Drug Detail:Ixinity (Coagulation factor ix [ koe-ag-yoo-lay-shun-fak-tor-nine ])
Generic Name: COAGULATION FACTOR IX RECOMBINANT HUMAN 250[iU] in 5mL;
Dosage Form: injection
Drug Class: Miscellaneous coagulation modifiers
For intravenous use after reconstitution only.
Dose
- Each vial of IXINITY has the recombinant factor IX (rFIX) potency in international units (IU) stated on the vial.
- Dosage and duration of treatment for factor IX products depend on the severity of the factor IX deficiency, the location and extent of bleeding, the patient’s clinical condition, age, and pharmacokinetic parameters of factor IX, such as incremental recovery and half-life.
Initial Dose
Calculate the initial dose of IXINITY based on the empirical finding that one international unit (IU) of IXINITY per kg body weight increases the circulating level of factor IX by 0.98 international units/dL (IU/dL) of plasma in adults and children.
| Initial Dose = body weight (kg) x desired factor IX increase (% of normal or IU/dL) |
| × reciprocal of observed recovery (IU/kg per IU/dL) |
Incremental Recovery in Previously Treated Patients (PTPs)
Base calculation of the dose on the patient’s individual incremental recovery using serial factor IX activity assays, to account for the wide range of inter-individual differences in incremental recovery and the type of aPTT reagent used for the assay. Titrate the dose based on the patient’s clinical response and individual pharmacokinetics, in particular incremental recovery and half-life.
For an incremental recovery of 0.98 IU/dL per IU/kg, calculate the dose as follows:
| Dose (IU) = body weight (kg) x desired factor IX increase (% of normal or IU/dL) |
| × 1.02 dL/kg |
Examples (assuming patient’s baseline factor IX level is < 1% of normal):
- A dose of 4550 international units (IUs) of IXINITY administered to a 70 kg patient
should be expected to result in a peak post-infusion factor IX increase of
4550 IU × (0.98 IU/dL per IU/kg)/(70 kg) = 64 IU/dL (approximately 60% of normal) - A peak of 70% is required in a 60 kg patient. The appropriate dose would be
(60 kg × 70 IU/dL)/(0.98 IU/dL per IU/kg) = 4286 IU
- Monitor factor IX activity to ensure that the desired factor IX activity level has been achieved [see Warnings and Precautions (5.5)].
- Titrate doses using factor IX activity and pharmacokinetic parameters such as half-life and incremental recovery, as well as by taking the clinical situation into consideration, to adjust the dose and frequency of repeated infusions as appropriate.
- Factor IX activity measurements in the clinical laboratory may be affected by the type of activated partial thromboplastin time (aPTT) reagent or laboratory standard used [see Warnings and Precautions (5.5)].
On-demand Treatment and Control of Bleeding Episodes and Perioperative Management of Bleeding
Guides for dosing IXINITY in the on-demand treatment and control of bleeding episodes (Table 1) and perioperative management (Table 2) are provided in the tables below. Individual patients may vary in their response to factor IX and may demonstrate different levels of in vivo recovery and different half-lives.
For surgical procedures, initiate treatment with IXINITY early enough pre-operatively to achieve and maintain the desired factor IX level before starting the procedure.
Routine Prophylaxis
The dose for previously treated patients (PTPs) is 40 to 70 IU/kg twice weekly. Adjust the dose based on the individual patient’s age, bleeding pattern, and physical activity.
