Drug Detail:Zydelig (Idelalisib [ eye-del-a-lis-ib ])
Drug Class: PI3K inhibitors
Highlights of Prescribing Information
ZYDELIG® (idelalisib) tablets, for oral use
Initial U.S. Approval: 2014
WARNING: FATAL AND SERIOUS TOXICITIES: HEPATIC, SEVERE DIARRHEA, COLITIS, PNEUMONITIS, INFECTIONS, and INTESTINAL PERFORATION
See full prescribing information for complete boxed warning.
- Fatal and/or serious hepatotoxicity occurred in 16% of Zydelig-treated patients. Monitor hepatic function prior to and during treatment. Interrupt and then reduce or discontinue Zydelig. (5.1)
- Fatal and/or serious and severe diarrhea or colitis occurred in 20% of Zydelig-treated patients. Monitor for the development of severe diarrhea or colitis. Interrupt and then reduce or discontinue Zydelig. (5.2)
- Fatal and/or serious pneumonitis occurred in 4% of Zydelig-treated patients. Monitor for pulmonary symptoms and bilateral interstitial infiltrates. Interrupt or discontinue Zydelig. (5.3)
- Fatal and/or serious infections occurred in 48% of Zydelig-treated patients. Monitor for signs and symptoms of infection. Interrupt Zydelig if infection is suspected. (5.4)
- Fatal and serious intestinal perforation can occur in Zydelig-treated patients across clinical trials. Discontinue Zydelig if intestinal perforation is suspected. (5.5)
Recent Major Changes
| Indications and Usage, Relapsed Follicular B-cell non-Hodgkin Lymphoma – Accelerated Approval Indication Removed (1) 2/2022 |
| Indications and Usage, Relapsed Small Lymphocytic Lymphoma – Accelerated Approval Indication Removed (1) 2/2022 |
Indications and Usage for Zydelig
Zydelig is a kinase inhibitor indicated for the treatment of patients with:
- Relapsed chronic lymphocytic leukemia (CLL), in combination with rituximab, in patients for whom rituximab alone would be considered appropriate therapy due to other co-morbidities. (1)
Limitations of use:
Zydelig is not indicated and is not recommended for first-line treatment of any patient, including patients with CLL, small lymphocytic lymphoma (SLL), follicular lymphoma (FL), and other indolent non-Hodgkin lymphomas. (1, 6.1)
Zydelig is not indicated and is not recommended in combination with bendamustine and rituximab, or in combination with rituximab for the treatment of patients with FL, SLL, and other indolent non-Hodgkin lymphomas. (6.1)
Zydelig Dosage and Administration
Recommended dosage: 150 mg orally twice daily. (2.1)
Dosage Forms and Strengths
Tablets: 100 mg, 150 mg. (3)
Contraindications
History of serious hypersensitivity reactions to idelalisib, including anaphylaxis, or history of toxic epidermal necrolysis with any drug. (4)
Warnings and Precautions
- Severe Cutaneous Reactions: Monitor patients for the development of severe cutaneous reactions. Permanently discontinue Zydelig if confirmed. (2.2, 5.6)
- Hypersensitivity Reactions: Permanently discontinue Zydelig and institute appropriate supportive measures. (2.2, 5.7)
- Neutropenia: Monitor blood counts. Interrupt Zydelig until resolution and resume at reduced dose. (2.2, 5.8)
- Embryo-fetal Toxicity: May cause fetal harm. Advise females of reproductive potential of potential risk to a fetus and to use effective contraception. (5.9, 8.1, 8.3)
Adverse Reactions/Side Effects
- The most common adverse reactions (incidence ≥30%) in patients treated with Zydelig in combination trials are diarrhea, pneumonia, pyrexia, fatigue, rash, cough, and nausea. (6.1)
- Common laboratory abnormalities are neutropenia, ALT elevations and AST elevations. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Gilead Sciences, Inc. at 1-800-GILEAD-5 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
- Strong CYP3A Inhibitors: Additional monitoring required if alternative therapy is not available. (7.1)
- Strong CYP3A Inducers: Avoid coadministration of strong CYP3A inducers. (7.1)
- CYP3A Substrates: Avoid coadministration of sensitive CYP3A substrates. (7.2)
Use In Specific Populations
Lactation: Advise not to breastfeed. (8.2)
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 2/2022
Full Prescribing Information
WARNING: FATAL AND SERIOUS TOXICITIES: HEPATIC, SEVERE DIARRHEA, COLITIS, PNEUMONITIS, INFECTIONS, and INTESTINAL PERFORATION
Fatal and/or serious hepatotoxicity occurred in 16% of Zydelig-treated patients. Monitor hepatic function prior to and during treatment. Interrupt and then reduce or discontinue Zydelig as recommended [see Dosage and Administration (2.2), Warnings and Precautions (5.1)].
