Drug Detail:Zetonna nasal spray (Ciclesonide nasal [ sik-le-son-ide ])
Drug Class: Nasal steroids
Highlights of Prescribing Information
ZETONNA® (ciclesonide) nasal aerosol
Initial U.S. Approval: 2006
Indications and Usage for Zetonna
ZETONNA is a corticosteroid indicated for:
- •
- treatment of symptoms associated with seasonal allergic rhinitis in adult and pediatric patients 12 years of age and older. (1.1)
- •
- Treatment of symptoms associated with perennial allergic rhinitis in adult and pediatric patients 12 years of age and older. (1.1)
Zetonna Dosage and Administration
For nasal use only
- •
- Recommended dosage: 1 actuation (37 mcg of ciclesonide) per nostril once daily. (2.1)
- •
- Priming: Gently shake and prime ZETONNA before using for the first time or when not used for 10 consecutive days. (2)
Dosage Forms and Strengths
Nasal aerosol: 37 mcg of ciclesonide per actuation. (3)
Contraindications
Patients with a known hypersensitivity to ciclesonide or any of the ingredients of ZETONNA. (4)
Warnings and Precautions
- •
- Local nasal adverse reactions, including epistaxis, ulceration, nasal septal perforations, Candida albicans infection, impaired wound healing. Prior to initiating therapy, examine patients for evidence of septal perforation, erosions, ulceration, nasal surgery, and trauma. Avoid spraying ZETONNA directly onto the nasal septum. Avoid use in patients with recent septal perforation, nasal erosion, nasal ulcers, nasal surgery, or nasal trauma. Monitor patients periodically for signs of adverse effects on the nasal mucosa. Discontinue ZETONNA if erosions, ulcerations or perforations occur. (5.1)
- •
- Development of glaucoma or cataracts: Monitor patients closely with a change in vision or with a history of increased intraocular pressure, glaucoma, or cataracts. (5.2)
- •
- Hypersensitivity reactions have been reported following administration of ciclesonide with manifestations such as angioedema, with swelling of the lips, tongue and pharynx. (5.3)
- •
- Potential worsening of existing tuberculosis; fungal, bacterial, viral, or parasitic infections; or ocular herpes simplex. More serious or even fatal course of chicken pox or measles in susceptible individuals. Use caution in patients with the above because of the potential for worsening of these infections. (5.4)
- •
- Hypercorticism and adrenal suppression: May occur with higher than recommended dosages or in susceptible individuals at recommended dosages. If such changes occur, discontinue ZETONNA slowly. (5.5)
- •
- Potential reduction in growth velocity in children: Monitor growth routinely in pediatric patients receiving ZETONNA. (5.6, 8.4)
Adverse Reactions/Side Effects
The most common adverse reactions (≥2% incidence) included nasal discomfort, headache and epistaxis. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Covis Pharma at 1-866-488-4423 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 2/2023
Related/similar drugs
prednisone, fluticasone nasal, cetirizine, loratadine, promethazine, ZyrtecFull Prescribing Information
1. Indications and Usage for Zetonna
2. Zetonna Dosage and Administration
2.1 Administration Information
Administer ZETONNA by the nasal route only. Avoid spraying in eyes or directly onto the nasal septum.
Priming:
- •
- Prior to initial use, ZETONNA must be primed by actuating 3 times.
- •
- If ZETONNA is not used for 10 consecutive days, it must be primed by actuating 3 times.
- •
- If ZETONNA is dropped, the canister and actuator may become separated. If this happens, reassemble ZETONNA and spray 1 test spray into the air before using.
Cleaning:
Clean outside of nose piece with a clean, dry tissue or cloth weekly; do not wash or put in water. Illustrated patient’s instructions for proper use accompany each package of ZETONNA.
4. Contraindications
ZETONNA is contraindicated in patients with a known hypersensitivity to ciclesonide or any of the ingredients of ZETONNA [see Warnings and Precautions (5.3)].
5. Warnings and Precautions
5.1 Local Nasal Adverse Reactions
Epistaxis and Nasal Ulceration: In clinical trials of 2 to 26 weeks in duration, epistaxis was observed more frequently in patients treated with ZETONNA than those who received placebo. In the 26-week open-label extension of the perennial allergic rhinitis trial, nasal ulceration was identified in 4 of 824 patients administered ZETONNA (148 mcg) [see Adverse Reactions (6)].
The occurrence of local nasal adverse events was further evaluated in a separate, postmarketing 26-week randomized, open-label, active-controlled nasal and ocular safety trial conducted in patients with perennial allergic rhinitis. In this study epistaxis was observed in 6% of patients treated with ZETONNA and nasal ulceration was identified in 3 of 367 patients administered ZETONNA [see Adverse Reactions (6)].
Nasal Septal Perforation: Nasal septal perforation has been reported in patients following the nasal application of ZETONNA. Three short-term placebo-controlled trials (2 weeks) and one long-term (26 weeks with placebo control and 26 weeks open-label extension without placebo control) trial were conducted in patients with seasonal and perennial allergic rhinitis. Nasal septal perforations were reported in 2 patients out of 2335 patients treated with ZETONNA compared with none of 892 patients treated with placebo. No nasal septal perforations were reported in 367 patients treated with ZETONNA in a postmarketing 26-week, open-label, active-controlled trial in patients with perennial allergic rhinitis [see Adverse Reactions (6)].
Before starting ZETONNA conduct a nasal examination to ensure that patients are free of nasal disease other than allergic rhinitis. Periodically monitor patients with nasal examinations during treatment for adverse effects in the nasal cavity. If an adverse reaction (e.g. erosion, ulceration, perforation) is noted, discontinue ZETONNA. Avoid spraying ZETONNA directly onto the nasal septum.
Candida Infection: Localized infections of the nose or pharynx with Candida albicans has occurred from the use of ciclesonide. If such an infection occurs with ZETONNA, treat it with appropriate local therapy and discontinue ZETONNA.
Impaired Wound Healing: Because of the inhibitory effect of corticosteroids on wound healing, patients who have experienced recent nasal septal ulcers, nasal surgery, or nasal trauma should not use ZETONNA until healing has occurred.
5.2 Glaucoma and Cataracts
Nasal and inhaled corticosteroids, including ZETONNA, can result in the development of glaucoma and cataracts. Therefore, close monitoring is warranted in patients with a change in vision or with a history of increased intraocular pressure, glaucoma, or cataracts.
5.3 Hypersensitivity Reactions
ZETONNA is contraindicated in patients with a known hypersensitivity to ciclesonide or any of the ingredients of ZETONNA. Hypersensitivity reactions including angioedema, with swelling of the lips, tongue and pharynx, have occurred after nasal administration of ZETONNA. Discontinue ZETONNA if such reactions occur.
5.4 Immunosuppression and Risk of Infections
Patients who are using drugs that suppress the immune system are more susceptible to infections than healthy individuals. Chickenpox and measles, for example, can have a more serious or even fatal course in susceptible children or adults using corticosteroids. In children or adults who have not had these diseases or been properly immunized, particular care should be taken to avoid exposure. How the dose, route, and duration of corticosteroid administration affect the risk of developing a disseminated infection is not known. The safety and effectiveness of ZETONNA have not been established in pediatric patients less than 12 years of age and ZETONNA is not indicated for use in this population. The contribution of the underlying disease or prior corticosteroid treatment to the risk is also not known. If a patient is exposed to chickenpox, prophylaxis with varicella zoster immune globulin (VZIG) may be indicated. If a patient is exposed to measles, prophylaxis with pooled intramuscular immunoglobulin (IG) may be indicated (see the respective Prescribing Information for VZIG and IG). If chickenpox develops, treatment with antiviral agents may be considered.
