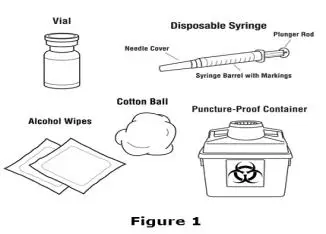
Drug Detail:Aranesp (Darbepoetin alfa [ dar-be-poe-e-tin-al-fa ])
Drug Class: Recombinant human erythropoietins
Highlights of Prescribing Information
ARANESP® (darbepoetin alfa) injection, for intravenous or subcutaneous use
Initial U.S. Approval: 2001
WARNING: ESAs INCREASE THE RISK OF DEATH, MYOCARDIAL INFARCTION, STROKE, VENOUS THROMBOEMBOLISM, THROMBOSIS OF VASCULAR ACCESS AND TUMOR PROGRESSION OR RECURRENCE
See full prescribing information for complete boxed warning.
Chronic Kidney Disease:
-
In controlled trials, patients experienced greater risks for death, serious adverse cardiovascular reactions, and stroke when administered erythropoiesis-stimulating agents (ESAs) to target a hemoglobin level of greater than 11 g/dL (5.1).
-
No trial has identified a hemoglobin target level, Aranesp dose, or dosing strategy that does not increase these risks (2.2).
- Use the lowest Aranesp dose sufficient to reduce the need for red blood cell (RBC) transfusions (5.1).
Cancer:
-
ESAs shortened overall survival and/or increased the risk of tumor progression or recurrence in clinical studies of patients with breast, non-small cell lung, head and neck, lymphoid, and cervical cancers (5.2).
-
Use the lowest dose to avoid RBC transfusions (2.3).
-
Use ESAs only for anemia from myelosuppressive chemotherapy (1.2).
-
ESAs are not indicated for patients receiving myelosuppressive chemotherapy when the anticipated outcome is cure (1.3).
- Discontinue following the completion of a chemotherapy course (2.3).
Recent Major Changes
- Warnings and Precautions, Increased Mortality and/or Increased Risk of Tumor Progression or Recurrence in Patients with Cancer (5.2) 1/2019
Indications and Usage for Aranesp
Aranesp is an erythropoiesis-stimulating agent (ESA) indicated for the treatment of anemia due to:
- Chronic Kidney Disease (CKD) in patients on dialysis and patients not on dialysis (1.1).
- The effects of concomitant myelosuppressive chemotherapy, and upon initiation, there is a minimum of two additional months of planned chemotherapy (1.2).
Limitations of Use
Aranesp has not been shown to improve quality of life, fatigue, or patient well-being (1.3).
Aranesp is not indicated for use:
- In patients with cancer receiving hormonal agents, biologic products, or radiotherapy, unless also receiving concomitant myelosuppressive chemotherapy (1.3).
- In patients with cancer receiving myelosuppressive chemotherapy when the anticipated outcome is cure (1.3).
- In patients with cancer receiving myelosuppressive chemotherapy in whom the anemia can be managed by transfusion (1.3).
- As a substitute for RBC transfusions in patients who require immediate correction of anemia (1.3).
Aranesp Dosage and Administration
- Recommended starting dose for patients with CKD on dialysis (2.2):
- 0.45 mcg/kg intravenously or subcutaneously weekly, or
- 0.75 mcg/kg intravenously or subcutaneously every 2 weeks
- Intravenous route is recommended for patients on hemodialysis
- Recommended starting dose for patients with CKD not on dialysis (2.2):
- 0.45 mcg/kg intravenously or subcutaneously at 4 week intervals
- Recommended starting dose for pediatric patients with CKD:
- 0.45 mcg/kg intravenously or subcutaneously weekly
- patients with CKD not on dialysis may also be initiated at 0.75 mcg/kg every 2 weeks
- Recommended starting dose for patients with cancer on chemotherapy (2.3):
- 2.25 mcg/kg subcutaneously weekly, or
- 500 mcg subcutaneously every 3 weeks
Dosage Forms and Strengths
Single-dose vials
- Injection: 25 mcg, 40 mcg, 60 mcg, 100 mcg, 200 mcg and 300 mcg (3).
Single-dose prefilled syringes
- Injection: 10 mcg/0.4 mL, 25 mcg/0.42 mL, 40 mcg/0.4 mL,
60 mcg/0.3 mL, 100 mcg/0.5 mL, 150 mcg/0.3 mL, 200 mcg/0.4 mL,
300 mcg/0.6 mL, and 500 mcg/1 mL (3).
Contraindications
- Uncontrolled hypertension (4)
- Pure red cell aplasia (PRCA) that begins after treatment with Aranesp or other erythropoietin protein drugs (4)
- Serious allergic reactions to Aranesp (4)
Warnings and Precautions
- Increased Mortality, Myocardial Infarction, Stroke, and Thromboembolism: Using Aranesp to target a hemoglobin level of greater than 11 g/dL increases the risk of serious adverse cardiovascular reactions and has not been shown to provide additional benefit (5.1 and 14.1). Use caution in patients with coexistent cardiovascular disease and stroke (5.1).