Fatal and/or serious and severe diarrhea or colitis occurred in 20% of Zydelig-treated patients. Monitor for the development of severe diarrhea or colitis. Interrupt and then reduce or discontinue Zydelig as recommended [see Dosage and Administration (2.2), Warnings and Precautions (5.2)].
Fatal and/or serious pneumonitis occurred in 4% of Zydelig-treated patients. Monitor for pulmonary symptoms and bilateral interstitial infiltrates. Interrupt or discontinue Zydelig as recommended [see Dosage and Administration (2.2), Warnings and Precautions (5.3)].
Fatal and/or serious infections occurred in 48% of Zydelig-treated patients. Monitor for signs and symptoms of infection. Interrupt Zydelig if infection is suspected [see Dosage and Administration (2.2), Warnings and Precautions (5.4)].
Fatal and serious intestinal perforation can occur in Zydelig-treated patients across clinical trials. Discontinue Zydelig for intestinal perforation [see Warnings and Precautions (5.5)].
1. Indications and Usage for Zydelig
Zydelig is indicated, in combination with rituximab, for the treatment of patients with relapsed chronic lymphocytic leukemia (CLL) for whom rituximab alone would be considered appropriate therapy due to other co-morbidities.
2. Zydelig Dosage and Administration
2.1 Recommended Dosage
The recommended dosage of Zydelig is 150 mg administered orally twice daily with or without food until disease progression or unacceptable toxicity. The optimal and safe dosing regimen for patients who receive treatment longer than several months is unknown.
Swallow tablets whole.
If a planned dose of Zydelig is missed by less than 6 hours, take the missed dose as soon as possible and take the next dose as usual. If a dose of Zydelig is missed by more than 6 hours, skip the missed dose and take the next dose at the usual time.
2.2 Dosage Modifications for Adverse Reactions
Table 1 presents the dosage modification for specific adverse reactions.
For other severe or life-threatening adverse reactions, withhold Zydelig until resolution. If resuming Zydelig after interruption for other severe or life-threatening toxicities, reduce the dosage to 100 mg orally twice daily. Permanently discontinue Zydelig for recurrence of other severe or life-threatening Zydelig-related toxicity upon rechallenge.
| Abbreviations: ANC: absolute neutrophil count; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BID, twice daily; ULN, upper limit of normal; CMV, cytomegalovirus; DRESS, drug reaction with eosinophilia and systemic symptoms; PCR: polymerase chain reaction; PJP: Pneumocystis jirovecii pneumonia; SJS: Stevens-Johnson syndrome; TEN: toxic epidermal necrolysis | |||
|
|||
| ALT/AST | >3–5 × ULN | >5–20 × ULN | >20 × ULN |
| [see Warnings and Precautions (5.1)] | Maintain Zydelig dose. Monitor at least weekly until ≤1 × ULN. | Withhold Zydelig. Monitor at least weekly until ALT/AST are ≤1 × ULN, then may resume Zydelig at 100 mg BID. | Discontinue Zydelig permanently. |
| Bilirubin | >1.5–3 × ULN | >3–10 × ULN | >10 × ULN |
| [see Warnings and Precautions (5.1)] | Maintain Zydelig dose. Monitor at least weekly until ≤1 × ULN. | Withhold Zydelig. Monitor at least weekly until bilirubin is ≤1 × ULN, then may resume Zydelig at 100 mg BID. | Discontinue Zydelig permanently. |
| Diarrhea* | Moderate diarrhea | Severe diarrhea or hospitalization | Life-threatening diarrhea |
| [see Warnings and Precautions (5.2)] | Maintain Zydelig dose. Monitor at least weekly until resolved. | Withhold Zydelig. Monitor at least weekly until resolved, then may resume Zydelig at 100 mg BID. | Discontinue Zydelig permanently. |
| Pneumonitis | Any symptomatic pneumonitis | ||