Corticosteroids should be used with caution, if at all, in patients with active or quiescent tuberculosis infections of the respiratory tract; or in patients with untreated local or systemic fungal or bacterial infections; systemic viral or parasitic infections; or ocular herpes simplex because of the potential for worsening of these infections.
5.5 Hypercorticism and Adrenal Suppression
Hypercorticism and adrenal suppression may occur when nasal corticosteroids, including ZETONNA, are used at higher-than-recommended dosages [see Dosage and Administration (2)] or patients at risk for such effects.
5.6 Effect on Growth
Corticosteroids, including ZETONNA, may cause a reduction in growth velocity when administered to pediatric patients. The safety and effectiveness of ZETONNA have not been established in pediatric patients less than 12 years of age and ZETONNA is not indicated for use in this population. Monitor the growth routinely (e.g., via stadiometry) in pediatric patients receiving ZETONNA [see Pediatric Use (8.4)].
6. Adverse Reactions/Side Effects
The following clinically significant adverse reactions are described elsewhere in the labeling:
- •
- Epistaxis, Ulcerations, Nasal Septal Perforations, Candida albicans Infection, Impaired Wound Healing [see Warnings and Precautions (5.1)]
- •
- Glaucoma and Cataracts [see Warnings and Precautions (5.2)]
- •
- Immunosuppression and Risk of Infections [see Warnings and Precautions (5.4)]
- •
- Hypercorticism and Adrenal Suppression, including Growth Reduction [see Warnings and Precautions (5.5, 5.6), Use in Specific Populations (8.4)]
6.1 Clinical Trials Experience
The safety data described below for adult and pediatric patients 12 years of age and older are based on 4 clinical trials evaluating doses of ciclesonide from 74 to 282 mcg. Three of the clinical trials were 2 to 6 weeks in duration and one trial was 26 weeks in duration with an additional 26-week open-label extension. Data from the first 6 weeks of the 26-week trial were pooled with data from the three 2-week trials. Short-term data (2 to 6 weeks) included 3001 patients with seasonal and perennial allergic rhinitis, of these, 884 received ZETONNA 74 mcg once daily and 892 received placebo. The short-term data included 1098 (36.6%) males, 1903 (63.4%) females, 2587 (86.2%) Caucasians, 320 (10.7%) Blacks, 49 (1.6%) Asians, and 45 (1.5%) patients classified as Other. The 26-week trial was conducted in 1110 patients with perennial allergic rhinitis [394 (35.5%) males and 716 (64.5%) females, ages 12 to 78 years old] treated with ZETONNA 74 mcg, 148 mcg or placebo once daily. Of these patients, 298 were treated with 74 mcg ZETONNA, 505 with 148 mcg, and 307 with placebo. The racial distribution in this trial included 922 (83.1%) Caucasians, 146 (13.2%) Blacks, 18 (1.6%) Asians, and 24 (2.2%) patients classified as Other. The 26-week open-label extension included 824 patients [295 (35.8%) males and 529 (64.2%) females, ages 12 to 79 years old] given ZETONNA 148 mcg once daily. The racial distribution in the open-label extension included 690 (83.7%) Caucasians, 104 (12.6%) Blacks, 15 (1.8%) Asians, and 15 (1.8%) patients classified as Other.
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Short-Term (2-6 weeks) Trials:
In three short-term trials and the first 6 weeks of one long-term trial, conducted in the US, 884 patients with a history of seasonal or perennial allergic rhinitis were treated with ZETONNA 74 mcg daily. Adverse reactions did not differ appreciably based on age, gender, or race.
Table 1 displays reactions that occurred with an incidence of at least 2.0% and more frequently with ZETONNA 74 mcg than with placebo in seasonal or perennial allergic rhinitis clinical trials of 2 to 6 weeks duration.
| a Nasal discomfort includes both nasal discomfort and instillation site discomfort | ||
|
Adverse Reaction |
ZETONNA |
Placebo |
|
Nasal discomforta |
28 (3.2%) |
16 (1.8%) |
|
Headache |
27 (3.1%) |
11 (1.2%) |
|
Epistaxis |
26 (2.9%) |
24 (2.7%) |
When considering the data from higher doses evaluated in the short-term trials, epistaxis demonstrated a dose response. In addition, 2 patients treated with ZETONNA 74 mcg experienced nasal septal perforations in the short-term trials compared to no patients treated with placebo.
Approximately 1.2% of patients treated with ZETONNA 74 mcg in clinical trials discontinued because of adverse reactions; this rate was similar for patients treated with placebo. Discontinuations due to local adverse reactions were similar in ZETONNA 74 mcg treated patients (0.8%) compared to placebo treated patients (0.8%). Local adverse reactions leading to discontinuation that occurred only in ZETONNA treated patients included ear infection, nasal discomfort, nasal dryness, nasal mucosal/septum disorders, pharyngitis, streptococcal pharyngitis, sinus headache, and tonsillitis.
Long-Term (26-Week Double-Blind and 26-Week Open-Label) Safety Trial:
In one 26-week double-blind, placebo-controlled safety trial that included 1110 adult and pediatric patients (12 to 17 years of age) with perennial allergic rhinitis, additional adverse reactions, with an incidence of at least 2%, that occurred more frequently with ZETONNA than with placebo were upper respiratory tract infection, urinary tract infection, oropharyngeal pain, nasal mucosal/septum disorders, viral upper respiratory tract infection, cough, influenza, bronchitis, streptococcal pharyngitis, muscle strain, and nausea. Nasal discomfort (5.7%) and epistaxis (11.4%) were also more frequent in the 26-week safety trial compared to clinical trials 2 to 6 weeks in duration. Nasal mucosal/septum disorders and cough demonstrated a dose response.
Discontinuations due to adverse reactions were higher in ZETONNA treated patients compared to placebo treated patients and demonstrated a dose response. Local adverse reactions leading to discontinuation were also higher in ZETONNA 74 mcg treated patients (1.7%) compared to placebo treated patients (0.7%). The only local adverse reaction leading to discontinuation that occurred in ZETONNA treated patients and was not observed in the 2- to 6-week trials was upper respiratory tract infection.
A total of 824 patients with perennial allergic rhinitis who completed the 26-week double-blind trial enrolled into an open-label extension and received ZETONNA 148 mcg for 26 weeks. Additional adverse reactions, observed with an incidence of at least 2% were sinusitis, nasopharyngitis, and back pain.
A total of 4 nasal septal ulcerations were also reported in the 26-week open-label extension.
There were no reports of nasal septal perforations in the long-term safety trial.
Long-Term (6-Month Open-Label) Nasal Safety Trial:
Nasal and ocular safety was evaluated in one 26-week, postmarketing, randomized, open-label, active-controlled trial, in adult and pediatric patients 12-74 years of age with a history of perennial allergic rhinitis. A total of 737 patients were treated with ZETONNA 74 mcg or ciclesonide nasal spray 200 mcg once daily. The combined incidence of nasal mucosal or septum disorders, including erosions and ulcerations, was 3 (0.8%) for ZETONNA 74 mcg and 4 (1.1%) for ciclesonide nasal spray 200 mcg treated patients. There were no nasal septal perforations reported with either treatment. Ocular findings, including the development or worsening of lens opacities, increase in intraocular pressure, and worsening visual acuity, were also evaluated over the 26-week treatment period. The occurrence of ocular safety events was similar for the ZETONNA 74 mcg and ciclesonide nasal spray 200 mcg treatment groups.