- Increased Mortality and/or Increased Risk of Tumor Progression or Recurrence in Patients with Cancer (5.2).
- Hypertension: Control hypertension prior to initiating and during treatment with Aranesp (5.3).
- Seizures: Aranesp increases the risk for seizures in patients with CKD (5.4). Increase monitoring of these patients for changes in seizure frequency or premonitory symptoms (5.4).
- PRCA: If severe anemia and low reticulocyte count develop during Aranesp treatment, withhold Aranesp and evaluate for PRCA (5.6).
- Serious Allergic Reactions: Discontinue Aranesp and manage reactions (5.7).
- Severe Cutaneous Reactions: Discontinue Aranesp (5.8).
Adverse Reactions/Side Effects
- Patients with CKD: Adverse reactions in ≥ 10% of Aranesp-treated patients in clinical studies were hypertension, dyspnea, peripheral edema, cough, and procedural hypotension (6.1).
- Patients with Cancer Receiving Chemotherapy: Adverse reactions in ≥ 1% of Aranesp-treated patients in clinical studies were abdominal pain, edema, and thrombovascular events (6.1).
To report SUSPECTED ADVERSE REACTIONS, contact Amgen Medical Information at 1-800-77-AMGEN (1-800-772-6436) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 1/2019
Full Prescribing Information
WARNING: ESAs INCREASE THE RISK OF DEATH, MYOCARDIAL INFARCTION, STROKE, VENOUS THROMBOEMBOLISM, THROMBOSIS OF VASCULAR ACCESS AND TUMOR PROGRESSION OR RECURRENCE
Chronic Kidney Disease:
-
In controlled trials, patients experienced greater risks for death, serious adverse cardiovascular reactions, and stroke when administered erythropoiesis-stimulating agents (ESAs) to target a hemoglobin level of greater than 11 g/dL [see Warnings and Precautions (5.1)].
-
No trial has identified a hemoglobin target level, Aranesp dose, or dosing strategy that does not increase these risks [see Dosage and Administration (2.2)].
- Use the lowest Aranesp dose sufficient to reduce the need for red blood cell (RBC) transfusions [see Warnings and Precautions (5.1)].
Cancer:
-
ESAs shortened overall survival and/or increased the risk of tumor progression or recurrence in clinical studies of patients with breast, non-small cell lung, head and neck, lymphoid, and cervical cancers [see Warnings and Precautions (5.2)].
-
To decrease these risks, as well as the risk of serious cardiovascular and thromboembolic reactions, use the lowest dose needed to avoid RBC transfusions [see Dosage and Administration (2.3)].
-
Use ESAs only for anemia from myelosuppressive chemotherapy [see Indications and Usage (1.2)].
-
ESAs are not indicated for patients receiving myelosuppressive chemotherapy when the anticipated outcome is cure [see Indications and Usage (1.3)].
- Discontinue following the completion of a chemotherapy course [see Dosage and Administration (2.3)].
1. Indications and Usage for Aranesp
1.1 Anemia Due to Chronic Kidney Disease
Aranesp is indicated for the treatment of anemia due to chronic kidney disease (CKD), including patients on dialysis and patients not on dialysis.
1.2 Anemia Due to Chemotherapy in Patients with Cancer
Aranesp is indicated for the treatment of anemia in patients with non-myeloid malignancies where anemia is due to the effect of concomitant myelosuppressive chemotherapy, and upon initiation, there is a minimum of two additional months of planned chemotherapy.
1.3 Limitations of Use
Aranesp has not been shown to improve quality of life, fatigue, or patient well-being.
Aranesp is not indicated for use:
• In patients with cancer receiving hormonal agents, biologic products, or radiotherapy, unless also receiving concomitant myelosuppressive chemotherapy.
• In patients with cancer receiving myelosuppressive chemotherapy when the anticipated outcome is cure.
• In patients with cancer receiving myelosuppressive chemotherapy in whom the anemia can be managed by transfusion.
• As a substitute for RBC transfusions in patients who require immediate correction of anemia.
2. Aranesp Dosage and Administration
2.2 Patients with Chronic Kidney Disease
In controlled trials, patients experienced greater risks for death, serious adverse cardiovascular reactions, and stroke when administered erythropoiesis-stimulating agents (ESAs) to target a hemoglobin level of greater than 11 g/dL. No trial has identified a hemoglobin target level, Aranesp dose, or dosing strategy that does not increase these risks. Individualize dosing and use the lowest dose of Aranesp sufficient to reduce the need for RBC transfusions [see Warnings and Precautions (5.1)]. Physicians and patients should weigh the possible benefits of decreasing transfusions against the increased risks of death and other serious cardiovascular adverse events [see Boxed Warning and Clinical Studies (14)].
