Drug Detail:Amphadase (Hyaluronidase (injection) [ hye-al-ure-on-i-dase ])
Drug Class: Miscellaneous uncategorized agents
Highlights of Prescribing Information
Amphadase® (hyaluronidase injection)
Initial U.S. Approval: 2005
Recent Major Changes
• Dosage and Administration (2) --- 3/2012
• Contraindications, Hypersensitivity (4.1) --- 3/2012
• Warnings and Precautions, Spread of Localized Infections (5.1) --- 3/2012
• Warnings and Precautions, Ocular Damage (5.2) --- 3/2012
• Warnings and Precautions, Enzyme Inactivation with Intravenous Administration (5.3) --- 3/2012
Indications and Usage for Amphadase
Amphadase® is indicated as an adjuvant
• in subcutaneous fluid administration for achieving hydration (1.1)
• to increase absorption and dispersion of other injected drugs (1.2)
• in subcutaneous urography for improving resorption of radiopaque agents (1.3)
Amphadase Dosage and Administration
• Subcutaneous Fluid Administration
Insert needle with aseptic precautions. With tip lying free and movable between skin and muscle, begin clysis; fluid should start in readily without pain or lump. Then inject Amphadase®
(hyaluronidase injection) into rubber tubing close to needle. (2.1)
• Absorption and Dispersion of Injected Drugs
Absorption and dispersion of other injected drugs may be enhanced by adding 50-300 Units, most typically 150 U hyaluronidase, to the injection solution. (2.2)
• Subcutaneous Urography
The subcutaneous route of administration of urographic contrast media is indicated when intravenous administration cannot be successfully accomplished, particularly in infants and small children. With the patient prone, 75 U of Amphadase® (hyaluronidase injection) is injected subcutaneously over each scapula, followed by injection of the contrast medium at the same sites. (2.3)
Dosage Forms and Strengths
150 USP units/mL single dose vials (3)
Contraindications
Hypersensitivity (4.1)
Warnings and Precautions
• Spread of Localized Infection (5.1)
• Ocular Damage (5.2)
• Enzyme Inactivation with Intravenous Administration (5.3)
Adverse Reactions/Side Effects
Allergic and anaphylactic-like reactions have been reported, rarely (6)
To report SUSPECTED ADVERSE REACTIONS, contact Amphastar Pharmaceuticals, Inc. at 1-800-423-4136 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
• Furosemide, the benzodiazepines and phenytoin are incompatible with hyaluronidase (7.1)
• Hyaluronidase should not be used to enhance the absorption and dispersion of dopamine and/or alpha agonist drugs (7.2)
• Local anesthetics: Hyaluronidase hastens onset and shortens duration of effect, increases incidence of systemic reactions (7.3)
• Large doses of salicylates, cortisone, ACTH, estrogens or antihistamines may require larger amounts of hyaluronidase for equivalent dispersing effect (7.4)
Use In Specific Populations
Pediatric Use: The dosage of subcutaneous fluids administered is dependent upon the age, weight, and clinical condition of the patient. For premature infants or during the neonatal period, the daily dosage should not exceed 25 mL/kg of body weight, and the rate of administration should not be greater than 2 mL per minute. Special care must be taken in pediatric patients to avoid over hydration by controlling the rate and total volume of the infusion. (2.1, 8.4)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 11/2016
Full Prescribing Information
2. Amphadase Dosage and Administration
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
6. Adverse Reactions/Side Effects
The following adverse reactions have been identified during post-approval use of hyaluronidase products. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. The most frequently reported adverse reactions have been local injection site reactions.
Hyaluronidase has been reported to enhance the adverse reactions associated with co-administered drug products. Edema has been reported most frequently in association with hypodermoclysis.
Allergic reactions (urticaria, angioedema) have been reported in less than 0.1% of patients receiving hyaluronidase. Anaphylactic-like reactions following retrobulbar block or intravenous injections have occurred, rarely.
7. Drug Interactions
It is recommended that appropriate references be consulted regarding physical or chemical incompatibilities before adding Amphadase® to a solution containing another drug.
11. Amphadase Description
Amphadase® is a preparation of purified bovine testicular hyaluronidase, a protein enzyme. The exact chemical structure of this enzyme is unknown. However, the amino acid sequence for the primary structure of the enzyme has been deduced from the sequence of purified peptides.