6.2 Post-marketing Experience
The following adverse reactions have been identified during post-approval use of other formulations of ciclesonide, ALVESCO® Inhalation Aerosol and OMNARIS® Nasal Spray. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
ALVESCO® Inhalation Aerosol: immediate or delayed hypersensitivity reactions such as angioedema with swelling of the lips, tongue, and pharynx.
OMNARIS® Nasal Spray: nasal congestion, nasal ulcer, and dizziness. Localized infections of the nose or mouth with Candida albicans have also occurred with OMNARIS® Nasal Spray.
7. Drug Interactions
In vitro studies and clinical pharmacology studies suggested that des-ciclesonide has no potential for metabolic drug interactions or protein binding-based drug interactions [see Clinical Pharmacology (12.3)]. In a drug interaction study, co-administration of orally inhaled ciclesonide and oral ketoconazole, a potent inhibitor of cytochrome P450 3A4, increased the exposure (AUC) of des-ciclesonide by approximately 3.6-fold at steady state, while levels of ciclesonide remained unchanged. Erythromycin, a moderate inhibitor of cytochrome P450 3A4, had no effect on the pharmacokinetics of either des-ciclesonide or erythromycin following oral inhalation of ciclesonide [see Clinical Pharmacology (12.3)].
8. Use In Specific Populations
8.1 Pregnancy
Risk Summary
There are no available data on ZETONNA use in pregnant women to assess a drug-associated risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes. There is low systemic exposure following ZETONNA administration at the recommended dose [see Clinical Pharmacology (12.3)].
In animal reproduction studies, ciclesonide, administered by the oral route to pregnant rats, during the period of organogenesis, did not cause any evidence of fetal harm at doses up to 120 times the maximum recommended human daily intranasal dose [MRHDID] of 74 mcg/day. Teratogenicity, characteristic of corticosteroids, decreased body weight and/or skeletal variations were observed in rabbit fetuses following administration of ciclesonide to pregnant rabbits by the subcutaneous route during the period of organogenesis at doses 1 times the MRHDID and higher on a mcg/m2 basis (see Data). No evidence of fetal harm was observed in rabbits at doses 0.3 times the MRHDID.
All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. In the United States general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Data
Animal Data
In an embryo-fetal development study in pregnant rats dosed by the oral route during the period of organogenesis from gestation days 6 to 15, ciclesonide did not cause any evidence of fetal harm at doses up to 120 times the MRHDID in adults (on a mcg/m2 basis with maternal oral dose up to 900 mcg/kg/day). Maternal toxicity, as evidenced by decreased body weight gain, was observed at 120 times the MRHDID in adults (on a mcg/m2 basis at a maternal dose of 900 mcg/kg/day); however, no adverse effects were observed at doses 40 times the MRHDID and lower (on a mcg/m2 basis with maternal oral doses of 300 mcg/kg/day and lower).
In two embryo-fetal development studies in pregnant rabbits dosed by the subcutaneous route during the period of organogenesis from gestation days 6 to 18, ciclesonide caused acampsia (flexures of legs) in fetuses at doses 1 times the MRHDID and higher (on a mcg/m2 basis with maternal oral doses of 5 mcg/kg/day and higher), decreased body weight, cleft palate, enlarged fontanelle, parchment-like skin, and incomplete ossification of bones in fetuses at doses 7 times the MHDID (on a mcg/m2 basis with a maternal subcutaneous dose of 25 mcg/kg/day), and embryo-fetal death at doses 25 times the MRHDID and higher (on a mcg/m2 basis with maternal subcutaneous doses of 100 mcg/kg/day and higher). No evidence of fetal harm was observed at a dose 0.25 times the MRHDID in adults (on a mcg/m2 basis at a maternal subcutaneous dose of 1 mcg/kg/day). Maternal toxicity was observed at doses 25 times the MRHDID in adults (on a mcg/m2 basis with maternal subcutaneous doses of 100 mcg/kg/day and lower); however, no evidence of toxicity was observed at doses 7 times the MRHDID and lower (on a mcg/m2 basis with maternal subcutaneous doses of 25 mcg/kg/day and lower).
In a prenatal and postnatal development study in pregnant rats dosed by the oral route from gestation day 6 to lactation day 20, ciclesonide produced no adverse developmental effects on offspring at doses up to approximately 120 times the MRHDID (on a mcg/m2 basis at maternal oral doses up to 900 mcg/kg/day).
8.2 Lactation
Risk Summary
There are no data on the presence of ciclesonide or its metabolite in human milk, the effects on the breastfed child, or the effects on milk production. It is not known whether administration of ciclesonide at the recommended dose could result in sufficient systemic absorption to produce detectable quantities in human milk [see Clinical Pharmacology (12.3)]. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for ZETONNA, and any potential adverse effects on the breastfed infant from ZETONNA, or from the underlying maternal condition.
Clinical Considerations
The molecular weight of the prodrug ciclesonide (approximately 541 g/mol) is small enough to be excreted into breast milk; however, its high plasma protein binding affinity and very short half-life suggest that minimal amounts will be present within the milk. Conversely, the half-life of the active metabolite des-ciclesonide (approximately 471 g/mol) suggests that exposure to the nursing infant will be greater than that of the prodrug ciclesonide. Although ciclesonide and des‑ciclesonide have negligible oral bioavailability (both less than 1% for each) due to low gastrointestinal absorption and high first-pass metabolism, the relative anti‑inflammatory activity of des-ciclesonide is 12-times greater than that of dexamethasone. The effects of this exposure on a nursing infant are unknown; however, like all corticosteroids, suppression of the HPA function is a potential complication.
Data
Human Data
At recommended doses, the nasal administration of ciclesonide results in negligible serum concentrations of ciclesonide. However, the known active metabolite (des‑ciclesonide) is detected in the serum of some patients after nasal inhalation of ciclesonide. The bioanalytical assay used has a lower limit of quantification of 25 pg/mL and 10 pg/mL, for ciclesonide and des-ciclesonide, respectively.
Animal Data
A study with 14C-ciclesonide showed milk exposure of rat pups to 0.006% of the dose secreted in milk.
8.4 Pediatric Use
The safety and effectiveness for seasonal and perennial allergic rhinitis in pediatric patients 12 years of age and older have been established. Use of ZETONNA for this indication is supported by evidence from placebo-controlled, double-blind trials in patients 12 years and older with allergic rhinitis [see Clinical Studies (14)].
The safety and effectiveness of ZETONNA have not been established in pediatric patients younger than 12 years of age.