For all patients with CKD
When initiating or adjusting therapy, monitor hemoglobin levels at least weekly until stable, then monitor at least monthly. When adjusting therapy consider hemoglobin rate of rise, rate of decline, ESA responsiveness and hemoglobin variability. A single hemoglobin excursion may not require a dosing change.
- Do not increase the dose more frequently than once every 4 weeks. Decreases in dose can occur more frequently. Avoid frequent dose adjustments.
- If the hemoglobin rises rapidly (e.g., more than 1 g/dL in any 2-week period), reduce the dose of Aranesp by 25% or more as needed to reduce rapid responses.
- For patients who do not respond adequately, if the hemoglobin has not increased by more than 1 g/dL after 4 weeks of therapy, increase the dose by 25%.
- For patients who do not respond adequately over a 12-week escalation period, increasing the Aranesp dose further is unlikely to improve response and may increase risks. Use the lowest dose that will maintain a hemoglobin level sufficient to reduce the need for RBC transfusions. Evaluate other causes of anemia. Discontinue Aranesp if responsiveness does not improve.
For adult patients with CKD on dialysis:
- Initiate Aranesp treatment when the hemoglobin level is less than 10 g/dL.
- If the hemoglobin level approaches or exceeds 11 g/dL, reduce or interrupt the dose of Aranesp.
- The recommended starting dose is 0.45 mcg/kg intravenously or subcutaneously as a weekly injection or 0.75 mcg/kg once every 2 weeks as appropriate. The intravenous route is recommended for patients on hemodialysis.
For adult patients with CKD not on dialysis:
- Consider initiating Aranesp treatment only when the hemoglobin level is less than 10 g/dL and the following considerations apply:
° The rate of hemoglobin decline indicates the likelihood of requiring a RBC transfusion and,
° Reducing the risk of alloimmunization and/or other RBC transfusion-related risks is a goal.
- If the hemoglobin level exceeds 10 g/dL, reduce or interrupt the dose of Aranesp, and use the lowest dose of Aranesp sufficient to reduce the need for RBC transfusions.
- The recommended starting dose is 0.45 mcg/kg body weight intravenously or subcutaneously given once at four week intervals as appropriate.
For pediatric patients with CKD:
- Initiate Aranesp treatment when the hemoglobin level is less than 10 g/dL.
- If the hemoglobin level approaches or exceeds 12 g/dL, reduce or interrupt the dose of Aranesp.
- The recommended starting dose for pediatric patients (less than 18 years) is 0.45 mcg/kg body weight administered as a single subcutaneous or intravenous injection once weekly; patients not receiving dialysis may be initiated at a dose of 0.75 mcg/kg once every 2 weeks.
When treating patients who have chronic kidney disease and cancer, physicians should refer to Warnings and Precautions (5.1 and 5.2).
Conversion from Epoetin alfa to Aranesp in patients with CKD on dialysis
Aranesp is administered less frequently than epoetin alfa.
- Administer Aranesp once weekly in patients who were receiving epoetin alfa 2 to 3 times weekly.
- Administer Aranesp once every 2 weeks in patients who were receiving epoetin alfa once weekly.
Estimate the starting weekly dose of Aranesp for adults and pediatric patients on the basis of the weekly epoetin alfa dose at the time of substitution (see Table 1). Maintain the route of administration (intravenous or subcutaneous injection).
| Previous Weekly Epoetin alfa Dose (Units/week) | Aranesp Dose (mcg/week) | |
| Adult | Pediatric | |
| < 1,500 | 6.25 | * |
| 1,500 to 2,499 | 6.25 | 6.25 |
| 2,500 to 4,999 | 12.5 | 10 |
| 5,000 to 10,999 | 25 | 20 |
| 11,000 to 17,999 | 40 | 40 |
| 18,000 to 33,999 | 60 | 60 |
| 34,000 to 89,999 | 100 | 100 |
| ≥ 90,000 | 200 | 200 |
*For pediatric patients receiving a weekly epoetin alfa dose of < 1,500 Units/week, the available data are insufficient to determine an Aranesp conversion dose.
Conversion from Epoetin alfa to Aranesp in patients with CKD not on dialysis
Refer to Table 1. The dose conversion depicted in Table 1 does not accurately estimate the once monthly dose of Aranesp.
2.3 Patients on Cancer Chemotherapy
Initiate Aranesp in patients on cancer chemotherapy only if the hemoglobin is less than 10 g/dL, and if there is a minimum of two additional months of planned chemotherapy.
Use the lowest dose of Aranesp necessary to avoid RBC transfusions.
Recommended Starting Dose
The recommended starting dose and schedules are:
- 2.25 mcg/kg every week subcutaneously until completion of a chemotherapy course.
