment (eGFR< 15 mL/min/1.73 m2) or end-stage renal disease. (4, 8.6)
- Moderate or severe hepatic impairment (Child-Pugh B or C). (4, 8.7)
- A history of bowel obstruction, symptomatic gallbladder disease, suspected sphincter of Oddi dysfunction, or abdominal adhesions. (4)
- Hypersensitivity to tegaserod. (4)
Warnings and Precautions
- Cardiovascular Ischemic Events, Including Major Adverse Cardiovascular Events (MACE): The potential risks of treatment must be balanced with expectations in improvements in symptoms of IBS-C. Discontinue ZELNORM treatment in patients who experience a myocardial infarction, stroke, transient ischemic attack or angina. (4) Evaluate the risks and benefits of continued treatment in patients who develop clinical or other evidence of cardiovascular ischemic heart disease and/or experience changes in health status that could increase cardiovascular risk during treatment. (4, 5.1)
- Ischemic Colitis: Monitor for rectal bleeding, bloody diarrhea, and new or worsening abdominal pain and discontinue ZELNORM if symptoms develop. (5.2)
- Volume Depletion Associated with Diarrhea: Avoid use in patients with severe diarrhea. Instruct patients to discontinue ZELNORM and contact their healthcare provider if severe diarrhea, hypotension or syncope occur. (5.3)
- Suicidal Ideation and Behavior: Monitor patients for clinical worsening of depression and emergence of suicidal thoughts and behaviors, especially during the initial few months of treatment. Instruct patients to immediately discontinue ZELNORM and contact their healthcare provider if their depression is persistently worse or they are experiencing emergent suicidal thoughts or behaviors. (5.4)
Adverse Reactions/Side Effects
Most common adverse reactions (>2%) are headache, abdominal pain, nausea, diarrhea, flatulence, dyspepsia, and dizziness. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact US WorldMeds at 1-855-697-9232 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Use In Specific Populations
Lactation: Breastfeeding not recommended. (8.2)
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 3/2019
Related/similar drugs
Linzess, Amitiza, lubiprostone, Trulance, linaclotide, IbsrelaFull Prescribing Information
1. Indications and Usage for Zelnorm
ZELNORM is indicated for the treatment of adult women less than 65 years of age with irritable bowel syndrome with constipation (IBS-C).
2. Zelnorm Dosage and Administration
The recommended dosage of ZELNORM in adult women less than 65 years of age is 6 mg taken twice daily orally at least 30 minutes before meals [see Clinical Pharmacology (12.3)]. Discontinue ZELNORM in patients who have not had adequate control of symptoms after 4 to 6 weeks of treatment.
3. Dosage Forms and Strengths
ZELNORM Tablets: 6 mg tegaserod; supplied as whitish to slightly yellowish, round flat tablet with a beveled edge engraved with "ZEL" and "6".
4. Contraindications
ZELNORM is contraindicated in patients with:
- A history of myocardial infarction (MI), stroke, transient ischemic attack (TIA), or angina [see Warnings and Precautions (5.1)]
- A history of ischemic colitis or other forms of intestinal ischemia [see Warnings and Precautions (5.2)]
- Severe renal impairment (eGFR< 15 mL/min/1.73 m2) or end-stage renal disease [see Use in Specific Populations (8.6)]
- Moderate and severe hepatic impairment (Child-Pugh B or C) [see Use in Specific Populations (8.7)]
- A history of bowel obstruction, symptomatic gallbladder disease, suspected sphincter of Oddi dysfunction, or abdominal adhesions [see Adverse Reactions (6.2)]
- Hypersensitivity to tegaserod [see Adverse Reactions (6.2)]
5. Warnings and Precautions
5.1 Cardiovascular Ischemic Events, Including Major Adverse Cardiovascular Events (MACE)
Stroke, MI, and cardiovascular death (major adverse cardiovascular events [MACE]) have been reported in adults taking ZELNORM who had an increased risk of developing an adverse cardiovascular event based on their medical history [see Adverse Reactions (6.1)].
ZELNORM is contraindicated in patients with a history of MI, stroke, TIA, or angina [see Contraindications (4)]. Assess female patients less than 65 years of age for a history of cardiovascular disease and cardiovascular risk factors prior to treatment with ZELNORM [see Adverse Reactions (6.1)]. The potential risks of treatment must be balanced with expectations in improvements in symptoms of IBS-C.