Effectiveness was not demonstrated for ZETONNA in pediatric patients 6 through 11 years of age. These patients were evaluated in 2 randomized, double blind, parallel placebo-controlled clinical trials in 1693 pediatric patients with allergic rhinitis. Of the 2 trials, 1 was 2 weeks in duration and evaluated the efficacy of 2 doses of ZETONNA (37 mcg and 74 mcg once daily) in 847 patients with seasonal allergic rhinitis. The second clinical trial was 12 weeks in duration and evaluated the efficacy of 2 doses of ZETONNA (37 mcg and 74 mcg once daily) in 846 patients with perennial allergic rhinitis. The trials were similar in design to the trials conducted in pediatric patients 12 years and older and adults. The primary efficacy endpoint was the difference from placebo in the change from baseline of the average morning and evening reflective total nasal symptom scores (rTNSS) averaged over 2 weeks of treatment in the seasonal allergic rhinitis trial and over the first 6 weeks of treatment in the perennial allergic rhinitis trial. In the 2-week trial in patients with seasonal allergic rhinitis, treatment with ZETONNA at either dose failed to demonstrate efficacy. In the 12-week trial in patients with perennial allergic rhinitis, both ZETONNA 37 mcg and 74 mcg once daily demonstrated significant improvement in rTNSS compared to placebo with treatment differences of 0.59 (95% CI: 0.23, 0.95) and 0.47 (95% CI: 0.11, 0.83), respectively.
The effect of ZETONNA on the HPA axis was evaluated in one placebo-controlled clinical study of 6 weeks in duration in children 6 to11 years of age with perennial allergic rhinitis [see Clinical Pharmacology (12.2)].
Studies in pediatric patients under 6 years of age have not been conducted.
Effect on Growth
Controlled clinical trials have shown that nasal corticosteroids may cause a reduction in growth velocity in pediatric patients. This effect has been observed in the absence of laboratory evidence of hypothalamic-pituitary-adrenal (HPA)-axis suppression, suggesting that growth velocity is a more sensitive indicator of systemic corticosteroid exposure in pediatric patients than some commonly used tests of HPA-axis function. The long-term effects of this reduction in growth velocity associated with nasal corticosteroids, including the impact on final adult height, are unknown. The potential for “catch-up” growth following discontinuation of treatment with nasal corticosteroids has not been adequately studied. The growth of pediatric patients receiving nasal corticosteroids, including ZETONNA, should be monitored routinely (e.g., via stadiometry). A 52-week, multi-center, double-blind, randomized, placebo-controlled parallel-group trial was conducted to assess the effect of orally inhaled ciclesonide (ALVESCO® Inhalation Aerosol) on growth rate in 609 pediatric patients with mild persistent asthma, aged 5 to 8.5 years. Treatment groups included orally inhaled ciclesonide 40 mcg or 160 mcg or placebo given once daily. Growth was measured by stadiometer height during the baseline, treatment and follow-up periods. The primary comparison was the difference in growth rates between ciclesonide 40 and 160 mcg and placebo groups. Conclusions cannot be drawn from this trial because compliance could not be assured. Ciclesonide blood levels were also not measured during the one-year treatment period. There was no difference in efficacy measures between the placebo and the orally inhaled ciclesonide (ALVESCO® Inhalation Aerosol) groups.
The potential growth effects of prolonged treatment should be weighed against clinical benefits obtained and the availability of safe and effective noncorticosteroid treatment alternatives. To minimize the systemic effects of nasal corticosteroids, each patient should be titrated to the lowest dose that effectively controls his/her symptoms.
The potential for ZETONNA to cause growth suppression in susceptible patients or when given at higher than recommended dosages cannot be ruled out.
8.5 Geriatric Use
Clinical trials of ZETONNA did not include sufficient numbers of patients age 65 and over to determine whether they responded differently from younger patients. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
10. Overdosage
Chronic overdosage may result in signs or symptoms of hypercorticism [see Warnings and Precautions (5.5)]. There are no data on the effects of acute or chronic overdosage with ZETONNA.
11. Zetonna Description
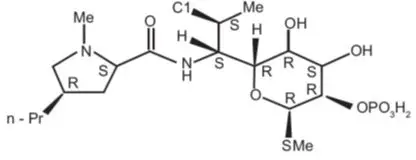
The active component of ZETONNA is ciclesonide, a non-halogenated glucocorticoid having the chemical name pregna -1,4-diene-3,20-dione, 16,17-[[R-cyclohexylmethylene]bis(oxy)]-11-hydroxy-21-(2-methyl-1-oxopropoxy)-,(11ß,16α)-. Ciclesonide is delivered as the R-epimer. The empirical formula is C32H44O7 and its molecular weight is 540.7. Its structural formula is as follows:
Ciclesonide is a white to yellow-white powder. It is soluble in dehydrated alcohol, acetone, dichloromethane, and chloroform.
ZETONNA is comprised of a pressurized, metered-dose aerosol canister and actuator, which is fitted with a dose indicator. ZETONNA is intended for nasal use only. Each canister contains a solution of ciclesonide in propellant HFA-134a (1,1,1,2 tetrafluoroethane) and ethanol. After priming, ZETONNA delivers 50 mcg of ciclesonide from the valve and 37 mcg of ciclesonide from the actuator. This product delivers 50 microliters (59.3 milligrams) of solution as fine particle mist from the valve with each actuation.
12. Zetonna - Clinical Pharmacology
12.1 Mechanism of Action
Ciclesonide is a pro-drug that is enzymatically hydrolyzed to a pharmacologically active metabolite, C21-desisobutyryl-ciclesonide (des-ciclesonide or RM1) following nasal application. Des-ciclesonide has anti-inflammatory activity with affinity for the glucocorticoid receptor that is 120 times higher than the parent compound.
The precise mechanism through which ciclesonide affects allergic rhinitis symptoms is not known. Corticosteroids have been shown to have a wide range of effects on multiple cell types (e.g., mast cells, eosinophils, neutrophils, macrophages, and lymphocytes) and mediators (e.g., histamine, eicosanoids, leukotrienes, and cytokines) involved in allergic inflammation.
12.2 Pharmacodynamics
Adrenal Function: In a 6-week, randomized, double-blind, placebo-controlled, parallel-group trial in adult and pediatric patients aged 12 to 73 years of age with perennial allergic rhinitis, daily doses of 148 mcg and 282 mcg of ZETONNA were compared to placebo. Dexamethasone 6 mg was used as an active control during the last 4 days of the trial. Adrenal function was assessed by 24-hr serum cortisol AUC before and after the treatment. At the end of 6 weeks of treatment, the LS means (SE) change from baseline in serum cortisol AUC(0-24) was ‑5.0 (4.6) mcg•hour/dL, -2.6 (4.6) mcg•hour/dL, and -4.6 (5.0) mcg•hour/dL for placebo (n=57), 148 mcg ZETONNA (n=60), and 282 mcg ZETONNA (n=50), respectively. The LS means difference from placebo for the change from baseline in serum cortisol AUC(0-24) was ‑2.4 mcg•hour/dL (95% CI: -15.1, 10.2) and -0.5 mcg•hour/dL (95% CI: ‑13.9, 13.0) for 148 mcg/day and 282 mcg/day treatments, respectively. The effects observed with the active control (dexamethasone, n=18) validate the sensitivity of the study to assess the effect of ciclesonide on the HPA axis.
In a 6-week, randomized, double-blind, placebo-controlled, parallel-group trial in patients 6 to 11 years of age with perennial allergic rhinitis, a daily dose of 74 mcg of ZETONNA was compared to placebo. Adrenal function was assessed by 24-hr serum cortisol AUC before and after the treatment. At the end of 6 weeks of treatment, the LS means (SE) change from baseline in serum cortisol AUC(0-24) was 5.9 (5.6) mcg•hour/dL and 1.7 (5.2) mcg•hour/dL for placebo and ZETONNA, respectively. The LS means difference from placebo for the change from baseline in serum cortisol AUC(0-24) was 7.6 mcg•hour/dL (95% CI: -7.4, 22.6).