- 500 mcg every 3 weeks subcutaneously until completion of a chemotherapy course.
| Dose Adjustment | Weekly Schedule | Every 3 Week Schedule |
| Reduce dose by 40% | Reduce dose by 40% |
| If hemoglobin exceeds a level needed to avoid RBC transfusion |
|
|
| If hemoglobin increases by less than 1 g/dL and remains below 10 g/dL after 6 weeks of therapy | Increase dose to 4.5 mcg/kg/week | No dose adjustment |
| Discontinue Aranesp | Discontinue Aranesp |
3. Dosage Forms and Strengths
Aranesp is a clear, colorless solution available as:
Single-dose vials
- Injection: 25 mcg, 40 mcg, 60 mcg, 100 mcg, 200 mcg and 300 mcg
Single-dose prefilled syringes
- Injection: 10 mcg/0.4 mL, 25 mcg/0.42 mL, 40 mcg/0.4 mL, 60 mcg/0.3 mL, 100 mcg/0.5 mL,
150 mcg/0.3 mL, 200 mcg/0.4 mL, 300 mcg/0.6 mL, and 500 mcg/1 mL
4. Contraindications
Aranesp is contraindicated in patients with:
- Uncontrolled hypertension [see Warnings and Precautions (5.3)].
- Pure red cell aplasia (PRCA) that begins after treatment with Aranesp or other erythropoietin protein drugs [see Warnings and Precautions (5.6)].
- Serious allergic reactions to Aranesp [see Warnings and Precautions (5.7)].
5. Warnings and Precautions
5.1 Increased Mortality, Myocardial Infarction, Stroke, and Thromboembolism
- In controlled clinical trials of patients with CKD comparing higher hemoglobin targets (13 - 14 g/dL) to lower targets (9 - 11.3 g/dL), Aranesp and other ESAs increased the risk of death, myocardial infarction, stroke, congestive heart failure, thrombosis of hemodialysis vascular access, and other thromboembolic events in the higher target groups.
- Using Aranesp to target a hemoglobin level of greater than 11 g/dL increases the risk of serious adverse cardiovascular reactions and has not been shown to provide additional benefit [see Clinical Studies (14.1)]. Use caution in patients with coexistent cardiovascular disease and stroke [see Dosage and Administration (2.2)]. Patients with CKD and an insufficient hemoglobin response to ESA therapy may be at even greater risk for cardiovascular reactions and mortality than other patients. A rate of hemoglobin rise of greater than 1 g/dL over 2 weeks may contribute to these risks.
- In controlled clinical trials of patients with cancer, Aranesp and other ESAs increased the risks for death and serious adverse cardiovascular reactions. These adverse reactions included myocardial infarction and stroke.
- In controlled clinical trials, ESAs increased the risk of death in patients undergoing coronary artery bypass graft surgery (CABG) and the risk of deep venous thrombosis (DVT) in patients undergoing orthopedic procedures.
The design and overall results of the 3 large trials comparing higher and lower hemoglobin targets are shown in Table 3.
| Normal Hematocrit Study (NHS)
(N = 1265) | CHOIR
(N = 1432) | TREAT
(N = 4038) |
|
| Time Period of Trial | 1993 to 1996 | 2003 to 2006 | 2004 to 2009 |
| Population | Adult patients with CKD on hemodialysis with coexisting CHF or CAD, hematocrit 30 ± 3% on epoetin alfa | Adult patients with CKD not on dialysis with hemoglobin < 11 g/dL not previously administered epoetin alfa | Adult patients with CKD not on dialysis with type II diabetes, hemoglobin ≤ 11 g/dL |
| Hemoglobin Target; Higher vs. Lower (g/dL) | 14.0 vs. 10.0 | 13.5 vs. 11.3 | 13.0 vs. ≥ 9.0 |
| Median (Q1, Q3) Achieved Hemoglobin level (g/dL) | 12.6 (11.6, 13.3) vs. 10.3 (10.0, 10.7) | 13.0 (12.2, 13.4) vs. 11.4 (11.1, 11.6) | 12.5 (12.0, 12.8) vs. 10.6 (9.9, 11.3) |
| Primary Endpoint | All-cause mortality or non-fatal MI | All-cause mortality, MI, hospitalization for CHF, or stroke | All-cause mortality, MI, myocardial ischemia, heart failure, and stroke |
| Hazard Ratio or Relative Risk (95% CI) | 1.28 (1.06 - 1.56) | 1.34 (1.03 - 1.74) | 1.05 (0.94 - 1.17) |
| Adverse Outcome for Higher Target Group | All-cause mortality | All-cause mortality | Stroke |
| Hazard Ratio or Relative Risk (95% CI) | 1.27 (1.04 - 1.54) | 1.48 (0.97 - 2.27) | 1.92 (1.38 - 2.68) |
Patients with Chronic Kidney Disease
Normal Hematocrit Study (NHS): A prospective, randomized, open-label study of 1265 patients with chronic kidney disease on dialysis with documented evidence of congestive heart failure or ischemic heart disease was designed to test the hypothesis that a higher target hematocrit (Hct) would result in improved outcomes compared with a lower target Hct. In this study, patients were randomized to epoetin alfa treatment targeted to a maintenance hemoglobin of either 14 ± 1 g/dL or 10 ± 1 g/dL. The trial was terminated early with adverse safety findings of higher mortality in the high hematocrit target group. Higher mortality (35% vs. 29%) was observed for the patients randomized to a target hemoglobin of 14 g/dL than for the patients randomized to a target hemoglobin of 10 g/dL. For all-cause mortality, the HR = 1.27; 95% CI (1.04, 1.54); p=0.018. The incidence of nonfatal myocardial infarction, vascular access thrombosis, and other thrombotic events was also higher in the group randomized to a target hemoglobin of 14 g/dL.