| [see Warnings and Precautions (5.3)] | Discontinue Zydelig in patients with any severity of symptomatic pneumonitis. | ||
| Infections | Grade 3 or higher sepsis or pneumonia | ||
| [see Warnings and Precautions (5.4)] | Interrupt Zydelig until infection has resolved. | ||
| Evidence of CMV infection or viremia | |||
| Interrupt Zydelig in patients with evidence of active CMV infection of any grade or viremia (positive PCR or antigen test) until the viremia has resolved. If Zydelig is resumed, monitor patients by PCR or antigen test for CMV reactivation at least monthly. | |||
| Evidence of PJP infection | |||
| Interrupt Zydelig in patients with suspected PJP infection of any grade. Permanently discontinue Zydelig if PJP infection is confirmed. |
|||
| Intestinal Perforation | Evidence of intestinal perforation | ||
| [see Warnings and Precautions (5.5)] | Permanently discontinue Zydelig in patients who experience intestinal perforation. | ||
| Severe Cutaneous Reactions | Suspected/Confirmed SJS, TEN, DRESS, or other severe or life-threatening (Grade ≥3) cutaneous reactions | ||
| [see Warnings and Precautions (5.6)] | Interrupt Zydelig in patients with suspected SJS, TEN, or DRESS until the etiology of the reaction has been determined. Permanently discontinue Zydelig in patients with confirmed SJS, TEN, or DRESS, or other severe or life-threatening (Grade ≥3) cutaneous reactions. |
||
| Hypersensitivity Reactions | Evidence of hypersensitivity reactions | ||
| [see Warnings and Precautions (5.7)] | Permanently discontinue Zydelig in patients who develop serious hypersensitivity reactions. | ||
| Neutropenia | ANC 1.0 to <1.5 Gi/L | ANC 0.5 to <1.0 Gi/L | ANC <0.5 Gi/L |
| [see Warnings and Precautions (5.8)] | Maintain Zydelig dose. | Maintain Zydelig dose. Monitor ANC at least weekly. | Interrupt Zydelig. Monitor ANC at least weekly until ANC ≥0.5 Gi/L, then may resume Zydelig at 100 mg BID. |
| Thrombocytopenia | Platelets 50 to <75 Gi/L | Platelets 25 to <50 Gi/L | Platelets <25 Gi/L |
| [see Adverse Reactions (6.1)] | Maintain Zydelig dose. | Maintain Zydelig dose. Monitor platelet counts at least weekly. | Interrupt Zydelig. Monitor platelet count at least weekly. May resume Zydelig at 100 mg BID when platelets ≥25 Gi/L. |
No dosage modification is recommended for lymphocytosis, which has been observed in some patients taking Zydelig. This observed lymphocytosis is a pharmacodynamic effect and should not be considered progressive disease in the absence of other clinical findings.
3. Dosage Forms and Strengths
Tablets:
- 100 mg: orange, oval-shaped, film-coated tablet debossed with "GSI" on one side and "100" on the other side.
- 150 mg: pink, oval-shaped, film-coated tablet debossed with "GSI" on one side and "150" on the other side.
4. Contraindications
Zydelig is contraindicated in patients with a history of serious hypersensitivity reactions to idelalisib, including anaphylaxis, or patients with a history of toxic epidermal necrolysis with any drug [see Warnings and Precautions (5.6, 5.7)] .
5. Warnings and Precautions
5.1 Hepatotoxicity
Fatal and/or serious hepatotoxicity occurred in 16% of patients treated with Zydelig in combination with rituximab or with unapproved combination therapies. Elevations in ALT or AST greater than 5 times the upper limit of normal have occurred [see Adverse Reactions (6.1)]. These findings were generally observed within the first 12 weeks of treatment and were reversible with dose interruption. After resumption of treatment at a lower dose, 26% of patients had recurrence of ALT and AST elevations. Discontinue Zydelig for recurrent hepatotoxicity.
Avoid concurrent use of Zydelig with other drugs that may cause liver toxicity.