12.3 Pharmacokinetics
Absorption: Ciclesonide and des-ciclesonide have negligible oral bioavailability (both less than 1%) due to low gastrointestinal absorption and high first-pass metabolism. The nasal administration of ciclesonide at recommended doses results in negligible serum concentrations of ciclesonide. However, the known active metabolite (des-ciclesonide) is detected in the serum of some patients after nasal inhalation of ciclesonide. The bioanalytical assay used has a lower limit of quantification of 10 pg/mL, for both ciclesonide and des-ciclesonide, respectively.
The low systemic exposure of des-ciclesonide following ciclesonide administration was confirmed in a crossover trial in 29 healthy adults. The median Cmax of des-ciclesonide was 59 pg/mL following a single dose of ciclesonide (296 mcg) compared to 602 pg/mL following a single dose of orally inhaled ciclesonide (320 mcg) and 12 pg/mL following a single dose of ciclesonide aqueous nasal spray (300 mcg). The pharmacokinetics of nasally administered ciclesonide have been assessed in perennial allergic rhinitis patients resulting in similar exposure compared to healthy subjects.
Distribution: Following intravenous administration of 800 mcg of ciclesonide, the volumes of distribution of ciclesonide and des-ciclesonide were approximately 2.9 L/kg and 12.1 L/kg, respectively. The percentage of ciclesonide and des-ciclesonide bound to human plasma proteins averaged ≥ 99% each, with ≤ 1% of unbound drug detected in the systemic circulation. Des-ciclesonide is not significantly bound to human transcortin.
Elimination: Following intravenous administration of 800 mcg of ciclesonide, the clearance values of ciclesonide and des-ciclesonide were high (approximately 152 L/hr and 228 L/hr, respectively). 14C-ciclesonide was predominantly excreted via the feces after intravenous administration (66%) indicating that excretion through bile is the major route of elimination. Approximately 20% or less of drug related radioactivity was excreted in the urine.
Metabolism: Ciclesonide is hydrolyzed to a biologically active metabolite, des-ciclesonide, by esterases. Des-ciclesonide undergoes further metabolism in the liver to additional metabolites mainly by the cytochrome P450 (CYP) 3A4 isozyme and to a lesser extent by CYP 2D6. The full range of potentially active metabolites of ciclesonide has not been characterized. After intravenous administration of 14C-ciclesonide, 19.3% of the resulting radioactivity in the plasma is accounted for by ciclesonide or des-ciclesonide; the remainder may be a result of other, as yet, unidentified multiple metabolites.
Specific Populations
Patients with Hepatic Impairment: Compared to healthy subjects, the systemic exposure (Cmax and AUC) in patients with liver impairment increased in the range of 1.4 to 2.7-fold after ex-actuator administration of 1280 mcg ciclesonide via oral inhalation. Dose adjustment in liver impairment is not necessary.
Patients with Renal Impairment: Trials in renally-impaired patients were not conducted since renal excretion of des-ciclesonide is a minor route of elimination (≤ 20%).
Drug Interaction Studies: Ciclesonide inhibited human recombinant cytochrome P450 enzymes at high concentration (3 microM) in vitro, but clinically relevant metabolic interactions are not anticipated. Based on in vitro studies in human liver microsomes, ciclesonide and des-ciclesonide appear to have no inhibitory or induction potential on the metabolism of other drugs metabolized by cytochrome P450 enzymes. In vitro studies demonstrated that the plasma protein binding of des-ciclesonide was not affected by warfarin or salicylic acid, indicating no potential for protein binding-based drug interactions.
In a drug interaction study, co-administration of orally inhaled ciclesonide and oral ketoconazole, a strong inhibitor of cytochrome P450 3A4, increased the exposure (AUC) of the active metabolite of ciclesonide, des-ciclesonide, by approximately 3.6-fold at steady state, while levels of ciclesonide remained unchanged.
In another drug interaction study, co-administration of orally inhaled ciclesonide and oral erythromycin, a moderate inhibitor of cytochrome P450 3A4, had no effect on the pharmacokinetics of either des-ciclesonide or erythromycin.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Two-year carcinogenicity studies in B6C3F1 mice and Wistar rats were conducted to assess the carcinogenic potential of ciclesonide. Ciclesonide demonstrated no tumorigenic potential in a study with mice that received oral doses up to 900 mcg/kg/day (approximately 60 times the MRHDID in adult and pediatric patients ≥ 12 years of age on a mcg/m2 basis), and a study with rats that received inhalation doses up to 193 mcg/kg/day (approximately 25 times the MRHDID in adult and pediatric patients ≥ 12 years of age on a mcg/m2 basis).
Ciclesonide was not mutagenic in an Ames test or in the Chinese hamster lung V79 cell/hypoxanthine-guanine phosphoribosyl transferase (HGPRT) forward mutation assay and was not clastogenic in a human lymphocyte chromosomal aberration assay or in an in vitro micronucleus test. However, ciclesonide was clastogenic in an in vivo mouse micronucleus test. The concurrent reference corticosteroid (dexamethasone) in this study showed similar findings.
Fertility and reproductive performance were unaffected in male and female rats dosed by the oral route up to 900 mcg/kg/day (approximately 120 times the MRHDID in adults based on mcg/m2).
14. Clinical Studies
14.1 Seasonal and Perennial Allergic Rhinitis
Adults and Pediatric Patients 12 Years of Age and Older:
The efficacy of ZETONNA was evaluated in one randomized, double-blind, parallel-group, multicenter, placebo-controlled dose-ranging trial (74 mcg, 148 mcg, and 282 mcg once daily) and 3 confirmatory trials (74 mcg and 148 mcg once daily) in adult and pediatric patients 12 years and older with allergic rhinitis. Efficacy endpoints were evaluated at 2 weeks for the two seasonal allergic rhinitis trials and at 6 weeks for the perennial allergic rhinitis trial. These trials were all conducted in the United States. A total of 3001 patients were included in these 4 trials. The dose-ranging trial included a total of 513 patients [193 males (37.6%) and 320 females (62.4%)], of whom 65 (12.7%) were pediatric patients 12 years and older. The three confirmatory trials included a total of 2488 patients (905 males and 1583 females) of whom 170 were pediatric patients, ages 12 to 18 years. Patients enrolled in the trials were 12 to 81 years of age with a history of seasonal or perennial allergic rhinitis, a positive skin test to at least one relevant allergen, and active symptoms of allergic rhinitis at study entry. Assessment of efficacy in these trials was based on patient recording of four nasal symptoms (runny nose, nasal itching, sneezing, and nasal congestion) on a 0 to 3 categorical severity scale (0 = absent, 1 = mild, 2 = moderate, and 3 = severe) as reflective or instantaneous total nasal symptom scores (rTNSS and iTNSS respectively). Reflective scoring required the patients to record symptom severity over the previous 12 hours; the instantaneous scoring required patients to record symptom severity over the previous 10 minutes.