| Adapted from Srivastava et al. 2013 (1). | |||
| Type of Bleeding Episode | Desired Peak Factor IX Level (% of normal or IU/dL) |
Dosing Interval (hours) |
Duration of therapy (days) |
| Minor Early bleeds: uncomplicated hemarthroses and superficial muscle (except iliopsoas) with no neurovascular compromise, other soft tissue |
30-60 | 24 | 1-3, until healing is achieved |
| Moderate Hemarthrosis of longer duration, recurrent hemarthrosis, mucous membranes, deep lacerations, hematuria |
40-60 | 24 | 2-7, until healing is achieved |
| Major or Life Threatening Iliopsoas, deep muscle with neurovascular injury, substantial blood loss, CNS, pharyngeal, retropharyngeal, retroperitoneal |
60-100 | 12-24 | 2-14, until healing is achieved |
| Adapted from Srivastava et al. 2013 (1). | |||
| Type of Surgery | Desired Peak Factor IX Level (% of normal or IU/dL) |
Dosing Interval (hours) |
Duration of therapy (days) |
| Minor (including uncomplicated dental extractions) |
|||
| Pre-op | 50-80 | ||
| Post-op | 30-80 | 24 | 1-5, depending on type of procedure |
| Major | |||
| Pre-op | 60-80 | ||
| Post-op | 40-60 30-50 20-40 |
8-24 | 1-3 4-6 7-14 |
Preparation and Reconstitution
The procedures below are provided as general recommendations for the preparation and reconstitution of IXINITY.
Before starting reconstitution and administration you will need the following items that are included in each kit of IXINITY:
- One (or more) vial(s) of IXINITY 250, 500, 1000, 1500, 2000, or 3000 IU powder
- One (or more) 10 mL syringe(s), pre-filled with 5 mL of Sterile Water for Injection (pre-filled syringe) with plunger rod attached
- One sterile vial adapter with filter
In addition, you will need the following items that are not included in the kit:
- One sterile LUER-LOK™ syringe (administration syringe); additional or larger syringes may be required if pooling multiple vials
- Sterile alcohol swabs
- Sterile infusion set
- Sterile gauze pad
- Sterile bandage
Always work on a clean surface and wash your hands before performing the following procedures:
- Use aseptic technique during reconstitution procedure.
- Allow IXINITY and the pre-filled syringe to reach room temperature before use.
- Remove cap from the vial (See Figure A). Peel back the cover of the vial adapter package (leave the vial adapter in the package).
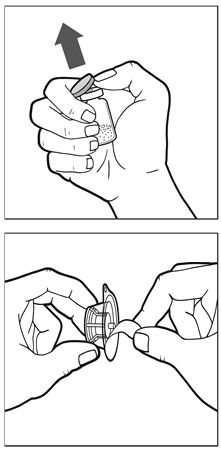
Figure A - Place the administration syringe, if using, and vial adapter on a clean flat work surface.
- Twist off the cap of the pre-filled syringe and place it on the clean flat surface (See Figure B).
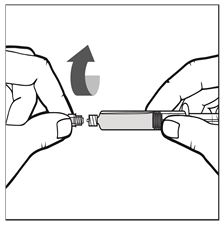
Figure B - Wipe the top of the IXINITY vial with an alcohol swab (or similar germicidal solution) and allow it to dry. Place on a clean, flat surface.
- Firmly hold the package containing the vial adapter on a clean, flat surface. Connect the pre-filled syringe to the vial adapter by pushing the syringe tip down onto the LUER-LOK in the center of the vial adapter, and screw until the syringe is secured (See Figure C).
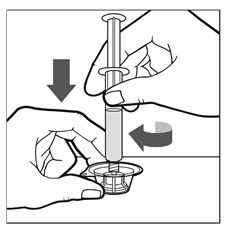
Figure C - Carefully lift up the combined syringe-and-vial-adapter and remove it from the plastic package (See Figure D).
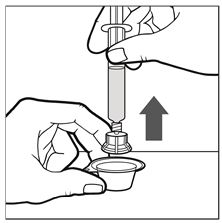
Figure D - With one hand, continue to hold the combined syringe-and-vial-adapter. With the other hand, hold the IXINITY vial tightly on a clean, flat surface. In a continuous motion, place the vial adapter over the IXINITY vial; firmly push the filter spike of the vial adapter through the center of IXINITY vial’s rubber circle until the clear plastic cap snaps onto the IXINITY vial (See Figure E). Push the plunger down to complete the transfer of all liquid from the syringe to the IXINITY vial.
