Generic name: elexacaftor, ivacaftor, and tezacaftor
Drug class: CFTR combinations
Dosage form: oral tablets, oral granules
Availability: Prescription only
Pregnancy & Lactation: Risk data available
Brand names: Elexacaftor, ivacaftor, and tezacaftor, Elexacaftor, tezacaftor, and ivacaftor (monograph)
What is Trikafta?
Trikafta is an oral combination medication that contains elexacaftor, ivacaftor, and tezacaftor, which may be used to treat adults and children aged 2 years and older with cystic fibrosis who have at least one copy of the F508del mutation in the cystic fibrosis transmembrane conductance regulator (CFTR) gene or another mutation that is responsive to treatment with Trikafta.
Cystic fibrosis (CF) is caused by mutations in the CF gene, which causes a specific protein, called the cystic fibrosis transmembrane conductance regulator (CFTR) protein to not work the way it should. Fewer CFTR proteins get to the cell surface and, if they do reach the cell surface, do not open the way they should, which prevents the proper movements of chloride ions in and out of the cell. As a result, thick sticky mucus builds up in the organs, including the lungs.
The three ingredients in Trikafta work together to improve the functioning of the CFTR protein:
- Elexacaftor and tezacaftor help more CFTR proteins reach the surface
- Ivacaftor helps the CFTR proteins stay open for longer at the cell surface, allowing more chloride ions to flow through, which thins the mucus.
Before you take this medicine, you may need an FDA-approved CF mutation test to make sure you have either the F508del mutation or at least one other eligible mutation.
Trikafta was first FDA-approved on October 21, 2019.
Warnings
Avoid in people with a known hypersensitivity to Trikafta or one of its components. Angioedema and anaphylaxis have been reported.
Use of Trikafta should be avoided in people with pre-existing advanced liver disease, (such as cirrhosis, portal hypertension, ascites, hepatic encephalopathy) unless the benefits outweigh the risks, as cases of liver failure leading to transplantation have been reported. Your healthcare provider will monitor your liver function throughout treatment.
Cataracts have been reported in pediatric patients treated with medications containing ivacaftor. Baseline and follow-up eye examinations should be conducted in children.
Trikafta may affect the way other medicines work, and other medicines may affect how Trikafta works. Tell your doctor about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
It is not known if Trikafta is safe and effective in children under 2 years of age.
How should I take Trikafta
Take Trikafta exactly as prescribed by your doctor. Follow all directions on your prescription label and read all medication guides or instruction sheets.
Trikafta must be taken with a meal or snack that contains fat to help your body absorb the medicine. Some ways you can add fat to your snack include:
- Pouring on extra virgin olive oil
- Mixing in sliced almonds
- Spreading on butter.
Or take it with a meal such as a bagel with peanut butter, macaroni cheese, a cheese sandwich, or hard-boiled eggs.
You will need frequent blood tests to check your liver function.
A child using Trikafta may need frequent eye exams to check for cataracts.
Dosing information
Trikafta is supplied as tablets for adults and children aged 6 years and older, and as granules for children aged 2 years to less than 6.
Trikafta tablets
Follow your doctor's dosing instructions very carefully. Your doctor may change your dose if you also take certain other medications or if you lose weight. Your doctor may tell you to take the orange tablets and the blue tablet on separate days. Do not change your dose or dosing schedule without your doctor's advice.
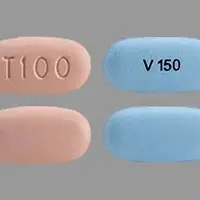
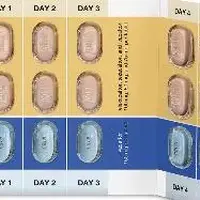
Your Trikafta tablets will come in a box of 4 weekly blister cards. Each blister card contains 21 tablets. The tablets are arranged so that you take 2 light orange/orange tablets in the morning and one light blue/blue tablet at night (the shade of tablet prescribed depends on your weight and age)
Children aged 6 through 11 years weighing 30kg (66lb) or more, and adults and children aged 12 years and older
- Two orange tablets in the morning and 1 blue tablet in the evening (about 12 hours later).
- Swallow Trikafta tablets whole and do not crush, chew, or break them. Take with a meal or snack containing fat, such as butter, peanut butter, eggs, nuts, meat, whole milk, cheese, or yogurt.
Children aged 6 through 11 years weighing less than 30 kg (66lb)
- Two light orange tablets in the morning and 1 light blue tablet in the evening (about 12 hours later)
- Swallow Trikafta tablets whole and do not crush, chew, or break them. Take with a meal or snack containing fat, such as butter, peanut butter, eggs, nuts, meat, whole milk, cheese, or yogurt.