Additional secondary efficacy variables were assessed, including the total ocular symptom score (TOSS) in the seasonal allergic rhinitis trials and the Rhinoconjunctivitis Quality of Life Questionnaire with Standardised Activities [RQLQ(S)] in both seasonal and perennial allergic rhinitis trials. TOSS is calculated as the sum of the patients’ scoring of the three individual ocular symptoms (itching, tearing, and redness) on a 0 to 3 categorical severity scale (0 = absent, 1 = mild, 2 = moderate, 3 = severe) as reflective (rTOSS) or instantaneous (iTOSS) scores. To assess efficacy, rTOSS and iTOSS were evaluated as described above for the TNSS. Patients perceptions of disease specific quality of life were evaluated through the use of the RQLQ(S), which assesses the impact of allergic rhinitis symptoms and treatment through 28 items in 7 domains (activities, sleep, non-nose/eye symptoms, practical problems, nasal symptoms, eye symptoms, and emotional) on a 7-point scale where 0 = not troubled and 6 = extremely troubled. An overall RQLQ(S) score is calculated from the average of the domain scores. An absolute difference of ≥ 0.5 in mean change from baseline over placebo is considered the minimally clinically important difference (MCID) for the RQLQ(S).
Dose-Ranging Trial: There was a 2-week placebo-controlled, double-blind, dose-ranging trial that evaluated efficacy of three doses of ZETONNA (74 mcg, 148 mcg, and 282 mcg once daily) in patients with seasonal allergic rhinitis. The primary efficacy endpoint was the difference from placebo in the change from baseline of the average of morning and evening reflective total nasal symptom score (rTNSS) averaged over the 2-week treatment period. The rTNSS showed a statistically significant estimated treatment difference from placebo of 0.81 (95% CI: 0.32, 1.29); 0.90 (95% CI: 0.40, 1.39); and 0.66 (95% CI: 0.16, 1.16) for 282 mcg, 148 mcg and 74 mcg, respectively.
Confirmatory Seasonal Allergic Rhinitis Trials: There were two 2-week placebo-controlled, double-blind confirmatory trials that evaluated efficacy of two doses of ZETONNA (74 mcg and 148 mcg once daily) in patients with seasonal allergic rhinitis. The primary efficacy endpoint was the difference from placebo in the change from baseline of the average of morning and evening rTNSS averaged over the 2-week treatment period. Table 2 displays the efficacy results from one of these trials in patients with seasonal allergic rhinitis. The other trial showed similar results. In these trials, ZETONNA 74 mcg once daily was statistically significantly different from placebo. Statistically significant differences in the morning pre-dose iTNSS indicate that the effect was maintained over the full 24-hour dosing interval. ZETONNA 74 mcg demonstrated a statistically significant decrease from baseline in the rTOSS compared to placebo. Similarly, a clinically significant decrease (≥ 0.5) from baseline compared to placebo for the RQLQ(S) was also shown. ZETONNA 148 mcg once daily did not provide an efficacy benefit over the 74 mcg once daily dose.
Confirmatory Perennial Allergic Rhinitis Trial: There was one 26-week placebo-controlled, double-blind trial that evaluated efficacy of two doses of ZETONNA (74 mcg and 148 mcg once daily) in patients with perennial allergic rhinitis. The primary efficacy endpoint was the difference from placebo in the change from baseline of the average of morning and evening rTNSS averaged over the first 6 weeks of treatment. In this trial, ZETONNA 74 mcg once daily was statistically significantly different from placebo (Table 2) in decreasing nasal symptom scores. Statistically significant differences in the morning pre-dose instantaneous total nasal symptom score indicate that the effect was maintained over the full 24‑hour dosing interval. ZETONNA 74 mcg did not demonstrate a clinically significant change from baseline in the overall RQLQ(S) compared to placebo. TOSS was not evaluated in this trial. ZETONNA 148 mcg once daily did not provide an efficacy benefit over the 74 mcg once daily dose.
| Treatment | N | Mean Baselinea | LS Mean Change from Baseline | Difference from Placebob | ||
|---|---|---|---|---|---|---|
| Estimate
(LS Mean) | 95% CI | p-valuec | ||||
| a Baseline for rTNSS, iTNSS, and rTOSS are averages of the AM and PM responses obtained during the Run-in Period up to 6 days prior to randomization and includes AM score prior to randomization. Baseline for morning iTNSS is the average of the AM responses obtained during the Run-in Period up to 6 days prior to randomization and includes the AM score prior to randomization. Baseline RQLQ(S) is from the randomization visit assessment. b Estimates (LS Mean), 95% Confidence Intervals, and p-values were obtained from ANCOVA analyses with treatment and center as fixed effects and baseline as covariate in the model. c P-values are significant at the 0.025 level based on Bonferroni correction. |
||||||
|
Seasonal Allergic Rhinitis |
||||||
|
Reflective Total Nasal Symptom Score |
||||||
|
Ciclesonide 74 mcg |
237 |
9.3 |
-1.5 |
0.9 |
0.6, 1.3 |
<0.001 |
|
Placebo |
235 |
9.1 |
-0.5 | |||
|
Instantaneous Total Nasal Symptom Score |
||||||
|
Ciclesonide 74 mcg |
237 |
8.7 |
-1.3 |
0.9 |
0.5, 1.3 |
<0.001 |
|
Placebo |
235 |
8.6 |
-0.5 | |||
|
Reflective Total Ocular Symptom Score |
||||||
|
Ciclesonide 74 mcg |
237 |
5.8 |
-0.8 |
0.5 |
0.3, 0.8 |
0.001 |
|
Placebo |
235 |
5.7 |
-0.2 | |||
|
Rhinoconjunctivitis Quality of Life Questionnaire with Standardized Activities |
||||||
|
Ciclesonide 74 mcg |
237 |
4.0 |
-0.8 |
0.6 |
0.4, 0.8 |
<0.001 |
|
Placebo |
234 |
4.0 |
-0.2 | |||
|
Perennial Allergic Rhinitis |
||||||
|
Reflective Total Nasal Symptom Score |
||||||
|
Ciclesonide 74 mcg |
298 |
8.5 |
-2.0 |
0.7 |
0.4, 1.0 |
<0.001 |
|
Placebo |
307 |
8.6 |
-1.3 | |||
|
Instantaneous Total Nasal Symptom Score |
||||||
|
Ciclesonide 74 mcg |
298 |
7.7 |
-1.8 |
0.6 |
0.3, 0.9 |
<0.001 |
|
Placebo |
307 |
7.7 |
-1.2 | |||
Onset of Action: Onset of action was evaluated in both 2-week seasonal and one 6-week perennial allergic rhinitis trials by frequent recording of instantaneous symptom score. In these trials, onset of effect was seen after 36 hours following the first dose. Maximum benefit is usually achieved within 1 to 2 weeks after initiation of dosing.
16. How is Zetonna supplied
ZETONNA (ciclesonide) 37 mcg nasal aerosol is supplied as a pressurized aluminum canister with a purple and white plastic actuator integrated with a dose indicator and a cap. Each actuation delivers 37 mcg of ciclesonide from the nasal actuator. The contents of one 6.1 gram canister provide 60 actuations, after initial priming.
Prior to initial use, or when not used for ten consecutive days, ZETONNA must be primed by actuating three times. If ZETONNA is dropped, the canister and actuator may become separated. If this happens, ZETONNA should be reassembled and actuated once into the air to test before using. The actuator and canister should be discarded after reaching zero in the indicator window since the amount of ciclesonide delivered per spray thereafter may be substantially less than the labeled dose.
The ZETONNA canister should only be used with the ZETONNA actuator. The actuator is fitted with a dose indicator and should not be used with other inhalation aerosol medications. The correct amount of medication in each inhalation cannot be ensured after the labeled number of actuations from the canister has been used, even though the inhaler may not feel completely empty and may continue to operate. Illustrated patient’s instructions for proper use accompany each package of ZETONNA.