CHOIR: A randomized, prospective trial, 1432 patients with anemia due to CKD who were not undergoing dialysis and who had not previously received epoetin alfa therapy were randomized to epoetin alfa treatment targeting a maintenance hemoglobin concentration of either 13.5 g/dL or 11.3 g/dL. The trial was terminated early with adverse safety findings. A major cardiovascular event (death, myocardial infarction, stroke, or hospitalization for congestive heart failure) occurred in 125 of the 715 patients (18%) in the higher hemoglobin group compared to 97 of the 717 patients (14%) in the lower hemoglobin group [hazard ratio (HR) 1.34, 95% CI: 1.03, 1.74; p = 0.03].
TREAT: A randomized, double-blind, placebo-controlled, prospective trial of 4038 patients with CKD not on dialysis (eGFR of 20 – 60 mL/min), anemia (hemoglobin levels ≤ 11 g/dL), and type 2 diabetes mellitus, patients were randomized to receive either Aranesp treatment or a matching placebo. Placebo group patients also received Aranesp when their hemoglobin levels were below 9 g/dL. The trial objectives were to demonstrate the benefit of Aranesp treatment of the anemia to a target hemoglobin level of 13 g/dL, when compared to a "placebo" group, by reducing the occurrence of either of two primary endpoints: (1) a composite cardiovascular endpoint of all-cause mortality or a specified cardiovascular event (myocardial ischemia, CHF, MI, and CVA) or (2) a composite renal endpoint of all-cause mortality or progression to end stage renal disease. The overall risks for each of the two primary endpoints (the cardiovascular composite and the renal composite) were not reduced with Aranesp treatment (see Table 3), but the risk of stroke was increased nearly two-fold in the Aranesp-treated group versus the placebo group: annualized stroke rate 2.1% vs. 1.1%, respectively, HR 1.92; 95% CI: 1.38, 2.68; p < 0.001. The relative risk of stroke was particularly high in patients with a prior stroke: annualized stroke rate 5.2% in the Aranesp treated group and 1.9% in the placebo group, HR 3.07; 95% CI: 1.44, 6.54. Also, among Aranesp-treated subjects with a past history of cancer, there were more deaths due to all causes and more deaths adjudicated as due to cancer, in comparison with the control group.
Patients with Cancer
An increased incidence of thromboembolic reactions, some serious and life-threatening, occurred in patients with cancer treated with ESAs.
In a randomized, placebo-controlled study (Study 2 in Table 4 [see Warnings and Precautions (5.2)]) of 939 women with metastatic breast cancer receiving chemotherapy, patients received either weekly epoetin alfa or placebo for up to a year. This study was designed to show that survival was superior when epoetin alfa was administered to prevent anemia (maintain hemoglobin levels between 12 and 14 g/dL or hematocrit between 36% and 42%). This study was terminated prematurely when interim results demonstrated a higher mortality at 4 months (8.7% vs. 3.4%) and a higher rate of fatal thrombotic reactions (1.1% vs. 0.2%) in the first 4 months of the study among patients treated with epoetin alfa. Based on Kaplan-Meier estimates, at the time of study termination, the 12-month survival was lower in the epoetin alfa group than in the placebo group (70% vs. 76%; HR 1.37, 95% CI: 1.07, 1.75; p = 0.012).
Patients Having Surgery
Aranesp is not approved for reduction of RBC transfusions in patients scheduled for surgical procedures.
An increased incidence of DVT in patients receiving epoetin alfa undergoing surgical orthopedic procedures was demonstrated. In a randomized, controlled study, 680 adult patients, not receiving prophylactic anticoagulation and undergoing spinal surgery, received epoetin alfa and standard of care (SOC) treatment (n = 340) or SOC treatment alone (n = 340). A higher incidence of DVTs, determined by either color flow duplex imaging or by clinical symptoms, was observed in the epoetin alfa group (16 [4.7%] patients) compared with the SOC group (7 [2.1%] patients). In addition to the 23 patients with DVTs included in the primary analysis, 19 [2.8%] patients experienced 1 other thrombovascular event (TVE) each (12 [3.5%] in the epoetin alfa group and 7 [2.1%] in the SOC group).
Increased mortality was observed in a randomized, placebo-controlled study of epoetin alfa in adult patients who were undergoing CABG surgery (7 deaths in 126 patients randomized to epoetin alfa versus no deaths among 56 patients receiving placebo). Four of these deaths occurred during the period of study drug administration and all 4 deaths were associated with thrombotic events.