Monitor ALT and AST in all patients every 2 weeks for the first 3 months of treatment, every 4 weeks for the next 3 months, then every 1 to 3 months thereafter. Monitor weekly for liver toxicity if the ALT or AST rises above 3 times the upper limit of normal until resolved. Withhold Zydelig if the ALT or AST is greater than 5 times the upper limit of normal, and continue to monitor AST, ALT and total bilirubin weekly until the abnormality is resolved [see Dosage and Administration (2.2)].
5.2 Severe Diarrhea or Colitis
Severe diarrhea or colitis (Grade 3 or higher) occurred in 20% of patients treated with Zydelig in combination with rituximab or with unapproved combination therapies [see Adverse Reactions (6.1)]. Diarrhea can occur at any time. Avoid concurrent use of Zydelig and other drugs that cause diarrhea. Diarrhea due to Zydelig responds poorly to antimotility agents. Median time to resolution ranged between 1 week and 1 month across trials, following interruption of Zydelig therapy and in some instances, use of corticosteroids [see Dosage and Administration (2.2)].
5.3 Pneumonitis
Fatal and serious pneumonitis occurred in patients treated with Zydelig [see Adverse Reactions (6.1)]. Clinical manifestations included interstitial infiltrates and organizing pneumonia. In randomized clinical trials of combination therapies, pneumonitis occurred in 4% of patients treated with Zydelig compared to 1% on the comparator arms. Time to onset of pneumonitis ranged from <1 to 15 months. Monitor patients on Zydelig for pulmonary symptoms. In patients taking Zydelig who present with pulmonary symptoms such as cough, dyspnea, hypoxia, interstitial infiltrates on a radiologic exam, or a decline by more than 5% in oxygen saturation, interrupt Zydelig until the etiology has been determined.
If symptomatic pneumonitis or organizing pneumonia is diagnosed, initiate appropriate treatment with corticosteroids and permanently discontinue Zydelig [see Dosage and Administration (2.2)].
5.4 Infections
Fatal and/or serious infections occurred in 48% of patients treated with Zydelig in combination with rituximab or with unapproved combination therapies [see Adverse Reactions (6.1)]. The most common infections were pneumonia, sepsis, and febrile neutropenia. Treat infections prior to initiation of Zydelig therapy. Monitor patients on Zydelig for signs and symptoms of infection, and interrupt Zydelig for Grade 3 or higher infection [see Dosage and Administration (2.2)].
Serious or fatal Pneumocystis jirovecii pneumonia (PJP) or cytomegalovirus (CMV) occurred in <1% of patients treated with Zydelig. Provide PJP prophylaxis during treatment with Zydelig. Interrupt Zydelig in patients with suspected PJP infection of any grade, and permanently discontinue Zydelig if PJP infection of any grade is confirmed. Regular clinical and laboratory monitoring for CMV infection is recommended in patients with history of CMV infection or positive CMV serology at the start of treatment with Zydelig. Interrupt Zydelig in the setting of positive CMV PCR or antigen test until the viremia has resolved. If Zydelig is subsequently resumed, patients should be monitored by PCR or antigen test for CMV reactivation at least monthly [see Dosage and Administration (2.2)].
5.5 Intestinal Perforation
Fatal and serious intestinal perforation occurred in Zydelig-treated patients. At the time of perforation, some patients had moderate to severe diarrhea. Advise patients to promptly report any new or worsening abdominal pain, chills, fever, nausea, or vomiting. Discontinue Zydelig permanently in patients who experience intestinal perforation.
5.6 Severe Cutaneous Reactions
Fatal cases of Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) have occurred in patients treated with Zydelig. Cases of drug reaction with eosinophilia and systemic symptoms (DRESS) have also occurred [see Adverse Reactions (6.2)]. Zydelig is contraindicated in patients with a history of toxic epidermal necrolysis [see Contraindications (4)]. If SJS, TEN, or DRESS is suspected, interrupt Zydelig until the etiology of the reaction has been determined. If SJS, TEN, or DRESS is confirmed, permanently discontinue Zydelig [see Dosage and Administration (2.2)].
Other severe or life-threatening (Grade ≥3) cutaneous reactions, including dermatitis exfoliative, rash, rash erythematous, rash generalized, rash macular, rash maculo-papular, rash papular, rash pruritic, exfoliative rash, and skin disorder, have been reported in patients treated with Zydelig. Monitor patients for the development of other severe or life-threatening cutaneous reactions and permanently discontinue Zydelig [see Dosage and Administration (2.2)].