Store at 25°C (77°F); excursions permitted to 15°C - 30°C (59°F - 86°F) [See USP Controlled Room Temp]. For optimal results, canister should be at room temperature when used.
Contents under pressure. Do not puncture. Do not use or store near heat or open flame. Exposure to temperatures above 49°C (120°F) may cause bursting. Never throw canister into fire or incinerator.
ZETONNA 37 mcg, 60 metered actuations; net fill weight 6.1 g. NDC Number 70515-737-60
17. Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
Local Nasal Adverse Reactions
Inform patients that treatment with ZETONNA may lead to adverse reactions, which include nasal septal perforation, epistaxis, and nasal ulceration. In addition, ciclesonide is associated with candidal infection, and nasal corticosteroids are associated with impaired wound healing. Do not spray ZETONNA directly onto the nasal septum. Patients who have experienced recent nasal septal perforation, nasal erosion, nasal ulcers, nasal surgery, or nasal trauma should not use ZETONNA until healing has occurred [see Warnings and Precautions (5.1)].
Glaucoma and Cataracts
Inform patients that glaucoma and cataracts are associated with nasal and inhaled corticosteroid use. Instruct patients to inform his/her health care provider if a change in vision is noted while using ZETONNA [see Warnings and Precautions (5.2)].
Immunosuppression and Risk of Infections
Warn patients who are on immunosuppressive doses of corticosteroids to avoid exposure to chickenpox or measles, and if exposed, to consult their physician without delay. Inform patients of potential worsening of existing tuberculosis, fungal, bacterial, viral or parasitic infections, or ocular herpes simplex [see Warnings and Precautions (5.4)].
Use Daily
Instruct patients to use ZETONNA on a regular, once daily basis since its effectiveness depends on its regular use. In clinical trials, the onset of effect was seen after 36 hours following the first dose. Maximum benefit is usually achieved within 1 to 2 weeks after initiation of dosing. Initial assessment of response should be made during this timeframe and periodically until the patient’s symptoms are stabilized. Instruct the patient to take the medication as directed, not exceed the prescribed dosage, and contact the physician if symptoms do not improve by a reasonable time or if the condition worsens.
Keep Spray Out of Eyes and Off Nasal Septum
Instruct patients to avoid spraying ZETONNA in their eyes or directly on the nasal septum.
Storage and Handling
Instruct patients to use the ZETONNA canister only with the ZETONNA actuator supplied with the product. The dose indicator display window will show a red zone when it is about time to replace the ZETONNA. Replace ZETONNA when the indicator shows zero.
Manufactured for:
Covis Pharma
Zug, 6300 Switzerland
Made the United Kingdom
© 2023 Covis Pharma. All rights reserved.
Patient Information
ZETONNA®<<Ze toe’ nah>>
(ciclesonide)
Note: For Use in the Nose Only.
- •
- Do not spray ZETONNA in your eyes or directly onto your nasal septum (the wall between the 2 nostrils).
- •
- Do not use your ZETONNA near heat or an open flame.
Read this Patient Information leaflet before you start using ZETONNA and each time you get a refill. There may be new information. This information does not take the place of talking with your healthcare provider about your medical condition or your treatment. If you have any questions about ZETONNA, ask your healthcare provider or pharmacist.
What is ZETONNA?
ZETONNA is a prescription medicine that treats seasonal and year-round allergy symptoms in adults and children 12 years of age and older.
ZETONNA contains ciclesonide, which is a man-made (synthetic) corticosteroid. Corticosteroids are natural substances found in the body and reduce inflammation. When you spray ZETONNA into your nose, it may help reduce nasal symptoms of allergic rhinitis (inflammation of the lining of the nose) such as stuffy nose, runny nose, itching and sneezing. ZETONNA may also help you if you have red, itchy, and watery eyes.
It is not known if ZETONNA is safe and effective in children 11 years of age and younger.
Who should not use ZETONNA?
Do not use ZETONNA if you are allergic to ciclesonide or any of the ingredients in ZETONNA. See the end of this Patient Information leaflet for a complete list of ingredients in ZETONNA.
What should I tell my healthcare provider before using ZETONNA?
Before you use ZETONNA tell your healthcare provider if you:
- •
- have had recent nose problems such as a hole in the cartilage of your nose, nasal ulcers, nasal surgery, or nasal injury.
- •
- have or have had eye problems such as increased intraocular pressure, glaucoma, or cataracts.
- •
- have any infections including tuberculosis or ocular herpes simplex.
- •
- have not had or been vaccinated for chicken pox or measles.
- •
- are pregnant or plan to become pregnant. It is not known if ZETONNA will harm your unborn baby. Talk to your healthcare provider about the best way to feed your baby if you are using ZETONNA.
- •
- are breastfeeding or plan to breastfeed. It is not known if ZETONNA passes into your breast milk. Talk to your healthcare provider about the best way to feed your baby if you are using ZETONNA.
Tell your healthcare provider about all the medicines you take, including prescription and non-prescription medicines, vitamins, and herbal supplements.
Know the medicines you take. Keep a list of them to show your doctors and pharmacist when you get a new medicine.
How should I use ZETONNA?
- •
- Read the Instructions for Use at the end of this leaflet for specific information about the right way to use ZETONNA.
- •
- Use ZETONNA exactly as your healthcare provider tells you to use it. Do not take more of your medicine or take it more often than your healthcare provider tells you.
- •
- ZETONNA is used 1 time each day, 1 spray in each nostril. Do not use more than a total of 1 spray in each nostril per day.
- •
- ZETONNA may begin to work within 36 hours after you take your first dose. Maximum benefit is usually achieved within 1 to 2 weeks after initiation of dosing.
- •
- If your symptoms do not improve or get worse, call your healthcare provider.
What are the possible side effects of ZETONNA?
ZETONNA may cause serious side effects, including:
- •
- nose bleeds and nasal ulcers. Call your healthcare provider right away if you start to have more nose bleeds or nasal ulcers.
- •
-
hole in the cartilage in the nose (nasal septal perforation). Stop using ZETONNA and call your doctor right away if you have symptoms of a nasal perforation. Symptoms of nasal perforation may include:
- •
- crusting in the nose
- •
- nosebleeds
- •
- runny nose
- •
- whistling sound when you breathe
- •
- thrush (Candida), a fungal infection in your nose, mouth, or throat. Tell your healthcare provider if you have any redness or white colored patches in your mouth or throat.
- •
- slow wound healing. You should not use ZETONNA until your nose has healed, if you have a sore in your nose, if you have had surgery in your nose, or if your nose has been injured.
- •
- eye problems such as glaucoma and cataracts. If you have a history of glaucoma or cataracts or have a family history of eye problems, you should have regular eye exams while you use ZETONNA.
- •
-
immune system problems that may increase your risk of infections. You are more likely to get infections if you take medicines that may weaken your body’s ability to fight infections. Avoid contact with people who have contagious diseases such as chicken pox or measles while you use ZETONNA. Symptoms of an infection may include:
- •
- fever
- •
- pain
- •
- aches
- •
- chills
- •
- feeling tired
- •
- nausea
- •
- vomiting
- •
-
adrenal insufficiency. Adrenal insufficiency is a condition in which the adrenal glands do not make enough steroid hormones. Call your healthcare provider right away if you experience the following symptoms of adrenal insufficiency:
- •
- tiredness
- •
- weakness
- •
- dizziness
- •
- nausea
- •
- vomiting
- •
- slowed or delayed growth in children. A child’s growth should be checked regularly while using ZETONNA.