5.2 Increased Mortality and/or Increased Risk of Tumor Progression or Recurrence in Patients with Cancer
ESAs resulted in decreased locoregional control/progression-free survival (PFS) and/or overall survival (OS) (see Table 4).
Adverse effects on PFS and/or OS were observed in studies of patients receiving chemotherapy for breast cancer (Studies 1, 2, and 4), lymphoid malignancy (Study 3), and cervical cancer (Study 5); in patients with advanced head and neck cancer receiving radiation therapy (Studies 6 and 7), and in patients with non-small cell lung cancer or various malignancies who were not receiving chemotherapy or radiotherapy (Studies 8 and 9).
|
Study/Tumor/(n) |
Hemoglobin Target | Hemoglobin
(Median; Q1, Q3*) |
Primary Efficacy Outcome |
Adverse Outcome for ESA-containing Arm |
| Chemotherapy | ||||
| Study 1
Metastatic breast cancer (n = 2098) | ≤12 g/dL† | 11.6 g/dL; 10.7, 12.1 g/dL | Progression-free survival (PFS) | Decreased progression-free and overall survival |
| Study 2
Metastatic breast cancer (n = 939) | 12-14 g/dL | 12.9 g/dL; 12.2, 13.3 g/dL | 12-month overall survival | Decreased 12-month survival |
| Study 3
Lymphoid malignancy (n = 344) | 13-15 g/dL (M) 13-14 g/dL (F) | 11 g/dL; 9.8, 12.1 g/dL | Proportion of patients achieving a hemoglobin response | Decreased overall survival |
| Study 4
Early breast cancer (n = 733) | 12.5-13 g/dL | 13.1 g/dL; 12.5, 13.7 g/dL | Relapse-free and overall survival | Decreased 3-year relapse-free and overall survival |
| Study 5
Cervical cancer (n = 114) | 12-14 g/dL | 12.7 g/dL; 12.1, 13.3 g/dL | Progression-free and overall survival and locoregional control | Decreased 3-year progression-free and overall survival and locoregional control |
| Radiotherapy Alone | ||||
| Study 6
Head and neck cancer (n = 351) | ≥ 15 g/dL (M) ≥ 14 g/dL (F) | Not available | Locoregional progression-free survival | Decreased 5-year locoregional progression-free and overall survival |
| Study 7
Head and neck cancer (n = 522) | 14-15.5 g/dL | Not available | Locoregional disease control | Decreased locoregional disease control |
| No Chemotherapy or Radiotherapy | ||||
| Study 8
Non-small cell lung cancer (n = 70) | 12-14 g/dL | Not available | Quality of life | Decreased overall survival |
| Study 9
Non-myeloid malignancy (n = 989) | 12-13 g/dL | 10.6 g/dL; 9.4, 11.8 g/dL | RBC transfusions | Decreased overall survival |
*Q1 = 25th percentile
Q3 = 75th percentile
†This study did not include a defined hemoglobin target. Doses were titrated to achieve and maintain the lowest hemoglobin level sufficient to avoid transfusion and not to exceed 12 g/dL.
Decreased Overall Survival
Study 2 was described in the previous section [see Warnings and Precautions (5.1)]. Mortality at 4 months (8.7% vs. 3.4%) was significantly higher in the epoetin alfa arm. The most common investigator-attributed cause of death within the first 4 months was disease progression; 28 of 41 deaths in the epoetin alfa arm and 13 of 16 deaths in the placebo arm were attributed to disease progression. Investigator-assessed time to tumor progression was not different between the 2 groups. Survival at 12 months was significantly lower in the epoetin alfa arm (70% vs. 76%; HR 1.37, 95% CI: 1.07, 1.75; p = 0.012).
Study 3 was a randomized, double-blind study (darbepoetin alfa vs. placebo) conducted in 344 anemic patients with lymphoid malignancy receiving chemotherapy. With a median follow-up of 29 months, overall mortality rates were significantly higher among patients randomized to darbepoetin alfa as compared to placebo (HR 1.36, 95% CI: 1.02, 1.82).
Study 8 was a multicenter, randomized, double-blind study (epoetin alfa vs. placebo) in which patients with advanced non-small cell lung cancer receiving only palliative radiotherapy or no active therapy were treated with epoetin alfa to achieve and maintain hemoglobin levels between 12 and 14 g/dL. Following an interim analysis of 70 patients (planned accrual 300 patients), a significant difference in survival in favor of the patients in the placebo arm of the study was observed (median survival 63 vs. 129 days; HR 1.84; p = 0.04).
Study 9 was a randomized, double-blind study (darbepoetin alfa vs. placebo) in 989 anemic patients with active malignant disease, neither receiving nor planning to receive chemotherapy or radiation therapy. There was no evidence of a statistically significant reduction in proportion of patients receiving RBC transfusions. The median survival was shorter in the darbepoetin alfa treatment group than in the placebo group (8 months vs. 10.8 months; HR 1.30, 95% CI: 1.07, 1.57).