Discontinue ZELNORM in patients who experience an MI, stroke, TIA, or angina [see Contraindications (4)]. Evaluate the risks and benefits of continued use of ZELNORM in patients who develop clinical or other evidence of cardiovascular ischemic heart disease (e.g., coronary artery disease) and/or experience changes in health status that could increase cardiovascular risk during treatment with ZELNORM.
5.2 Ischemic Colitis
Ischemic colitis and other forms of intestinal ischemia have been reported postmarketing in patients receiving ZELNORM [see Adverse Reactions (6.2)]. In some cases, hospitalization was required. Discontinue ZELNORM in patients who develop symptoms of ischemic colitis, such as rectal bleeding, bloody diarrhea, or new or worsening abdominal pain. Evaluate patients experiencing these symptoms promptly and perform appropriate diagnostic testing. Do not reinitiate ZELNORM in patients who develop findings consistent with ischemic colitis or other forms of intestinal ischemia [see Contraindications (4)].
5.3 Volume Depletion Associated with Diarrhea
Diarrhea is one of the most common adverse reactions in ZELNORM-treated patients from the pooled IBS-C double-blind placebo-controlled trials. Diarrhea resulted in discontinuation in 1.6% of ZELNORM-treated patients compared to 0% in placebo [see Adverse Reactions (6)].
In post-marketing experience, serious consequences of diarrhea including hypovolemia, hypotension, and syncope have been reported in patients treated with ZELNORM. In some cases, these complications have required hospitalization for rehydration. Avoid use of ZELNORM in patients who are currently experiencing or frequently experience diarrhea. Instruct patients to discontinue ZELNORM and contact their healthcare provider if severe diarrhea, hypotension, or syncope occur.
5.4 Suicidal Ideation and Behavior
Suicide, suicidal attempt and ideation, and self-injurious behavior have been reported in clinical trials of IBS-C and other gastrointestinal motility disorders. The frequency of suicidal ideation or attempts with tegaserod treatment (8 patients out of 10,003) was higher than placebo (1 patient out of 5,425) [see Adverse Reactions (6.1)]. Suicidal ideation/behavior in clinical trials was proportionately more frequent among patients receiving antidepressant medication.
Monitor all ZELNORM-treated patients for clinical worsening of depression and emergence of suicidal thoughts and behaviors, especially during the initial few months of treatment. Counsel family members and caregivers of patients to monitor for changes in behavior and to alert the healthcare provider. Instruct patients to immediately discontinue ZELNORM and contact their healthcare provider if their depression is persistently worse or they are experiencing emergent suicidal thoughts or behaviors.
6. Adverse Reactions/Side Effects
The following adverse reactions are discussed in more detail elsewhere in the labeling:
- Cardiovascular Ischemic Events, including MACE [see Warnings and Precautions (5.1)]
- Ischemic Colitis [see Warnings and Precautions (5.2)]
- Volume Depletion Associated with Diarrhea [see Warnings and Precautions (5.3)]
- Suicidal Ideation and Behavior [see Warnings and Precautions (5.4)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
6.2 Postmarketing Experience
The following adverse reactions have been identified during postapproval use of ZELNORM. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Ischemic colitis, mesenteric ischemia, gangrenous bowel and rectal bleeding [see Warnings and Precautions (5.2)]
- Severe diarrhea resulting in syncope, hypotension, hypovolemia, electrolyte disorders [see Warnings and Precautions (5.3)]
- Sphincter of Oddi spasm, bile duct stone, cholecystitis with elevated transaminases, elevation in ALT, AST and bilirubin, hepatitis [see Contraindications (4)]
- Alopecia
- Hypersensitivity reactions, including anaphylaxis [see Contraindications (4)]
8. Use In Specific Populations
8.4 Pediatric Use
Safety and effectiveness of ZELNORM in pediatric patients have not been established.
8.6 Renal Impairment
ZELNORM is contraindicated in patients with severe renal impairment (eGFR < 15 mL/min/1.73 m2) or end stage renal disease [see Contraindications (4)]. The Cmax and AUC of the tegaserod metabolite, 5-methoxyindole-3-carboxylic acid glucuronide (M29), are substantially increased in severe renal impairment [see Clinical Pharmacology (12.3)].
No dosage adjustment is recommended in patients with mild to moderate renal impairment (eGFR ≥ 30 mL/min/1.73 m2).