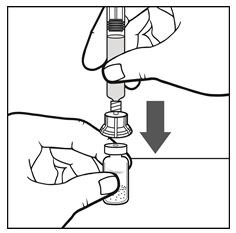
Figure E - With the syringe and the vial still attached, gently swirl, in a circular motion, the IXINITY vial until the product is fully dissolved/reconstituted (See Figure F).
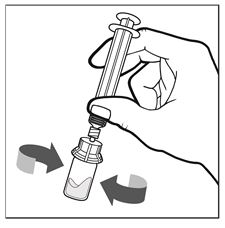
Figure F - Remove the pre-filled syringe (now empty) from the vial adapter by turning it counterclockwise until it is completely detached (See Figure G).
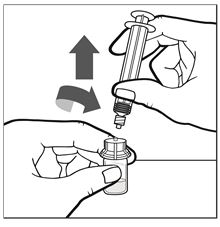
Figure G - Remove the administration syringe from its packaging.
- Leave the vial adapter attached to the vial and attach the administration syringe to the vial adapter by turning clockwise until it is securely attached (See Figure H).
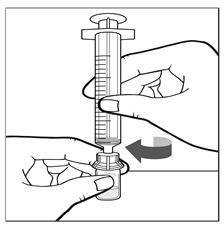
Figure H - Keeping the administration syringe plunger pressed, turn the IXINITY vial upside down. Draw the solution from the vial through the filter spike in the vial adapter by pulling the plunger back slowly until all solution is transferred into the administration syringe (See Figure I).
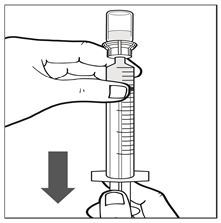
Figure I - Keep the administration syringe plunger facing downwards and prevent it from moving. With one hand hold the vial-and-vial-adapter, and with the other hand firmly grasp the barrel of the administration syringe and unscrew the syringe from the vial adapter (See Figure J).
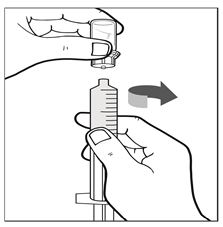
Figure J - If only dosing with a single vial, proceed to administer IXINITY via intravenous infusion; otherwise proceed to Pooling Instructions.
POOLING INSTRUCTIONS
- 1.
- If two or more vials are required to achieve the required dose, remove the pre-filled syringe from the vial adapter on the reconstituted second vial by turning it counterclockwise until it is completely detached.
- 2.
- Leave the vial adapter attached to the vial and attach the administration syringe containing the reconstituted IXINITY from the first vial by turning it clockwise until it is securely in place.
- 3.
- Turn the IXINITY vial upside down and slowly pull on the plunger rod to draw the solution into the administration syringe (see Figure I).
- 4.
- Continue with remaining vials, if required. Once pooling is complete, proceed to administer IXINITY via intravenous infusion.
- After reconstitution of the lyophilized powder, all dosage strengths should yield a clear, colorless solution without visible particles. Discard if visible particulate matter or discoloration is observed.
- Infuse reconstituted solution immediately or within 3 hours of storage at room temperature after reconstitution. Do not refrigerate after reconstitution.
- Do not touch the syringe tip or the inside of the cap. Place the syringe containing the IXINITY solution on the clean surface, making sure that the tip does not touch anything.
Administration
For intravenous use after reconstitution only.
- Inspect parenteral drug products visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
- Do not mix IXINITY with other medicinal products for infusion.
- Attach the administration syringe containing the reconstituted IXINITY solution to a sterile infusion set.
- Adapt the infusion rate to the comfort level of each patient, not exceeding 10 mL per minute.
- Record the name and batch number of the product in the patient record.
Dispose of any unused product or waste material in accordance with local requirements.