- •
- allergic reactions. Call your healthcare provider right away if you experience swelling of the lips, tongue, or throat.
The most common side effects with ZETONNA include:
- •
- Nasal discomfort
- •
- Headache
- •
- Nose bleeds
Tell your doctor if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of ZETONNA.
For more information, ask your doctor or pharmacist.
Call your healthcare provider for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store ZETONNA?
- •
- Store ZETONNA at room temperature between 59°F and 86°F (15°C to 30°C).
- •
- Do not puncture the ZETONNA canister.
- •
- Do not store the ZETONNA canister near heat or a flame. Temperatures above 120°F (49°C) may cause the canister to burst.
- •
- Do not throw the ZETONNA canister into a fire or an incinerator.
- •
- Safely throw away medicine that is out of date or no longer needed.
- •
- Keep ZETONNA clean and dry at all times.
Keep ZETONNA and all medicines out of the reach of children.
General Information About the Safe and Effective Use of ZETONNA
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use ZETONNA for a condition for which it was not prescribed. Do not give ZETONNA to other people, even if they have the same symptoms that you have. It may harm them.
This Patient Information summarizes the most important information about ZETONNA. If you would like more information, talk with your healthcare provider. You can ask your pharmacist or healthcare provider for information about ZETONNA that is written for health professionals. You may want to read this leaflet again. Please DO NOT THROW IT AWAY until you have finished your medicine.
What are the ingredients in ZETONNA?
Active ingredient: ciclesonide
Inactive ingredients: HFA propellant and ethanol
Instructions for Use
ZETONNA® <<Ze toe’ nah>>
(ciclesonide)
Read these Instructions for Use for ZETONNA before you start using it and each time you get a refill. There may be new information. This leaflet does not take the place of talking to your doctor about your medical condition or treatment.
Note: For Use in the Nose Only.
- •
- Do not spray ZETONNA in your eyes or directly onto your nasal septum (the wall between your 2 nostrils).
- •
- Do not use your ZETONNA near heat or an open flame.
The Parts of Your ZETONNA
ZETONNA comes as a canister fitted into a nasal actuator with a dose indicator. Do not use the actuator with a canister of medicine from any other inhaler. Do not use the ZETONNA canister with an actuator from any other inhaler. (See Figure A)
Priming Your ZETONNA For Use
- •
- Remove ZETONNA from its package.
- •
- Before you use ZETONNA for the first time or if you have not used your medicine for 10 days in a row, you will need to prime your ZETONNA.
- •
- Open the purple plastic dust cap by gently squeezing both sides and pulling the cap away from the nasal actuator. Hold the nasal actuator upright. (See Figure B)
- •
- Spray 3 times into the air away from the face, by pressing down fully on the top of the canister three times (See Figure C). Make sure the canister returns to its original position after each spray.
Using Your ZETONNA
- Step 1. Open the purple plastic dust cap.
- Step 2. Hold the nasal actuator upright, with the nose piece pointing upwards, between your thumb and forefinger (and middle finger) (See Figure D ).
- Step 3. Tilt your head back slightly and insert the end of the nose piece into 1 nostril, pointing it slightly toward the outside nostril wall away from the nasal septum (the wall between the 2 nostrils), while holding your other nostril closed with 1 finger (See Figure E ). Do not get any spray in your eyes or directly on your nasal septum.
- Step 4. Press down on the canister to release 1 spray and at the same time breathe in gently through the nostril. Hold your breath for a few seconds then breathe out slowly through your mouth.
- Step 5. Remove the nose piece from your nostril. Make sure the canister has returned to its original position and repeat steps 2-4 for the second spray in your other nostril.
- Step 6. Replace the protective purple dust cap on the nasal actuator.
- Step 7. Avoid blowing your nose for the next 15 minutes.
Cleaning Your Nasal Actuator
The outside of the nose piece should be cleaned weekly, by wiping with a clean, dry tissue or cloth (see Figure F).
Do not wash or put any part of the ZETONNA canister or actuator in water.
How to Tell if Your ZETONNA Is Empty
- •
- Each canister of ZETONNA contains enough medicine for you to spray medicine 60 times (or 30 times for sample size product). This does not count the first 3 priming sprays.
- •
- The actuator of your ZETONNA is fitted with a dose indicator which shows you how much medicine is left after each use. The dose indicator will display the number of sprays remaining in groups of 5 or 10 actuations.
- •
- The display window will begin showing a green color. As you continue to use the medicine, the window will show a yellow color. A yellow color in the window means that you need to replace your medicine soon. When the medicine is almost empty, the window will show a red color.
- •
- When the window shows red and you see the dose indicator read zero, “0” (see Figure G), you should throw away the canister and nasal actuator.
- •
- Do not throw your ZETONNA canister in the fire or an incinerator.
- •
- Do not use your ZETONNA after zero is shown in the window of the dose indicator even though it may look like there is medicine left in the canister. You may not get the right amount of medicine.
- •
- Talk with your healthcare provider before your supply of ZETONNA runs out to see if you should get a refill of your medicine.
What to Do if You Drop Your ZETONNA
- •
- If you drop your ZETONNA, the canister may become separated from the actuator. If this happens, insert the canister into the actuator as shown in Figure H, test spray once into the air away from your face, then use as described above.
- •
- If the ZETONNA is dropped, the dose counter may not work. It is recommended to keep track of the number of sprays taken from your ZETONNA based on your records.
This Patient Information and Instructions for Use have been approved by the U.S. Food and Drug Administration.
Manufactured for:
Covis Pharma
Zug, 6300 Switzerland
Made in the United Kingdom
© 2023 Covis Pharma. All rights reserved.
PRINCIPAL DISPLAY PANEL - Carton Label
NDC 70515-737-60
Net Contents: 6.1 g
Zetonna™
(ciclesonide) Nasal Aerosol
37 mcg per actuation
For Intranasal Use Only.
Zetonna™ Nasal Aerosol Canister
Should Be Used with Zetonna™
Nasal Aerosol Actuator Only.
60 Metered Actuations
Rx Only
COVIS
Each 6.1 g canister of Zetonna™
Nasal Aerosol contains a solution of
ciclesonide in propellant HFA-134a
(1,1,1,2 tetrafluoroethane) and
ethanol. The 37 mcg strength of
Zetonna™ Nasal Aerosol delivers
37 mcg of ciclesonide from the
actuator.
Usual Dosage:
See Prescribing Information.
Avoid spraying in eyes.
CONTENTS UNDER PRESSURE.
Do not puncture. Do not use or store
near heat or open flame. Exposure
to temperatures above 49°C (120°F)
may cause bursting. Never throw
canister into fire or incinerator.
Store at 25°C (77°F); excursions
between 15°–30°C (59°–86°F)
are permitted [see USP]. For optimal
results, canister should be at room
temperature when used.
Pharmacist: Dispense with Patient’s
Instructions for Use from package
insert.
Keep out of reach of children.
Manufactured for
Covis Pharma
Zug, 6300 Switzerland
Made in the United Kingdom
© 2020 Covis Pharma All rights reserved.
Zetonna™
(ciclesonide) Nasal Aerosol
37 mcg per actuation
60 Metered Actuations
GTIN: XXXXXXXXXXXXXX
SN: XXXXXXXXXXXXXX
EXP: MM-YYYY
LOT: XXXXXXX
| ZETONNA
ciclesonide aerosol, metered |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Covis Pharma US, Inc (118094829) |