10. Overdosage
Single oral doses of 120 mg (20 times the recommended dose) of ZELNORM were administered to three healthy subjects in one study. All three subjects developed diarrhea and headache. Two of these subjects also reported intermittent abdominal pain and one developed orthostatic hypotension. In 28 healthy subjects exposed to 90 to 180 mg per day of ZELNORM (7.5 to 15 times the recommended daily dosage) for several days, adverse reactions were diarrhea (100%), headache (57%), abdominal pain (18%), flatulence (18%), nausea (7%), and vomiting (7%).
Based on the large distribution volume and high protein binding of tegaserod, it is unlikely that tegaserod could be removed by dialysis. In cases of overdosage, treat symptomatically and institute supportive measures as appropriate.
11. Zelnorm Description
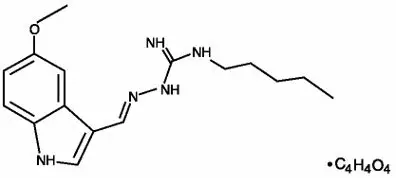
ZELNORM oral tablets contain tegaserod, a serotonin-4 (5-HT4) receptor agonist, as the hydrogen maleate salt. As the maleate salt, tegaserod is chemically designated as 3-(5-methoxy-1H-indol-3-ylmethylene)-N-pentylcarbazimidamide hydrogen maleate. Its empirical formula is C16H23N5O∙C4H4O4. The molecular weight is 417.47 and the structural formula is:
Tegaserod as the maleate salt is a white to off-white crystalline powder and is slightly soluble in ethanol and very slightly soluble in water. Each ZELNORM tablet contains 6 mg of tegaserod (equivalent to 8.31 mg of tegaserod maleate) and the following inactive ingredients: colloidal silicon dioxide, crospovidone, glyceryl behenate, hypromellose, and lactose monohydrate.
12. Zelnorm - Clinical Pharmacology
12.1 Mechanism of Action
Tegaserod is an agonist of serotonin type-4 (5-HT4) receptors that stimulates the peristaltic reflex and intestinal secretion, inhibits visceral sensitivity, enhances basal motor activity, and normalizes impaired motility throughout the gastrointestinal tract.
Based on in vitro binding affinity and functional assessment, at clinically relevant plasma concentrations, tegaserod is an antagonist at 5-HT2B receptors in humans. It is expected to have minimal binding to 5-HT1 receptors. Tegaserod has no affinity for 5-HT3 or dopamine receptors.
The main metabolite, M29, has negligible affinity for 5-HT4 receptors in vitro.
In vivo studies showed that tegaserod enhanced basal motor activity and normalized impaired motility throughout the gastrointestinal tract. In addition, studies demonstrated that tegaserod moderated visceral sensitivity during colorectal distension in animals.
12.3 Pharmacokinetics
The pharmacokinetics of tegaserod in IBS-C patients are comparable to those in healthy subjects. The mean (±SD) peak tegaserod concentration (Cmax) was 2.9 (±1.1) ng/mL, and mean (±SD) AUC was 10.5 (±4.6) h∙ng/mL following a single ZELNORM dose at 6 mg. Tegaserod systemic exposure at steady state increase proportionally over a dose range of 2 mg to 12 mg twice daily (0.3 to 2 times the approved recommended dosage). There was no significant accumulation (~10%) of tegaserod following the approved recommended dosage.
Drug Interaction Studies
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Tegaserod was not carcinogenic in rats given oral dietary doses up to 180 mg/kg/day (approximately 93 to 111 times the recommended dose based on AUC) for 110 to 124 weeks.
In mice, dietary administration of tegaserod for 104 weeks produced mucosal hyperplasia and adenocarcinoma of small intestine at 600 mg/kg/day (approximately 83 to 110 times the recommended dose based on AUC). There was no evidence of carcinogenicity at lower doses (3 to 35 times the recommended dose based on AUC).
Tegaserod was not genotoxic in the in vitro Chinese hamster lung fibroblast (CHL/V79) cell chromosomal aberration and forward mutation test, the in vitro rat hepatocyte unscheduled DNA synthesis (UDS) test or the in vivo mouse micronucleus test. The results of the Ames test for mutagenicity were equivocal.
Tegaserod at oral (dietary) doses up to 240 mg/kg/day (approximately 57 times the recommended dose based on AUC) in male rats and 150 mg/kg/day (approximately 42 times the recommended dose based on AUC) in female rats was found to have no effect on fertility and reproductive performance.
