Drug Detail:Jeuveau (Prabotulinumtoxina [ pra-bot-ue-lye-num-tox-in-ay ])
Generic Name: BOTULINUM TOXIN TYPE A 100[USP'U]
Dosage Form: powder for injection
Drug Class: Skeletal muscle relaxants
Instructions for Safe Use
The potency Units of JEUVEAU (prabotulinumtoxinA-xvfs) for injection are specific to the preparation and assay method utilized. They are not interchangeable with other preparations of botulinum toxin products and, therefore, units of biological activity of JEUVEAU cannot be compared to nor converted into units of any other botulinum toxin products assessed with any other specific assay method [see Warnings and Precautions (5.2) and Description (11)].
Retreatment of JEUVEAU should be administered no more frequently than every three months. Consideration of the cumulative dose is necessary when treating adult patients with JEUVEAU for glabellar lines if other botulinum toxin products are or have been used to treat other indications approved for those products.
The safe and effective use of JEUVEAU depends upon proper storage of the product, selection of the correct dose, and proper reconstitution and administration techniques. Physicians administering JEUVEAU must understand the relevant neuromuscular and/or orbital anatomy of the area involved and any alterations to the anatomy due to prior surgical procedures [see Warnings and Precautions (5.4)].
Preparation and Dilution Technique
JEUVEAU is supplied in a single-dose 100 Unit vial. Prior to intramuscular injection, reconstitute each vacuum-dried vial of JEUVEAU with only sterile, preservative-free 0.9% Sodium Chloride Injection, USP to obtain a reconstituted solution at a concentration of 4 Units/0.1 mL and a total treatment dose of 20 Units in 0.5 mL (see Table 1). Slowly inject the diluent into the vial. Discard the vial if a vacuum does not pull the diluent into the vial. Dispose of any unused saline. Gently mix JEUVEAU with 0.9% Sodium Chloride Injection USP by rotating the vial. JEUVEAU should be administered within 24 hours after reconstitution. During this time period, unused reconstituted JEUVEAU should be stored in a refrigerator between 2° to 8°C (36°F to 46°F) in the original carton to protect from light for up to 24 hours until time of use. Do not freeze reconstituted JEUVEAU. JEUVEAU vials are for single-dose only. After reconstitution, JEUVEAU should be used for only one injection session and for only one patient. Discard any remaining solution after administration.
|
|
|
Diluent* Added to 100 Unit Vial |
Resulting Dose Units per 0.1 mL |
|
2.5 mL |
4 Units |
Reconstituted JEUVEAU should be clear, colorless, and free of particulate matter otherwise it should not be injected. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration.
Administration
Glabellar facial lines arise from the activity of the corrugator and orbicularis oculi muscles. These muscles move the brow medially and the procerus and depressor supercilii pull the brow inferiorly. This creates a frown or “furrowed brow”. The location, size, and use of the muscles vary markedly among individuals. Lines induced by facial expression occur perpendicular to the direction of action of contracting facial muscles. An effective dose for facial lines is determined by gross observation of the patient’s ability to activate the superficial muscles injected.
In order to reduce the complication of eyelid ptosis the following steps should be taken:
- •
- Avoid injection near the levator palpebrae superioris, particularly in patients with larger brow depressor complexes.
- •
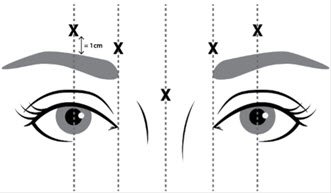
- Lateral corrugator injections should be placed at least 1 cm above the bony supraorbital ridge.
- •
- Ensure the injected volume/dose is accurate and where feasible kept to a minimum.
- •
- Avoid injecting JEUVEAU closer than 1 centimeter above the central eyebrow.
Draw at least 0.5 mL of the properly reconstituted JEUVEAU into a sterile syringe and expel any air bubbles in the syringe barrel. Remove the needle used to reconstitute the product and attach a 30-33 gauge needle. Confirm the patency of the needle. Inject a dose of 0.1 mL (4 Units) intramuscularly into each of five sites: the inferomedial and superior middle of each corrugator, and one in the mid-line of the procerus muscle for a total dose of 20 Units (See Figure 1).