Drug Detail:Butrans skin patch (Buprenorphine transdermal (skin patch) [ bue-pre-nor-feen ])
Generic Name: buprenorphine 10ug in 1h
Dosage Form: patch, extended release
Drug Class: Opioids (narcotic analgesics)
Important Dosage and Administration Information
BUTRANS should be prescribed only by healthcare professionals who are knowledgeable in the use of potent opioids for the management of chronic pain.
BUTRANS doses of 7.5, 10, 15, and 20 mcg/hour are only for use in patients who are opioid experienced and in whom tolerance to an opioid of comparable potency has been established. Patients who are opioid-experienced are those receiving, for one week or longer, daily opioid doses up to 80 mg/day of oral morphine or an equianalgesic dose of another opioid.
- Use the lowest effective dosage for the shortest duration consistent with individual patients treatment goals [see Warnings and Precautions (5)].
- Initiate the dosing regimen for each patient individually, taking into account the patient's severity of pain, patient response, prior analgesic treatment experience, and risk factors for addiction, abuse, and misuse [see Warnings and Precautions (5.1)].
- Monitor patients closely for respiratory depression, especially within the first 24-72 hours of initiating therapy and following dosage increases with BUTRANS [see Warnings and Precautions (5.3)].
- Instruct patients not to use BUTRANS if the pouch seal is broken or the patch is cut, damaged, or changed in any way and not to cut BUTRANS.
- Instruct patients to avoid exposing BUTRANS to external heat sources, hot water, or prolonged direct sunlight [see Warnings and Precautions (5.15)].
BUTRANS is for transdermal use (on intact skin) only. Each BUTRANS patch is intended to be worn for 7 days.
Patient Access to Naloxone for the Emergency Treatment of Opioid Overdose
Discuss the availability of naloxone for the emergency treatment of opioid overdose with the patient and caregiver and assess the potential need for access to naloxone, both when initiating and renewing treatment with BUTRANS [see Warnings and Precautions (5.3), Patient Counseling Information (17)].
Inform patients and caregivers about the various ways to obtain naloxone as permitted by individual state naloxone dispensing and prescribing requirements or guidelines (e.g., by prescription, directly from a pharmacist, or as part of a community-based program).
Consider prescribing naloxone, based on the patient’s risk factors for overdose, such as concomitant use of CNS depressants, a history of opioid use disorder, or prior opioid overdose. The presence of risk factors for overdose should not prevent the proper management of pain in any given patient [see Warnings and Precautions (5.1, 5.3, 5.5), Overdosage (10)].
Consider prescribing naloxone if the patient has household members (including children) or other close contacts at risk for accidental exposure or overdose.
Initial Dosage
Use of BUTRANS as the First Opioid Analgesic (opioid-naive patients)
Initiate treatment with BUTRANS with a 5 mcg/hour patch.
Conversion from Other Opioids to BUTRANS
Discontinue all other around-the-clock opioid drugs when BUTRANS therapy is initiated.
There is a potential for buprenorphine to precipitate withdrawal in patients who are already on opioids.
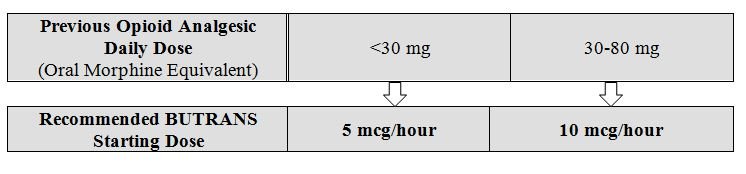
Prior Total Daily Dose of Opioid Less than 30 mg of Oral Morphine Equivalents per Day: Initiate treatment with BUTRANS 5 mcg/hour at the next dosing interval (see Table 1 below, middle column).
Prior Total Daily Dose of Opioid Between 30 mg to 80 mg of Oral Morphine Equivalents per Day:
Taper the patient’s current around-the-clock opioids for up to 7 days to no more than 30 mg of morphine or equivalent per day before beginning treatment with BUTRANS. Then initiate treatment with BUTRANS 10 mcg/hour at the next dosing interval (see Table 1 below, right column). Patients may use short-acting analgesics as needed until analgesic efficacy with BUTRANS is attained.
