Drug Detail:Beyfortus (cvx 306) (Nirsevimab (cvx 306) [ nir-sev-i-mab ])
Generic Name: NIRSEVIMAB 50mg in 0.5mL
Dosage Form: injection
Drug Class: Other immunostimulants
Recommended Dosage
Neonates and Infants: First RSV Season
For neonates and infants born during or entering the RSV season, administer BEYFORTUS starting from birth. For infants born outside the RSV season, administer BEYFORTUS once prior to the start of the RSV season considering duration of protection provided by BEYFORTUS [see Clinical Pharmacology (12.2)].
The recommended dosage of BEYFORTUS for neonates and infants born during or entering their first RSV season is based on body weight (see Table 1) and is administered as a single intramuscular (IM) injection.
|
Body Weight at Time of Dosing |
Recommended Dosage |
|
Less than 5 kg |
50 mg by IM injection |
|
5 kg and greater |
100 mg by IM injection |
Children Who Remain at Increased Risk for Severe RSV Disease: Second RSV Season
For children up to 24 months of age who remain at increased risk for severe RSV disease in their second RSV season, the recommended dosage of BEYFORTUS is a single 200 mg dose administered as two IM injections (2 x 100 mg).
Children Undergoing Cardiac Surgery with Cardiopulmonary Bypass
For children undergoing cardiac surgery with cardiopulmonary bypass, an additional dose of BEYFORTUS is recommended as soon as the child is stable after surgery to ensure adequate nirsevimab-alip serum levels. The recommended dosage of BEYFORTUS is administered as an IM injection.
First RSV season:
-
- •
- If surgery is within 90 days after receiving BEYFORTUS, the additional dose should be based on body weight at the time of the additional dose. Refer to Table 1 for weight-based dosing.
- •
- If more than 90 days have elapsed since receiving BEYFORTUS, the additional dose should be 50 mg regardless of body weight.
Second RSV season:
-
- •
- If surgery is within 90 days after receiving BEYFORTUS, the additional dose should be 200 mg, regardless of body weight.
- •
- If more than 90 days have elapsed since receiving BEYFORTUS, the additional dose should be 100 mg, regardless of body weight.
Administration Instructions
BEYFORTUS must be administered by a healthcare provider.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. BEYFORTUS is a clear to opalescent, colorless to yellow solution. Do not inject BEYFORTUS if the liquid is cloudy, discolored, or it contains large particles or foreign particulate matter.
Do not use if the BEYFORTUS pre-filled syringe has been dropped or damaged, the security seal on the carton has been broken, or the expiration date has passed.
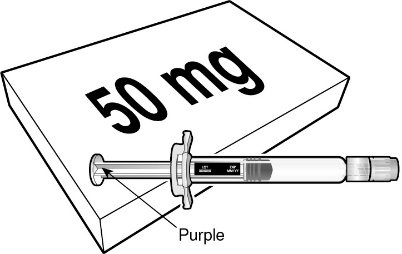
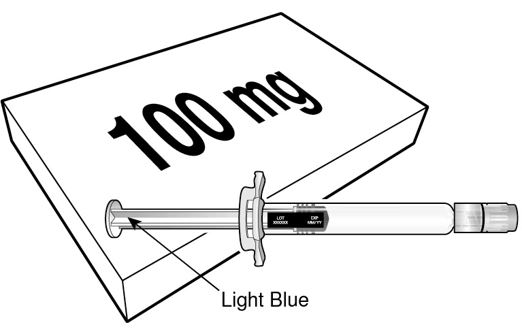
BEYFORTUS is available in a 50 mg and a 100 mg pre-filled syringe. Check the labels on the BEYFORTUS carton and pre-filled syringe to ensure the correct 50 mg or 100 mg product is being used.
Co-administration with Childhood Vaccines and Immunoglobulin Products
BEYFORTUS can be given concomitantly with childhood vaccines [see Clinical Pharmacology (12.3)]. When administered concomitantly with injectable vaccines, they should be given with separate syringes and at different injection sites. Do not mix BEYFORTUS with any vaccines or medications in the same syringe or vial.
There is no information regarding co-administration of BEYFORTUS with other immunoglobulin products. Palivizumab should not be administered to infants who have already received BEYFORTUS in the same season. There are no data regarding substitution of BEYFORTUS for palivizumab once prophylaxis treatment is initiated with palivizumab for the RSV season. BEYFORTUS may be administered prior to or during the second RSV season to children up to 24 months of age who remain vulnerable to severe RSV disease, and who received palivizumab in their first RSV season [see Adverse Reactions (6.1) and Clinical Studies (14.3)].
Administration Instructions for Single-Dose Pre-filled Syringe
|
BEYFORTUS 50 mg (50 mg/0.5 mL) pre‑filled syringe with a purple plunger rod. |
BEYFORTUS 100 mg (100 mg/mL) pre‑filled syringe with a light blue plunger rod. |
 |
 |
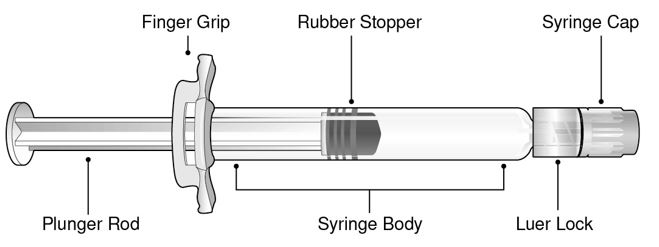
Refer to Figure 1 for pre-filled syringe components.
- Figure 1 Luer Lock Syringe Components
Step 1: Holding the Luer lock in one hand (avoid holding the plunger rod or syringe body), unscrew the syringe cap by twisting it counter‑clockwise with the other hand.
Step 2: Attach a Luer lock needle to the pre-filled syringe by gently twisting the needle clockwise onto the pre-filled syringe until slight resistance is felt.
Step 3: Hold the syringe body with one hand and carefully pull the needle cover straight off with the other hand. Do not hold the plunger rod while removing the needle cover or the rubber stopper may move. Do not touch the needle or let it touch any surface. Do not recap the needle or detach it from the syringe.
Step 4: Administer the entire contents of the BEYFORTUS pre-filled syringe as an IM injection, preferably in the anterolateral aspect of the thigh. The gluteal muscle should not be used as an injection site because of the risk of damage to the sciatic nerve.
Step 5: Discard syringe into a sharps container.
If two injections are required, repeat Steps 1-5 in a different injection site.