Drug Detail:Gammagard s/d (igiv) (Immune globulin (igiv) (intravenous) [ im-myoon-glob-yoo-lin ])
Generic Name: HUMAN IMMUNOGLOBULIN G 50mg in 1mL;
Dosage Form: injection
Drug Class: Immune globulins
For Intravenous Use Only
Preparation and Handling
Instructions for Reconstitution:
Allow GAMMAGARD S/D and diluent to reach room temperature before reconstitution and administration if refrigerated.
Reconstitution:
- 1.
- Remove caps from concentrate and diluent bottles to expose central portion of rubber stoppers and cleanse stoppers with germicidal solution.
- To make a 5% solution: Use the full volume of the diluent bottle
- To make a 10% solution: Remove half of the volume of the diluent bottle
Table 1 indicates the volume of diluent to be removed from the vial before attaching the transfer device to produce a 10% concentration. Using aseptic technique, withdraw the unnecessary volume of diluent using a sterile hypodermic syringe and needle. Discard the filled syringe into a suitable puncture proof container (sharps container).
| Concentration | 5 g bottle | 10 g bottle | |
|---|---|---|---|
| 5% | Do not remove any diluent for reconstitution of 5% Solution | ||
| 10% | 48 mL | 96 mL | |
- 2.
- Remove spike cap from one end of the transfer device. Do not touch spike.
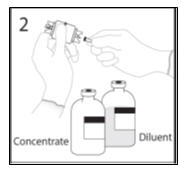
- 3a.
- Place the diluent bottle on a flat surface and hold the bottle to prevent slipping. Use exposed end of transfer device to spike diluent bottle perpendicularly through center of the stopper. CAUTION: Failure to insert spike into center of the stopper may result in dislodging of the stopper.
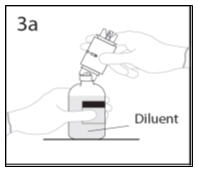
- 3b.
- Ensure that the collar collapses fully into the device by pushing down on the transfer device firmly. While holding onto transfer device, remove remaining spike cover from the other end of the transfer device. Do not touch spike.
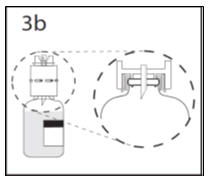
- 4.
- Hold diluent bottle with attached transfer device at an angle to the concentrate bottle to prevent spilling the diluent. Note: Do not hold diluent bottle upside down, for this can lead to diluent spillage.
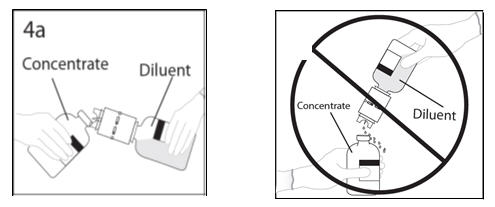
- 5a.
- Spike concentrate bottle through center of the stopper while quickly inverting the diluent bottle to minimize spilling out diluent. CAUTION: Failure to insert the spike into the center of the stopper may result in dislodging of the stopper and loss of vacuum.
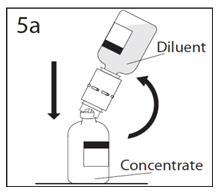
- 5b.
- Ensure that the collar collapses fully into the device by pushing down on the diluent bottle firmly.
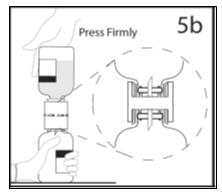
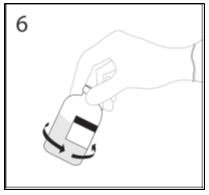
- 6.
- After transfer of diluent is complete, remove transfer device and empty diluent bottle. Immediately swirl the concentrate bottle gently to thoroughly mix contents. Repeat gentle rotation as long as undissolved product is observed. CAUTION: Do not shake. Avoid foaming. Discard transfer device after single use per local guidelines.
- Visually inspect parenteral drug product for particulate matter and discoloration prior to administration. Reconstituted GAMMAGARD S/D is a clear to slightly opalescent and colorless to pale yellow solution. Do not use if particulate matter and/or discoloration is observed.