Prior Total Daily Dose of Opioid Greater than 80 mg of Oral Morphine Equivalents per Day: BUTRANS 20 mcg/hour may not provide adequate analgesia for patients requiring greater than 80 mg/day oral morphine equivalents. Consider the use of an alternate analgesic.
Conversion from Methadone to BUTRANS
Close monitoring is of particular importance when converting from methadone to other opioid agonists. The ratio between methadone and other opioid agonists may vary widely as a function of previous dose exposure. Methadone has a long half-life and can accumulate in the plasma.
Titration and Maintenance of Therapy
Individually titrate BUTRANS to a dose that provides adequate analgesia and minimizes adverse reactions. Continually reevaluate patients receiving BUTRANS to assess the maintenance of pain control and the relative incidence of adverse reactions, as well as monitoring for the development of addiction, abuse, or misuse [see Warnings and Precautions (5.1)]. Frequent communication is important among the prescriber, other members of healthcare team, the patient, and the caregiver/family during periods of changing analgesic requirements, including initial titration. During chronic therapy, periodically reassess the continued need for opioid analgesics.
The minimum BUTRANS titration interval is 72 hours, based on the pharmacokinetic profile and time to reach steady state levels [see Clinical Pharmacology (12.3)].
The maximum BUTRANS dose is 20 mcg/hour. Do not exceed a dose of one 20 mcg/hour BUTRANS system due to the risk of QTc interval prolongation. In a clinical trial, BUTRANS 40 mcg/hour (given as two BUTRANS 20 mcg/hour systems) resulted in prolongation of the QTc interval [see Warnings and Precautions (5.12), Clinical Pharmacology (12.2)].
Patients who experience breakthrough pain may require dosage adjustment increase of BUTRANS, or may need rescue medication with an appropriate dose of an immediate-release analgesic. If the level of pain increases after dose stabilization, attempt to identify the source of increased pain before increasing the BUTRANS dose.
Because steady-state plasma concentrations are achieved within 72 hours, BUTRANS dosage may be adjusted every 3 days. Dose adjustments may be made in 5 mcg/hour, 7.5 mcg/hour, or 10 mcg/hour increments by using no more than two patches of the 5 mcg/hour, or 7.5 mcg/hour, or 10 mcg/hour system(s). The total dose from both patches should not exceed 20 mcg/hour. For the use of two patches, instruct patients to remove their current patch, and apply the two new patches at the same time, adjacent to one another at a different application site [see Dosage and Administration (2.7)].
If unacceptable opioid-related adverse reactions are observed, consider reducing the dosage. Adjust the dosage to obtain an appropriate balance between the management of pain and opioid-related adverse reactions.
Safe Reduction or Discontinuation of BUTRANS
Do not abruptly discontinue BUTRANS in patients who may be physically dependent on opioids. Rapid discontinuation of opioid analgesics in patients who are physically dependent on opioids has resulted in serious withdrawal symptoms, uncontrolled pain, and suicide. Rapid discontinuation has also been associated with attempts to find other sources of opioid analgesics, which may be confused with drug-seeking for abuse. Patients may also attempt to treat their pain or withdrawal symptoms with illicit opioids, such as heroin, and other substances.
When a decision has been made to decrease the dose or discontinue therapy in an opioid-dependent patient taking BUTRANS, there are a variety of factors that should be considered, including the dose of BUTRANS the patient has been taking, the duration of treatment, the type of pain being treated, and the physical and psychological attributes of the patient. It is important to ensure ongoing care of the patient and to agree on an appropriate tapering schedule and follow-up plan so that patient and provider goals and expectations are clear and realistic. When opioid analgesics are being discontinued due to a suspected substance use disorder, evaluate and treat the patient, or refer for evaluation and treatment of the substance use disorder. Treatment should include evidence-based approaches, such as medication assisted treatment of opioid use disorder. Complex patients with comorbid pain and substance use disorders may benefit from referral to a specialist.