5.7 Hypersensitivity Reactions
Serious hypersensitivity reactions, including anaphylaxis, have been reported in patients on Zydelig. Zydelig is contraindicated in patients with a history of serious hypersensitivity reactions to idelalisib, including anaphylaxis [see Contraindications (4)]. In patients who develop serious hypersensitivity reactions, permanently discontinue Zydelig [see Dosage and Administration (2.2)] and institute appropriate supportive measures.
5.8 Neutropenia
Grade 3 or 4 neutropenia occurred in 58% of patients treated with Zydelig in combination with rituximab or with unapproved combination therapies [see Adverse Reactions (6.1)]. Monitor blood counts at least every 2 weeks for the first 6 months of therapy, and at least weekly in patients while neutrophil counts are less than 1.0 Gi/L. Interrupt Zydelig until resolution and resume at reduced dose [see Dosage and Administration (2.2)].
5.9 Embryo-fetal Toxicity
Based on findings in animals and its mechanism of action, Zydelig may cause fetal harm when administered to a pregnant woman. In animal reproduction studies, administration of idelalisib to pregnant rats during organogenesis caused decreased fetal weight and congenital malformations at systemic exposures 12 times those reported in patients at the recommended dose of 150 mg twice daily.
Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment with Zydelig and for 1 month after the last dose [see Use in Specific Populations (8.1, 8.3)].
6. Adverse Reactions/Side Effects
The following clinically significant adverse reactions are described elsewhere in the labeling.
- Hepatotoxicity [see Warnings and Precautions (5.1)]
- Severe Diarrhea or Colitis [see Warnings and Precautions (5.2)]
- Pneumonitis [see Warnings and Precautions (5.3)]
- Infections [see Warnings and Precautions (5.4)]
- Intestinal Perforation [see Warnings and Precautions (5.5)]
- Severe Cutaneous Reactions [see Warnings and Precautions (5.6)]
- Hypersensitivity Reactions [see Warnings and Precautions (5.7)]
- Neutropenia [see Warnings and Precautions (5.8)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The pooled safety population described in the WARNINGS AND PRECAUTIONS reflect exposure to Zydelig at a dosage of 150 mg twice daily in 110 patients administered in combination with rituximab in Study 312-0116, and in combination with other drugs in 380 patients. Among 490 patients who received Zydelig, 74% were exposed for 6 months or longer and 50% were exposed for one year or longer. In this pooled safety population, the most common (> 30%) adverse reactions were diarrhea, pneumonia, pyrexia, fatigue, rash, cough, and nausea. Common laboratory abnormalities were neutropenia, ALT elevations and AST elevations.
6.2 Postmarketing Experience
The following adverse reactions have been identified during postapproval use of Zydelig. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Skin and Subcutaneous Disorders - Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and drug reaction with eosinophilia and systemic symptoms (DRESS)
7. Drug Interactions
7.1 Effects of Other Drugs on Zydelig
Table 6 lists the potential effects of the coadministration of strong CYP3A modulators on Zydelig.
| Strong CYP3A Inhibitors | |
| Clinical Impact |
|
| Prevention or Management |
|
| Strong CYP3A Inducers | |
| Clinical Impact |
|
| Prevention or Management | Avoid coadministration of Zydelig with strong CYP3A4 inducers. |
8. Use In Specific Populations
8.3 Females and Males of Reproductive Potential
Zydelig may cause fetal harm when administered to a pregnant woman [see Use in Specific Populations (8.1)].
8.4 Pediatric Use
Safety and effectiveness of Zydelig in pediatric patients have not been established.
8.5 Geriatric Use
Of the 490 patients with relapsed CLL who were treated with Zydelig in combination trials, 271 (55%) were 65 years of age and older. When comparing patients 65 years of age or older to younger patients with CLL, older patients had a higher incidence of discontinuation due to an adverse reaction (36% vs 28%), higher incidence of serious adverse reactions (73% vs 67%), and higher incidence of death (13% vs 9%).
8.6 Hepatic Impairment
No dose adjustment is recommended for patients with ALT or AST or bilirubin > upper limit of normal (ULN); however, limited safety and efficacy data are available for patients with baseline AST or ALT > 2.5 × ULN or bilirubin > 1.5 × ULN. Monitor patients with baseline hepatic impairment for adverse reactions [see Warnings and Precautions (5)]. Follow dosage modifications for adverse reactions [see Dosage and Administration (2.2)].