- Follow directions for use which accompany the administration set provided. If another administration set is used, ensure that the set contains a similar filter.
- Begin administration as soon as possible within 2 hours if reconstitution is performed aseptically outside of a sterile laminar air flow hood.
- Administer within 24 hours if reconstitution is performed aseptically inside of a sterile laminar flow hood and stored in the original glass container or pooled into ViaFlex bags under constant refrigeration (2°C to 8°C). Record the date and time of reconstitution/pooling. Discard partially used vials.
Dose
Primary Immunodeficiency (PI)
The recommended dose of GAMMAGARD S/D for patients with PI is 300-600 mg/kg infused at 3 to 4 week intervals.1,2,6 Adjust dose according to the clinical response; the frequency and dose of immunoglobulin may vary from patient to patient. No randomized controlled clinical trials are available to determine an optimum target trough serum IgG level.
B-cell Chronic Lymphocytic Leukemia (CLL)
The recommended dose of GAMMAGARD S/D for patients with hypogammaglobulinemia and/or recurrent bacterial infections due to B-cell CLL is 400 mg/kg body weight infused at every 3 to 4 week intervals.4
Idiopathic Thrombocytopenic Purpura (ITP)
The recommended dose of GAMMAGARD S/D for patients with chronic ITP is 1 g/kg. The need for additional doses can be determined by clinical response and platelet count. Up to three separate doses may be given on alternate days if required.
Kawasaki Syndrome
The recommended dose of GAMMAGARD S/D for patients with Kawasaki syndrome is either a single 1 g/kg dose or a dose of 400 mg/kg for four consecutive days beginning within seven days of the onset of fever, administered concomitantly with appropriate aspirin therapy (80-100 mg/kg/day in four divided doses).5,7,8
Measles Exposure
If a patient has been exposed to measles, it may be prudent to administer an extra dose of GAMMAGARD S/D as soon as possible and within 6 days of exposure. A dose of 400 mg/kg should provide a serum level > 240 mIU/mL of measles antibodies for at least two weeks.
If a patient is at risk of future measles exposure and receives a dose of less than 530 mg/kg every 3-4 weeks, the dose should be increased to at least 530 mg/kg. This should provide a serum level of 240 mIU/mL of measles antibodies for at least 22 days after infusion.
Administration
Administer GAMMAGARD S/D as soon after reconstitution as possible and administer the reconstituted material at room temperature.
The recommended initial 5% solution infusion rate is 0.5 mL/kg/hour. The infusion rate may be gradually increased to a maximum rate of 4 mL/kg/hour as tolerated for patients with no history of adverse reactions to IGIV and no significant risk factors for renal dysfunction or thrombotic complications. Patients who tolerate the 5% concentration at 4 mL/kg/hour can be infused with the 10% concentration starting at 0.5 mL/kg/hour. The rate can be increased gradually up to a maximum of 8 mL/kg/hour if no adverse effects occur.9
Monitor patient vital signs throughout the infusion. Certain adverse reactions such as headaches, flushing and changes in pulse rate and blood pressure may be related to the rate of infusion. Slow or stop the infusion if adverse reactions occur. If symptoms subside promptly, the infusion may be resumed at a lower rate that does not result in reoccurrence of the symptoms.
It is recommended that, if possible, the antecubital veins are used, especially for 10% solutions, to reduce the likelihood of discomfort at the infusion site. [See Adverse Reactions (6)]
Adverse reactions may occur more frequently in patients who receive human IGIV for the first time, upon switching brands, or if there has been a long hiatus since the previous infusion. In such cases, start at a lower rate and gradually increase as tolerated.
There are no prospective studies demonstrating that any concentration or rate of infusion is completely safe. However, the risk may be decreased at lower rates of infusion.10 For patients who are judged to be at risk of renal dysfunction or thrombotic complications, administer GAMMAGARD S/D at the minimum practicable rate of infusion and gradually titrate up to a more conservative maximal rate of less than 3.3 mg/kg/min (< 2mL/kg/hour of a 10% or < 4mL/kg/hour of a 5% solution). [See Warnings and Precautions (5.3)]