There are no standard opioid tapering schedules that are suitable for all patients. Good clinical practice dictates a patient-specific plan to taper the dose of the opioid gradually. For patients on BUTRANS who are physically opioid-dependent, initiate the taper by a small enough increment (e.g., no greater than 10% to 25% of the total daily dose) to avoid withdrawal symptoms, and proceed with dose-lowering at an interval of every 2 to 4 weeks. Patients who have been taking opioids for briefer periods of time may tolerate a more rapid taper.
It may be necessary to provide the patient with lower dosage strengths to accomplish a successful taper. Reassess the patient frequently to manage pain and withdrawal symptoms, should they emerge. Common withdrawal symptoms include restlessness, lacrimation, rhinorrhea, yawning, perspiration, chills, myalgia, and mydriasis. Other signs and symptoms also may develop, including irritability, anxiety, backache, joint pain, weakness, abdominal cramps, insomnia, nausea, anorexia, vomiting, diarrhea, or increased blood pressure, respiratory rate, or heart rate. If withdrawal symptoms arise, it may be necessary to pause the taper for a period of time or raise the dose of the opioid analgesic to the previous dose, and then proceed with a slower taper. In addition, monitor patients for any changes in mood, emergence of suicidal thoughts, or use of other substances.
When managing patients taking opioid analgesics, particularly those who have been treated for a long duration and/or with high doses for chronic pain, ensure that a multimodal approach to pain management, including mental health support (if needed), is in place prior to initiating an opioid analgesic taper. A multimodal approach to pain management may optimize the treatment of chronic pain, as well as assist with the successful tapering of the opioid analgesic [see Warnings and Precautions (5.3), Drug Abuse and Dependence (9.3)].
Patients with Hepatic Impairment
BUTRANS has not been evaluated in patients with severe hepatic impairment. As BUTRANS is only intended for 7-day application, consider use of an alternate analgesic that may permit more flexibility with the dosing in patients with severe hepatic impairment [see Warnings and Precautions (5.10), Use in Specific Populations (8.6), and Clinical Pharmacology (12.3)]
Administration of BUTRANS
- Instruct patients to apply immediately after removal from the individually sealed pouch. Instruct patients not to use BUTRANS if the pouch seal is broken or the patch is cut, damaged, or changed in any way. See the Instructions for Use for step-by-step instructions for applying BUTRANS.
- Apply BUTRANS to the upper outer arm, upper chest, upper back or the side of the chest. These 4 sites (each present on both sides of the body) provide 8 possible application sites. Rotate BUTRANS among the 8 described skin sites. After BUTRANS removal, wait a minimum of 21 days before reapplying to the same skin site [see Clinical Pharmacology (12.3)].
- Apply BUTRANS to a hairless or nearly hairless skin site. If none are available, the hair at the site should be clipped, not shaven. Do not apply BUTRANS to irritated skin. If the application site must be cleaned, clean the site with water only. Do not use soaps, alcohol, oils, lotions, or abrasive devices. Allow the skin to dry before applying BUTRANS.
- Incidental exposure of the BUTRANS patch to water, such as while bathing or showering is acceptable based on experience during clinical studies.
- If problems with adhesion of BUTRANS occur, the edges may be taped with first aid tape. If problems with lack of adhesion continue, the patch may be covered with waterproof or semipermeable adhesive dressings suitable for 7 days of wear.
- If BUTRANS falls off during the 7-day dosing interval, dispose of the transdermal system properly and place a new BUTRANS patch on at a different skin site.
- When changing the system, instruct patients to remove BUTRANS and dispose of it properly [see Dosage and Administration (2.8)].
- If the buprenorphine-containing adhesive matrix accidentally contacts the skin, instruct patients or caregivers to wash the area with water and not to use soap, alcohol, or other solvents to remove the adhesive because they may enhance the absorption of the drug.
Disposal Instructions
Patients should refer to the Instructions for Use for proper disposal of BUTRANS. Dispose of used and unused patches by following the instructions on the Patch-Disposal Unit that is packaged with the BUTRANS patches.
Alternatively, patients can dispose of used patches by folding the adhesive side of the patch to itself, then flushing the patch down the toilet immediately upon removal. Unused patches should be removed from their pouches, the protective liners removed, the patches folded so that the adhesive side of the patch adheres to itself, and immediately flushed down the toilet.
Patients should dispose of any patches remaining from a prescription as soon as they are no longer needed.




