Drug Detail:Lumason (Sulfur hexafluoride [ sul-fur-hex-a-flor-ide ])
Generic Name: SULFUR HEXAFLUORIDE 60.7mg in 1mg, DISTEAROYLPHOSPHATIDYLCHOLINE, DL- .19mg in 1mg, SODIUM 1, 2-DIPALMITOYL-SN-GLYCERO-3-PHOSPHO-(1'-RAC-GLYCEROL) .19mg in 1mg;
Dosage Form: injection
Drug Class: Diagnostic radiopharmaceuticals
2.1 Important Administration Instructions
Do not administer Lumason by intra-arterial injection [see Warnings and Precautions (5.3)].
2.2 Recommended Dosage
The recommended dose of Lumason after reconstitution is 2 mL administered as an intravenous bolus injection during echocardiography. During a single examination, a second injection of 2 mL may be administered to prolong contrast enhancement. Follow each Lumason injection with an intravenous flush using 5 mL of 0.9% Sodium Chloride Injection, USP.
The recommended dose of Lumason after reconstitution in pediatric patients is 0.03 mL per kg administered as an intravenous injection during echocardiography. During a single examination, a second injection of 0.03 mL per kg may be administered, if needed. Do not exceed 2 mL per injection. Follow Lumason injection with an intravenous flush using 5 mL of 0.9% Sodium Chloride Injection, USP.
The recommended dose of Lumason after reconstitution in adult patients is 2.4 mL administered as an intravenous injection during ultrasonography of the liver. During a single examination, a second injection of 2.4 mL may be administered, if needed. Follow Lumason injection with an intravenous flush using 5 mL of 0.9% Sodium Chloride Injection, USP.
The recommended dose of Lumason after reconstitution in pediatric patients is 0.03 mL per kg administered as an intravenous injection during ultrasonography of the liver. During a single examination, a second injection of 0.03 mL per kg may be administered, if needed. Do not exceed 2.4 mL per injection. Follow Lumason injection with an intravenous flush of 0.9% Sodium Chloride Injection, USP.
Ultrasonography of the Urinary Tract
The recommended dose of Lumason after reconstitution is 1 mL. The bladder may be refilled with 0.9% Sodium Chloride Injection, USP for a second cycle of voiding and imaging, without the need of a second Lumason administration.
2.3 Reconstitution Instructions
- Refer to Section 2.3.1 for instructions for using the single patient use kit with diluent provided
- Refer to Section 2.3.2 for instructions for using the 20-vial pack without diluent provided
2.3.1 Lumason Kit (single patient use kit)
- Inspect the Lumason kit and its components for signs of damage. Do not use the kit if the protective caps on the Lumason vial and prefilled syringe with 5 mL 0.9% Sodium Chloride Injection, USP are not intact or if the kit shows other signs of damage.
- Under aseptic conditions, reconstitute the Lumason vial using the following illustrated steps:
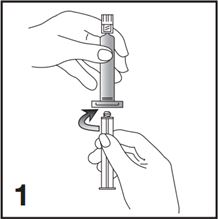
1. Connect the plunger rod to the prefilled 0.9% Sodium Chloride Injection, USP syringe barrel by screwing it clockwise into the syringe (see Figure 1).
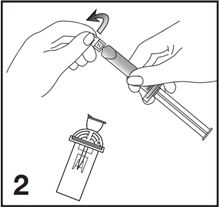
2. Open the Mini-Spike blister and remove the syringe tip cap (see Figure 2).
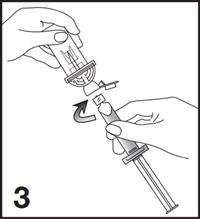
3. Remove the Mini-Spike green cap and connect the syringe to the Mini-Spike by screwing it in clockwise (see Figure 3).
4. Remove the Mini-Spike spike protection and position the spike in the center of the rubber stopper of the vial. Press firmly inward until the spike is fully inserted in the stopper (see Figure 4).

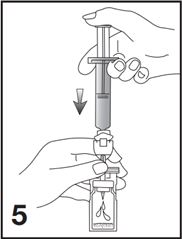
5. Empty the content of the syringe into the vial by pushing on the plunger rod (see Figure 5).
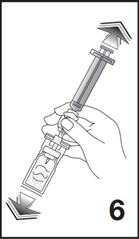
6. Shake vigorously for 20 seconds, mixing all the contents in the vial (see Figure 6). A homogeneous white milky liquid indicates formation of sulfur hexafluoride lipid microspheres.
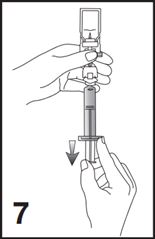
7. For preparation of doses greater than or equal to 1 mL, invert the system and slowly withdraw the intended volume of suspension into the syringe (see Figure 7). For preparation of doses less than 1 mL, withdraw 2 mL of the reconstituted suspension into the 5 mL syringe and measure the volume of Lumason to inject by using the 0.2 mL graduations between the 1 mL and 2 mL marks.
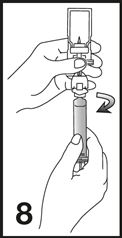
8. Unscrew the syringe from the Mini-Spike (see Figure 8). Peel and remove the diluent label to display the reconstituted product label. For intravenous administration, immediately connect the syringe to a dose administration line (20 G) and administer as directed under the Administration Instructions below. For intravesical administration, immediately connect the syringe to a sterile urinary catheter (6 French to 8 French) and administer as directed under the Administration Instructions below.
- Following reconstitution, Lumason suspension contains 1.5 to 5.6 x108 microspheres/mL with 45 mcg/mL of sulfur hexafluoride.