Decreased Progression-free Survival and Overall Survival
Study 1 was a randomized, open-label, multicenter study in 2,098 anemic women with metastatic breast cancer, who received first line or second line chemotherapy. This was a non-inferiority study designed to rule out a 15% risk increase in tumor progression or death of epoetin alfa plus standard of care (SOC) as compared with SOC alone. At the time of clinical data cutoff, the median progression free survival (PFS) per investigator assessment of disease progression was 7.4 months in each arm (HR 1.09, 95% CI: 0.99, 1.20), indicating the study objective was not met. There were more deaths from disease progression in the epoetin alfa plus SOC arm (59% vs. 56%) and more thrombotic vascular events in the epoetin alfa plus SOC arm (3% vs. 1%). At the final analysis, 1653 deaths were reported (79.8% of subjects in the epoetin alfa plus SOC group and 77.8% of subjects in the SOC group). Median overall survival in the epoetin alfa plus SOC group was 17.8 months compared with 18.0 months in the SOC alone group (HR 1.07, 95% CI: 0.97, 1.18).
Study 4 was a randomized, open-label, controlled, factorial design study in which darbepoetin alfa was administered to prevent anemia in 733 women receiving neo-adjuvant breast cancer treatment. A final analysis was performed after a median follow-up of approximately 3 years. The 3-year survival rate was lower (86% vs. 90%; HR 1.42, 95% CI: 0.93, 2.18) and the 3-year relapse-free survival rate was lower (72% vs. 78%; HR 1.33, 95% CI: 0.99, 1.79) in the darbepoetin alfa-treated arm compared to the control arm.
Study 5 was a randomized, open-label, controlled study that enrolled 114 of a planned 460 patients with cervical cancer receiving chemotherapy and radiotherapy. Patients were randomized to receive epoetin alfa to maintain hemoglobin between 12 and 14 g/dL or to RBC transfusion support as needed. The study was terminated prematurely due to an increase in thromboembolic adverse reactions in epoetin alfa-treated patients compared to control (19% vs. 9%). Both local recurrence (21% vs. 20%) and distant recurrence (12% vs. 7%) were more frequent in epoetin alfa-treated patients compared to control. Progression-free survival at 3 years was lower in the epoetin alfa-treated group compared to control (59% vs. 62%; HR 1.06, 95% CI: 0.58, 1.91). Overall survival at 3 years was lower in the epoetin alfa-treated group compared to control (61% vs. 71%; HR 1.28, 95% CI: 0.68, 2.42).
Study 6 was a randomized, placebo-controlled study in 351 patients with head and neck cancer where epoetin beta or placebo was administered to achieve target hemoglobins ≥ 14 and ≥ 15 g/dL for women and men, respectively. Locoregional progression-free survival was significantly shorter in patients receiving epoetin beta (HR 1.62, 95% CI: 1.22, 2.14; p = 0.0008) with medians of 406 days and 745 days in the epoetin beta and placebo arms respectively. Overall survival was significantly shorter in patients receiving epoetin beta (HR 1.39, 95% CI: 1.05, 1.84; p = 0.02).
Decreased Locoregional Control
Study 7 was a randomized, open-label, controlled study conducted in 522 patients with primary squamous cell carcinoma of the head and neck receiving radiation therapy alone (no chemotherapy) who were randomized to receive darbepoetin alfa to maintain hemoglobin levels of 14 to15.5 g/dL or no darbepoetin alfa. An interim analysis performed on 484 patients demonstrated that locoregional control at 5 years was significantly shorter in patients receiving darbepoetin alfa (RR 1.44, 95% CI: 1.06, 1.96; p = 0.02). Overall survival was shorter in patients receiving darbepoetin alfa (RR 1.28, 95% CI: 0.98, 1.68; p = 0.08).
Non-inferiority for Overall Survival and Progression-free Survival
In a randomized, double-blind, placebo-controlled study to demonstrate non-inferiority of overall survival for Aranesp compared to placebo in patients with anemia receiving chemotherapy for the treatment of advanced stage non-small cell lung cancer (NSCLC), a total of 2549 adult patients who were expected to receive ≥ 2 cycles of myelosuppressive chemotherapy and with a hemoglobin (Hb) ≤ 11.0 g/dL, were randomized 2:1 to Aranesp or placebo and treated to a maximum Hb of 12 g/dL.
Non-inferiority of Aranesp versus placebo was shown for overall survival (OS) and progression-free survival (PFS). The study was designed to rule out a 15% risk increase. The median OS for Aranesp versus placebo was 9.5 and 9.3 months, respectively (stratified hazard ratio 0.92; 95% CI: 0.84–1.01). The median PFS was 4.4 and 4.2 months, respectively (stratified hazard ratio 0.96; 95% CI: 0.87–1.05). Aranesp did not demonstrate superiority to placebo for OS or PFS.
