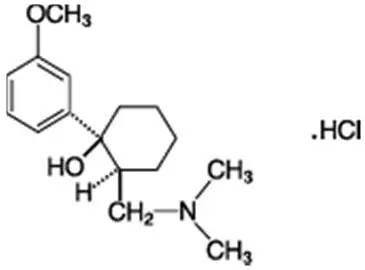
Drug Detail:Hyoscyamine (Hyoscyamine [ hye-oh-sye-a-meen ])
Drug Class: Anticholinergics / antispasmodics
Precautions
General: Use with caution and only when clearly indicated in patients with autonomic neuropathy, hyperthyroidism, coronary heart disease, congestive heart failure, cardiac arrhythmias, hypertension, and renal disease. Investigate any tachycardia before giving any anticholinergic drug since they may increase the heart rate. Use with caution in patients with hiatal hernia associated with reflux esophagitis.
Information for Patients: ANASPAZ may produce drowsiness, dizziness or blurred vision. Patients should observe caution before operating a motor vehicle or other machinery or performing other tasks requiring mental alertness.
Use of ANASPAZ may decrease sweating resulting in heat prostration, fever or heat stroke; febrile patients or those who may be exposed to elevated environmental temperatures should use caution.
Drug Interactions: Additive adverse effects resulting from cholinergic blockade may occur when ANASPAZ is administered concomitantly with other anti-muscarinics, amantadine, haloperidol, phenothiazines, monoamine oxidase (MAO) inhibitors, tricyclic antidepressants or some antihistamines.
Antacids may interfere with the absorption of ANASPAZ; take ANASPAZ before meals and antacids after meals.
Carcinogenesis, Mutagenesis, Impairment of Fertility: No long-term studies in animals have been performed to evaluate the carcinogenic, mutagenic or impairment of fertility potential of ANASPAZ in either males or females.
Pregnancy-Pregnancy Category C: Animal reproduction studies have not been conducted with ANASPAZ. It is also not known whether ANASPAZ can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. ANASPAZ should be given to a pregnant woman only if clearly needed.
Nursing Mothers: ANASPAZ is excreted in human milk. Caution should be exercised when ANASPAZ is administered to a nursing woman.
Pediatric Use: This product is not recommended for use in children under twelve years of age. Infants and young children are especially susceptible to the toxic effects of anticholinergics. Close supervision is recommended for infants and children with spastic paralysis or brain damage since an increased response to anticholinergics has been reported in these patients and dosage adjustments are often required. When anticholinergics are given to children where the environmental temperature is high, there is a risk of a rapid increase in body temperature because of these medications’ suppression of sweat gland activity.
A paradoxical reaction characterized by hyperexcitability may occur in children taking large doses of anticholinergics.
Geriatric Use: This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function. Sedating drugs may cause confusion and over-sedation in the elderly; elderly patients generally should be started on low doses of ANASPAZ and observed closely.
| ANASPAZ
hyoscyamine sulfate tablet, orally disintegrating |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - BF ASCHER AND CO INC (003854403) |