- Use immediately after reconstitution. If the suspension is not used immediately after reconstitution, resuspend the microspheres for a few seconds by hand agitation before the suspension is drawn into the syringe. Reconstituted suspension within a vial may be used for up to 3 hours from the time of its reconstitution. Maintain the vial containing the reconstituted suspension at room temperature 25°C (77°F); excursions permitted to 15° to 30°C (59° to 86°F).
2.3.2 Lumason Pack (20-vial pack)
*Please note: This presentation does not include pre-filled syringes of 0.9% Sodium Chloride Injection, USP (Diluent).
Lumason vials are to be used with the supplied Mini-Spike only.
Use only additive-free 0.9% Sodium Chloride Injection, USP for the reconstitution of Lumason.
- Inspect the Lumason components for signs of damage. Do not use the Lumason vial if the protective cap on the vial is not intact or other components in the pack show signs of damage.
- Use aseptic conditions for the preparation and administration of Lumason.
1. Obtain a 5 mL syringe, with luer lock tip, and fill with 5 mL of additive-free 0.9% Sodium Chloride Injection, USP (diluent) (see Figure 1).
- Two healthcare professionals (HCPs) should verify that the solution selected for reconstitution of Lumason is additive-free 0.9% Sodium Chloride Injection, USP.
- Ensure that any air in the syringe is expelled.
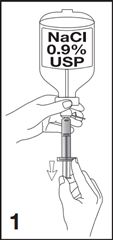
[Note: A prefilled syringe containing additive-free 0.9% Sodium Chloride Injection, USP may be used. Ensure that any air in the syringe is expelled.]
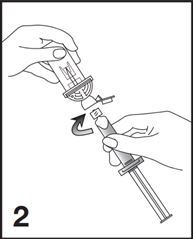
2. Remove the Mini-Spike green cap and connect the syringe to the Mini-Spike by screwing it in clockwise (see Figure 2).
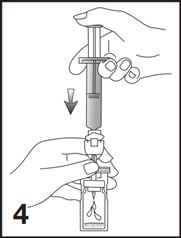
3. Remove the Mini-Spike spike protection and position the spike in the center of the rubber stopper of the vial. Press firmly inward until the spike is fully inserted in the stopper (see Figure 3).

4. Empty the entire 5 mL content of the syringe into the vial by pushing on the plunger rod (see Figure 4).
5. Shake vigorously for 20 seconds, mixing all the contents in the vial (see Figure 5). A homogeneous white milky liquid indicates formation of sulfur hexafluoride lipid microspheres.
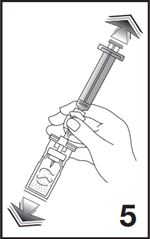
6. To obtain required dose, invert the system and slowly withdraw the intended volume of suspension into the syringe (see Figure 6).
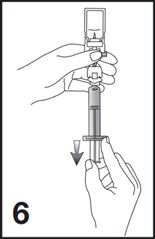
7. Unscrew the syringe from the Mini-Spike (see Figure 7).
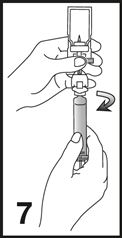
8. Label the syringe using the peel-off sticker provided.
9. For intravenous administration, immediately connect the syringe to a dose administration line (20G) and administer as directed under the Administration Instructions below. For intravesical administration, immediately connect the syringe to a sterile urinary catheter (6 French to 8 French) and administer as directed under the Administration Instructions below.
- Following reconstitution, Lumason suspension contains 1.5 to 5.6 x108 microspheres/mL with 45 mcg/mL of sulfur hexafluoride.
- Use immediately after reconstitution. If the suspension is not used immediately after reconstitution, resuspend the microspheres for a few seconds by hand agitation before the suspension is drawn into the syringe. Reconstituted suspension within a vial may be used for up to 3 hours from the time of its reconstitution. Maintain the vial containing the reconstituted suspension at room temperature 25°C (77°F); excursions permitted to 15° to 30°C (59° to 86°F).
2.4 Administration Instructions
Inspect visually for particulate matter and discoloration prior to administration, whenever solution and container permit. The reconstituted suspension is milky-white, and does not contain visible particulate matter. Do not use the single-patient use vial for more than one patient.
Administer Lumason as an intravenous bolus injection.
Intravesical Administration in Pediatric Patients
- Insert a sterile 6 French to 8 French urinary catheter into the bladder under sterile conditions;
- Empty the bladder of urine, and then fill the bladder with sterile 0.9% Sodium Chloride Injection, USP to approximately one third or half of its predicted total volume. The total bladder volume in children is calculated as [(age in years + 2) x 30] mL;
- Administer Lumason as an intravesical bolus injection through the urinary catheter;
- Continue filling the bladder with 0.9% Sodium Chloride Injection, USP until the patient has the urge to micturate or at the first sign of back pressure to the infusion.
- Immediately following the first voiding, the bladder may be refilled with 0.9% Sodium Chloride Injection, USP for a second cycle of voiding and imaging, without the need of a second Lumason administration.
2.5 Imaging Guidelines
After baseline non-contrast echocardiography is complete, adjust the mechanical index for the ultrasound device to 0.8 or lower. Continue ultrasound imaging following Lumason injection.
After identification of the target focal lesion on non-contrast ultrasound examination, hold transducer still while switching scanner to low mechanical index (≤ 0.4) contrast-specific imaging. Continue ultrasound imaging following Lumason injection.
Ultrasonography of the Urinary Tract
After baseline non-contrast ultrasound examination of the kidney and bladder, switch the scanner to low mechanical index (≤0.4) contrast specific imaging. Perform continuous alternate ultrasound imaging of the bladder, ureters, and kidneys during filling and voiding of the bladder.