Trikafta granules
For children aged 2 to less than 6 years, the dosage depends on weight.
14 kg (31lb) or more
- Morning: One white and orange higher-strength packet of granules (elexacaftor 100 mg/tezacaftor 50 mg/ivacaftor 75mg)
- Evening: One white and pink higher-strength packet of granules (ivacaftor 75mg)
Mix with 1 teaspoon (5mL) of soft food or liquid and give with a fat-containing food.
Less than 14 kg (31lb)
- Morning: One white and blue lower-strength packet of granules (elexacaftor 80 mg/tezacaftor 40 mg/ivacaftor 60mg)
- Evening: One white and green lower-strength packet of granules (ivacaftor 59.5 mg)
Mix with 1 teaspoon (5mL) of soft food or liquid and give with a fat-containing food.
Before Taking
Tell your doctor about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Trikafta may affect the way other medicines work and other medicines may affect how it works. The dose of Trikafta may need to be adjusted when taken with certain medicines. Ask your doctor or pharmacist for a list of these medicines if you are not sure.
Tell your doctor if you take:
- antibiotics such as rifampin or rifabutin
- seizure medications such as phenobarbital, carbamazepine, or phenytoin
- St. John’s wort
- antifungal medicines including ketoconazole, itraconazole, posaconazole, voriconazole, or fluconazole.
- antibiotics including telithromycin, clarithromycin, or erythromycin.
Know the medicines you take. Keep a list of them to show your doctor and pharmacist when you get a new medicine
To make sure Trikafta is safe for you, tell your doctor if you have ever had:
- an allergy to Trikafta or any of its components
- liver disease or
- kidney disease.
- are pregnant or plan to become pregnant. It is not known if Trikafta will harm your unborn baby. You and your doctor should decide if you should take Trikafta while you are pregnant
- are breastfeeding or planning to breastfeed. It is not known if Trikafta passes into your breast milk. You and your doctor should decide if you should take it while you are breastfeeding.
What happens if I miss a dose?
If 6 hours or less have passed since the missed morning or evening dose, then take the missed dose as soon as possible and continue on the original schedule.
If more than 6 hours have passed since:
- the missed morning dose, the patient should take the missed dose as soon as possible and should not take the evening dose. The next scheduled morning dose should be taken at the usual time.
- the missed evening dose, the patient should not take the missed dose. The next scheduled morning dose should be taken at the usual time.
Morning and evening doses should not be taken at the same time. Take the medicine as soon as you can, but do not take two doses at one time.
What happens if I overdose?
Seek emergency medical attention or call the Poison Help line at 1-800-222-1222.
What should I avoid while using Trikafta?
Avoid driving or hazardous activity until you know how Trikafta will affect you. Your reactions could be impaired.
Grapefruit may interact with Trikafta and lead to unwanted side effects. Avoid the use of grapefruit products.
Be sure to ask your healthcare provider or pharmacist if any new medication or supplement is compatible with Trikafta. Many medicines that interact with it.
Trikafta side effects
Get emergency medical help if you have signs of an allergic reaction to Trikafta: hives; difficulty breathing; swelling of your face, lips, tongue, or throat.
Call your doctor at once if you have:
- vision changes or
- liver problems - loss of appetite, stomach pain (upper right side), dark urine, jaundice (yellowing of the skin or eyes).
Common Trikafta side effects affecting more than 5% of patients may include:
- headache
- flu with symptoms such as fever, chills, or body aches
- colds with symptoms such as stuffy nose, sinus pain, sneezing, sore throat
- diarrhea, stomach pain
- rash
- abnormal lab tests.
This is not a complete list of side effects and others may occur. Call your doctor for medical advice about side effects. You may report side effects to the FDA at 1-800-FDA-1088.
See more: Trikafta Side EffectsWhat other drugs will affect Trikafta?
Sometimes it is not safe to use certain medications at the same time. Some drugs can affect your blood levels of other drugs you take, which may increase side effects or make the medications less effective.
Tell your doctor about all your other medicines, especially:
- Antibiotics, such as clarithromycin, erythromycin, or telithromycin
- Antifungal medicines, such as fluconazole, ketoconazole, itraconazole, posaconazole, or voriconazole
- Moderate to strong CYP3A Inhibitors, such as nefazodone, atazanavir, ritonavir, grapefruit juice, or verapamil
- Moderate to strong CYP3A inducers, such as glucocorticoids, rifampin, carbamazepine, phenobarbital, and phenytoin. Avoid coadministration.
This list is not complete. Other drugs may interact with the components of Trikafta (elexacaftor, ivacaftor, and tezacaftor) including prescription and over-the-counter medicines, vitamins, and herbal products. Not all possible drug interactions are listed here.