11. Zydelig Description
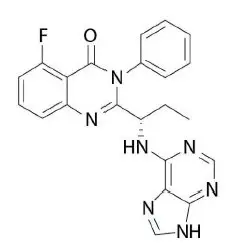
Idelalisib is a kinase inhibitor. The chemical name for idelalisib is 5-fluoro-3-phenyl-2-[(1S)-1-(9H-purin-6-ylamino)propyl]quinazolin-4(3H)-one. It has a molecular formula of C22H18FN7O and a molecular weight of 415.42 g/mol. Idelalisib has the following structural formula:
Idelalisib is a white to off-white solid with a pH-dependent aqueous solubility ranging from <0.1 mg/mL at pH 5–7 to over 1 mg/mL at pH 2 under ambient conditions.
Zydelig (idelalisib) tablets are for oral use. Each tablet contains either 100 mg or 150 mg of idelalisib with the following inactive ingredients: microcrystalline cellulose, hydroxypropyl cellulose, croscarmellose sodium, sodium starch glycolate, magnesium stearate and a tablet coating. The tablet coating consists of polyethylene glycol, talc, polyvinyl alcohol, and titanium dioxide and of FD&C Yellow #6/Sunset Yellow FCF Aluminum Lake (for the 100 mg tablet) and red iron oxide (for the 150 mg tablet).
12. Zydelig - Clinical Pharmacology
12.1 Mechanism of Action
Idelalisib is an inhibitor of phosphatidylinositol 3-kinase, PI3Kδ, which is expressed in normal and malignant B-cells. Idelalisib induced apoptosis and inhibited proliferation in cell lines derived from malignant B-cells and in primary tumor cells. Idelalisib inhibits several cell signaling pathways, including B-cell receptor (BCR) signaling and the CXCR4 and CXCR5 signaling, which are involved in trafficking and homing of B-cells to the lymph nodes and bone marrow. Treatment of lymphoma cells with idelalisib resulted in inhibition of chemotaxis and adhesion, and reduced cell viability.
12.3 Pharmacokinetics
Idelalisib exposure increased in a less than dose-proportional manner over a dose range of 50 mg to 350 mg twice daily (0.3 to 2.3 times the approved recommended dosage).
Following 150 mg twice daily administration of idelalisib, average (% coefficient of variation) maximum concentrations (Cmax) and area under the curve (AUC) at steady-state were 1861 (43%) ng/mL and 10598 (41%) ng∙h/mL for idelalisib.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Idelalisib was not carcinogenic in a 26-week study in transgenic mice when administered daily by oral gavage at doses up to 500 mg/kg/day in males and 1000 mg/kg/day in females. Idelalisib was not carcinogenic in a 2-year study in rats when administered daily by oral gavage at exposures 0.40/0.62-fold (male/female), compared to the exposure in patients with hematologic malignancies administered the recommended dose of 150 mg twice daily.
Idelalisib did not induce mutations in the bacterial mutagenesis (Ames) assay and was not clastogenic in the in vitro chromosome aberration assay using human peripheral blood lymphocytes. Idelalisib was genotoxic in males in the in vivo rat micronucleus study at a high dose of 2000 mg/kg.
Idelalisib may impair fertility in humans. In a fertility study, treated male rats (25, 50, or 100 mg/kg/day of idelalisib) were mated with untreated females. Decreased epididymidal and testicular weights were observed at all dose levels and reduced sperm concentration at the mid- and high doses; however, there were no adverse effects on fertility parameters. The low dose in males resulted in an exposure (AUC) that is approximately 50% of the exposure in patients at the recommended dose of 150 mg twice daily.
In a separate fertility study, treated female rats (25, 50, or 100 mg/kg/day of idelalisib) were mated with untreated males. There were no adverse effects on fertility parameters; however, there was a decrease in the number of live embryos at the high dose. The high dose in females resulted in an exposure (AUC) that is approximately 17-fold the exposure in patients at the recommended dose of 150 mg twice daily.