Amphadase® (hyaluronidase injection) is supplied as a sterile, clear, colorless, ready for use solution. Each vial contains 150 USP units of hyaluronidase per mL with 8.5 mg sodium chloride, 1 mg edetate disodium, 0.4 mg calcium chloride, monobasic sodium phosphate buffer, and not more than 0.1 mg thimerosal (mercury derivative).
Amphadase® has an approximate pH of 6.8 and an osmolality of 295 to 355 mOsm.
16. How is Amphadase supplied
Amphadase® (hyaluronidase injection) is supplied sterile as 150 USP units of hyaluronidase per mL in a 2 mL single-use glass vial with a gray rubber stopper and aluminum flip-off seal.
NDC 0548-9090-10, 1 mL vial, 10 vials/carton.
Store unopened in a refrigerator at 2° to 8°C (36° to 46° F).
17. Patient Counseling Information
17.1 Important Precautions Regarding Amphadase®
Instruct patient that Amphadase® is being used to increase the dispersion and absorption of fluids or other injected drugs, as appropriate to the intended use.
Instruct patient that there may be mild local injection site signs and symptoms, such as redness, swelling, itching, or pain localized to the site of injection.
17.2 What Patients Should Know About Adverse Reactions
The most frequently reported adverse reactions have been mild local injection site reactions such as redness, swelling, itching, or pain.
Anaphylactic-like reactions, and allergic reactions, such as hives, have been reported rarely in patients receiving hyaluronidases.
17.3 Patients Should Inform Their Doctors If Taking Other Medications
You may not receive furosemide, the benzodiazepines, phenytoin, dopamine and/or alpha agonists with Amphadase®. These medications have been found to be incompatible with hyaluronidase.
If you are taking salicylates (e.g., aspirin), steroids (e.g., cortisone or estrogens), or antihistamines your doctor may need to prescribe larger amounts of hyaluronidase for equivalent dispersing effect.
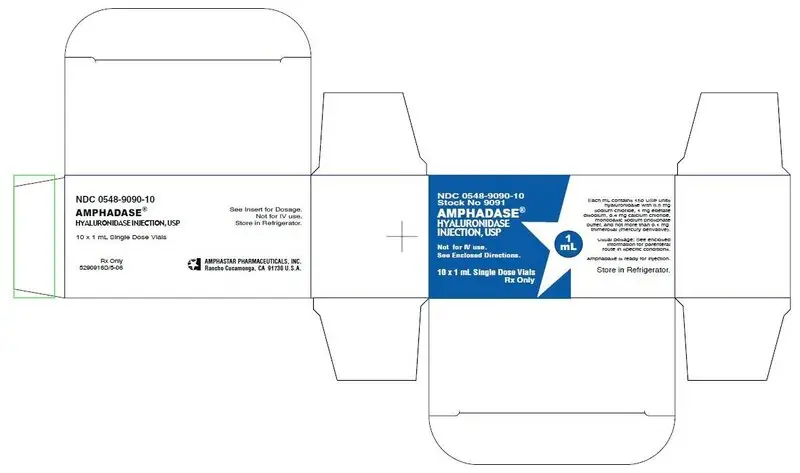
PRINCIPLE DISPLAY PANEL: Carton: 1mL
NDC 0548-9090-10
Stock No 9091
AMPHADASE® HYALURONIDASE INJECTION, USP
Not for IV use.
See Enclosed Directions.
10 x 1 mL Single Dose Vials
Rx Only
1 mL
Each mL contains 150 USP units hyaluronidase with 8.5 mg sodium chloride,
1 mg edetate disodium, 0.4 mg calcium chloride, monobasic sodium phosphate buffer, and
not more than 0.1 mg thimerosal (mercury derivative).
Usual dosage: See enclosed information for parenteral route and specific conditions.
Amphadase is ready for injection.
Store in Refrigerator
|
AMPHADASE
hyaluronidase injection |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Amphastar Pharmaceuticals, Inc. (024736733) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Amphastar Pharmaceuticals, Inc. | 024736733 | analysis(0548-9090) , manufacture(0548-9090) | |