13.2 Animal Toxicology and/or Pharmacology
Inhibition of the hERG (human Ether-a-go-go-Related Gene) channel was evident only in the micromolar concentration range with an IC50 of 13 micromolar (approximately 1300 times the Cmax in humans at the recommended dose). In in vitro studies, tegaserod had no effects on impulse conduction in isolated guinea pig papillary muscle at up to 100 times the Cmax in humans, Langendorff-perfused isolated rabbit heart (QT interval) at up to 1000 times the Cmax in humans, or human atrial myocytes at multiples up to 10 times the Cmax in humans. The major metabolite, M29, had no effect on QT in the Langendorff-perfused isolated rabbit heart at multiples up to 323 times the Cmax in humans.
In anesthetized and conscious dogs, tegaserod at doses up to 92 to 134 times the recommended dose based on Cmax did not alter heart rate, QRS interval duration, QTc or other ECG parameters. In chronic toxicology studies in rats and dogs, there were no treatment-related changes in cardiac morphology after tegaserod administration at doses up to 660 times the recommended dose based on AUC.
Although tegaserod is expected to bind to 5-HT2B receptors in humans at the recommended dose, there does not appear to be any potential for heart valve injury based on functional evidence of 5-HT2B receptor antagonism.
Studies with isolated coronary and mesenteric blood vessels from non-human primates and humans showed no vasoconstrictor effect at concentrations approximately 100 times the human Cmax. Tegaserod exhibited antagonism of 5-HT-mediated vasoconstriction via 5-HT1B receptors. In rat thoracic aortic rings that were pre-constricted with phenylephrine or norepinephrine, tegaserod produced vasorelaxation, with IC50 values 6 and 64 times the Cmax plasma concentrations in humans, respectively. No effects were observed in the basal tone of aortic rings at concentrations up to 1000 times the human Cmax.
In studies with an anesthetized rat model for measuring macro- and micro-circulation of the colon, intraduodenal dosing with tegaserod (approximately 7 times the recommended dose based on Cmax) produced no clinically relevant effect on blood pressure, heart rate, or vascular conductance.
16. How is Zelnorm supplied
ZELNORM is supplied as 6 mg tegaserod whitish to slightly yellowish, round flat tablets with a beveled edge engraved with "ZEL" and "6" .
Unit Dose (blister pack)
| NDC 27505-090-60 |
17. Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
|
Medication Guide |
||
|
What is the most important information I should know about ZELNORM? ZELNORM can cause serious side effects, including:
|
||
|
|
|
|
||
|
What is ZELNORM? ZELNORM is a prescription medicine used for the treatment of adult women less than 65 years of age who have irritable bowel syndrome with constipation (IBS-C). It is not known if ZELNORM is safe and effective in men with IBS-C. It is not known if ZELNORM is safe and effective in children. |
||
|
Do not take ZELNORM if you:
|
||
|
Before taking ZELNORM, tell your healthcare provider about all of your medical conditions, including if you:
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. |
||
|
How should I take ZELNORM?
|
||
|
What are the possible side effects of ZELNORM? ZELNORM can cause serious side effects, including:
The most common side effects of ZELNORM are: |
||
|
|
|
|
These are not all the possible side effects of ZELNORM. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
||
|
How should I store ZELNORM?
Keep ZELNORM and all medicines out of the reach of children. |
||
|
General information about the safe and effective use of ZELNORM. Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use ZELNORM for a condition for which it was not prescribed. Do not give ZELNORM to other people, even if they have the same symptoms that you have. It may harm them. You can ask your healthcare provider or pharmacist for information about ZELNORM that is written for health professionals. |
||
|
What are the ingredients in ZELNORM? Active ingredient: tegaserod Inactive Ingredients: colloidal silicon dioxide, crospovidone, glyceryl behenate, hypromellose, and lactose monohydrate Distributed by: US WorldMeds, LLC Louisville, KY 40241 Under License from Sloan Pharma S.a.r.l. Sloan Pharma S.a.r.l. is the exclusive licensee and US WorldMeds, LLC is the distributor of ZELNORM in the United States and Its territories. ©2019. ZELNORM is a trademark of Sloan Pharma S.a.r.l. For more information, call 1-855-697-9232. |
||
| ZELNORM
tegaserod tablet |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - US WorldMeds, LLC (087875626) |
| Registrant - Sloan Pharma S.a.r.l (480076470) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Corden Pharma GmbH | 312576524 | MANUFACTURE(27505-090) , ANALYSIS(27505-090) , PACK(27505-090) , LABEL(27505-090) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Novartis Pharma Produktions GmbH | 333288046 | API MANUFACTURE(27505-090) , ANALYSIS(27505-090) | |