13.2 Animal Toxicology and/or Pharmacology
Toxicities observed in animals and not reported in patients include cardiac toxicity (cardiomyopathy, inflammation, and increased heart weight) and pancreatic findings (inflammation, hemorrhage, and low-incidence acinar degeneration and hyperplasia). These findings were observed in Sprague-Dawley rats in toxicology studies at exposures (AUCs) higher than those reported in patients at the recommended dose of 150 mg twice daily. Cardiac inflammation was mainly seen in a 28-day study in rats, the other findings were observed in the 13-week and/or 6-month studies.
14. Clinical Studies
Zydelig was evaluated in a randomized, double-blind, placebo-controlled study GS-US-312-0116 (referred to as 312-0116) (NCT01539512) in 220 patients with relapsed CLL who required treatment and were unable to tolerate standard chemoimmunotherapy due to coexisting medical conditions, reduced renal function as measured by creatinine clearance < 60 mL/min, or NCI CTCAE Grade ≥ 3 neutropenia or Grade ≥ 3 thrombocytopenia resulting from myelotoxic effects of prior therapy with cytotoxic agents. Patients were randomized 1:1 to receive 8 doses of rituximab (first dose at 375 mg/m2, subsequent doses at 500 mg/m2 every 2 weeks for 4 infusions and every 4 weeks for an additional 4 infusions) in combination with either an placebo taken orally twice daily or with Zydelig 150 mg taken orally twice daily until disease progression or unacceptable toxicity.
The median age was 71 years (range 47, 92) with 78% over 65, 66% were male, and 90% were White. The median time since diagnosis was 8.5 years. The median number of prior therapies was 3. Nearly all (96%) patients had received prior anti-CD20 monoclonal antibodies. The most common (>15%) prior regimens were: bendamustine + rituximab (BR) (98 patients, 45%), fludarabine + cyclophosphamide + rituximab (75 patients, 34%), single-agent rituximab (67 patients, 31%), fludarabine + rituximab (37 patients, 17%), and chlorambucil (36 patients, 16%). The median CIRS (Cumulative Illness Rating Scale) score was 8 (range 0–17), and 85% of patients had a score of >6. Median Karnofsky score was 80. Median estimated Creatinine Clearance (eCLcr) was 63.6 mL/min, with 41% of patients having an eCLcr of <60 mL/min. At screening, 19.5% of patients had a platelet count of <50 × 109/L, and 13.2% had an absolute neutrophil count (ANC) of <1 × 109/L.
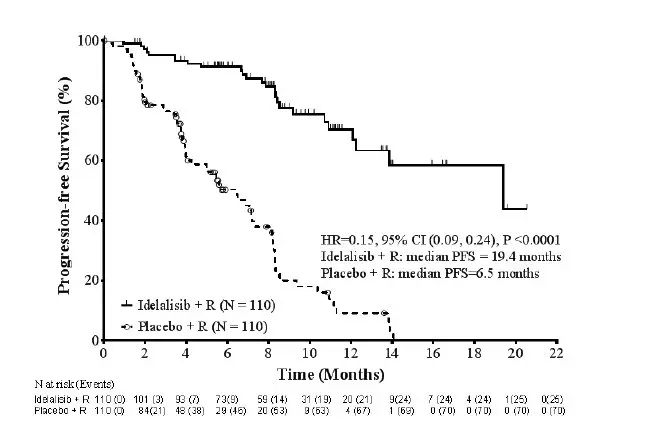
The efficacy of Zydelig was based on progression free survival (PFS), as assessed by an independent review committee (IRC). The trial was stopped for efficacy following the first pre-specified interim analysis. Results of a second interim analysis continued to show a statistically significant improvement for Zydelig + R compared to placebo + R for the major efficacy outcome measure of PFS (HR: 0.18, 95% CI [0.10, 0.32], p <0.0001).
At the final analysis, with a median follow-up of 8.3 months for the Zydelig + R group, and 5.6 months for the placebo + R group, the median PFS for the Zydelig + R group was 19.4 months (95% CI: 12.3, Not Reached) versus 6.5 months (95% CI: 4.0, 7.3) for the placebo + R group (HR: 0.15, 95% CI [0.09, 0.24], p < 0.0001).
Updated efficacy results are shown in Table 7 and the Kaplan-Meier curve for PFS is shown in Figure 1.
| Zydelig + R N = 110 | Placebo + R N = 110 |
||
|---|---|---|---|
| PFS: progression-free survival; NR: not reached; ORR: overall response rate; PR: partial response; DOR: duration of response | |||
|
|||
| PFS | Median (months) (95% CI) | 19.4 (12.3, NR) | 6.5 (4.0, 7.3) |
| Hazard ratio (95% CI) | 0.15 (0.09, 0.24) | ||
| P-value | < 0.0001 * | ||
| ORR† | (All PRs) | 92 (83.6%) | 17 (15.5%) |
| 95% CI | 75.4, 90.0 | 9.3, 23.6 | |
| Odds Ratio (95% CI) | 27.8 (13.4, 57.5) | ||
| P-value | <0.0001 | ||
| DOR | Median (months) (95% CI) | NR (12, NR) | 6.2 (2.8, 6.5) |
Figure 1 Kaplan-Meier Plot of IRC-Assessed PFS for Study 312-0116
16. How is Zydelig supplied
Zydelig tablets supplied as follows:
| Tablet Strength | Package Configuration | NDC No. | Description of Tablet; Debossed on Tablet |
|---|---|---|---|
| 150 mg | High density polyethylene (HDPE) bottle with a polyester fiber coil, capped with a child-resistant closure. Each bottle contains 60 film-coated tablets. | 61958-1702-1 | Oval shaped; pink; "150" on one side and "GSI" on the other side |
| 100 mg | 61958-1701-1 | Oval-shaped; orange; "100" on one side and "GSI" on the other side |
17. Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
| This Medication Guide has been approved by the U.S. Food and Drug Administration. | Revised: 2/2022 | |
| MEDICATION GUIDE ZYDELIG® (zye-DEL-ig) (idelalisib) tablets |
||
| What is the most important information I should know about Zydelig? Zydelig can cause serious side effects that can lead to death, including:
See "What are the possible side effects of Zydelig?" for more information about side effects. |
||
| What is Zydelig?
Zydelig is a prescription medicine used to treat people with chronic lymphocytic leukemia (CLL), in combination with rituximab, when CLL comes back after prior cancer treatment and when rituximab treatment alone may be used due to other health problems. Zydelig should not be used as the first medicine to treat anyone, including people with CLL, small lymphocytic lymphoma (SLL), follicular lymphoma (FL), and other slow growing (indolent) non-Hodgkin lymphomas. Zydelig should not be used in combination with bendamustine and rituximab, or in combination with rituximab to treat people with FL, SLL, and other indolent non-Hodgkin lymphomas. It is not known if Zydelig is safe and effective in children. |
||
Do not take Zydelig if you:
|
||
Before taking Zydelig, tell your healthcare provider about all of your medical conditions, including if you:
|
||
How should I take Zydelig?
|
||
| What are the possible side effects of Zydelig?
Zydelig can cause serious side effects, including:
|
||
|
|
|
|
||
| The most common side effects of Zydelig when used in combination with rituximab include: | ||
|
|
|
| Tell your healthcare provider if you have any side effect that bothers you or that does not go away. These are not all the possible side effects of Zydelig. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
||
How should I store Zydelig?
|
||
| General information about the safe and effective use of Zydelig.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use Zydelig for a condition for which it was not prescribed. Do not give Zydelig to other people, even if they have the same symptoms you have. It may harm them. You can ask your healthcare provider or pharmacist for information about Zydelig that is written for health professionals. |
||
| What are the ingredients in Zydelig?
Active ingredient: idelalisib Inactive ingredients: microcrystalline cellulose, hydroxypropyl cellulose, croscarmellose sodium, sodium starch glycolate, and magnesium stearate. The tablet coating contains polyethylene glycol, talc, polyvinyl alcohol, titanium dioxide and FD&C Yellow #6 or Sunset Yellow FCF Aluminum Lake (for the 100 mg tablet) and red iron oxide (for the 150 mg tablet). Manufactured and distributed by: Gilead Sciences, Inc. Foster City, CA 94404 ©2022 Gilead Sciences, Inc. All rights reserved For more information, call 1-800-445-3235 or go to www.Zydelig.com. 205858-GS-005-MG |
||
| ZYDELIG
idelalisib tablet, film coated |
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| ZYDELIG
idelalisib tablet, film coated |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Labeler - Gilead Sciences, Inc. (185049848) |