Thrombovascular events were more frequent with Aranesp than placebo group (5.3% Aranesp, 4.1% placebo). No new safety signals were identified [see Warnings and Precautions (5.1)].
5.3 Hypertension
Aranesp is contraindicated in patients with uncontrolled hypertension. In Aranesp clinical studies, approximately 40% of patients with CKD required initiation or intensification of antihypertensive therapy during the early phase of treatment. Hypertensive encephalopathy and seizures have been reported in patients with CKD receiving Aranesp.
Appropriately control hypertension prior to initiation of and during treatment with Aranesp. Reduce or withhold Aranesp if blood pressure becomes difficult to control. Advise patients of the importance of compliance with antihypertensive therapy and dietary restrictions [see Patient Counseling Information (17)].
5.4 Seizures
Aranesp increases the risk of seizures in patients with CKD. During the first several months following initiation of Aranesp, monitor patients closely for premonitory neurologic symptoms. Advise patients to contact their healthcare practitioner for new-onset seizures, premonitory symptoms, or change in seizure frequency.
5.6 Pure Red Cell Aplasia
Cases of PRCA and of severe anemia, with or without other cytopenias that arise following the development of neutralizing antibodies to erythropoietin have been reported in patients treated with Aranesp. This has been reported predominantly in patients with CKD receiving ESAs by subcutaneous administration. PRCA has also been reported in patients receiving ESAs for anemia related to hepatitis C treatment (an indication for which Aranesp is not approved).
If severe anemia and low reticulocyte count develop during treatment with Aranesp, withhold Aranesp and evaluate patients for neutralizing antibodies to erythropoietin. Contact Amgen (1-800-77-AMGEN) to perform assays for binding and neutralizing antibodies. Permanently discontinue Aranesp in patients who develop PRCA following treatment with Aranesp or other erythropoietin protein drugs. Do not switch patients to other ESAs.
5.7 Serious Allergic Reactions
Serious allergic reactions, including anaphylactic reactions, angioedema, bronchospasm, skin rash, and urticaria may occur with Aranesp. Immediately and permanently discontinue Aranesp and administer appropriate therapy if a serious allergic or anaphylactic reaction occurs.
5.8 Severe Cutaneous Reactions
Blistering and skin exfoliation reactions including Erythema multiforme and Stevens-Johnson Syndrome (SJS)/Toxic Epidermal Necrolysis (TEN), have been reported in patients treated with ESAs (including Aranesp) in the post-marketing setting. Discontinue Aranesp therapy immediately if a severe cutaneous reaction, such as SJS/TEN, is suspected.
6. Adverse Reactions/Side Effects
The following clinically significant adverse reactions are discussed in greater detail in other sections of the label:
- Increased Mortality, Myocardial Infarction, Stroke, and Thromboembolism [see Warnings and Precautions (5.1)]
- Increased Mortality and/or Increased Risk of Tumor Progression or Recurrence in Patients with Cancer [see Warnings and Precautions (5.2)]
- Hypertension [see Warnings and Precautions (5.3)]
- Seizures [see Warnings and Precautions (5.4)]
- Pure Red Cell Aplasia [see Warnings and Precautions (5.6)]
- Serious Allergic Reactions [see Warnings and Precautions (5.7)]
- Severe Cutaneous Reactions [see Warnings and Precautions (5.8)]
6.1 Clinical Trial Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of other drugs and may not reflect the rates observed in practice.
Patients with Chronic Kidney Disease
Adult Patients
Adverse reactions were determined based on pooled data from 5 randomized, active-controlled studies of Aranesp with a total of 1357 patients (Aranesp 766, epoetin alfa 591). The median duration of exposure for patients receiving Aranesp was 340 days, with 580 patients exposed for greater than 6 months and 360 patients exposed for greater than 1 year. The median (25th, 75th percentiles) weight-adjusted dose of Aranesp was 0.50 mcg/kg (0.32, 0.81). The median (range) age for patients administered Aranesp was 62 years (18 to 88). In the Aranesp group, 55% were male, 72% were white, 83% were receiving dialysis, and 17% were not receiving dialysis.
Table 5 lists adverse reactions occurring in ≥ 5% of patients treated with Aranesp.
| Adverse Reaction | Patients Treated with Aranesp (n = 766) |
| Hypertension | 31% |
| Dyspnea | 17% |
| Peripheral edema | 17% |
| Cough | 12% |
| Procedural hypotension | 10% |
| Angina pectoris | 8% |
| Vascular access complications | 8% |
| Fluid overload | 7% |
| Rash/Erythema | 5% |
| Arteriovenous graft thrombosis | 5% |
Rates of adverse reactions with Aranesp therapy were similar to those observed with other recombinant erythropoietins in these studies.
Pediatric Patients
Adverse reactions were determined based on pooled data from 2 randomized, controlled trials [see Clinical Studies (14.1)]. In one study, Aranesp was administered to 81 pediatric patients with CKD who had stable hemoglobin concentrations while previously receiving epoetin alfa. In a second study, Aranesp was administered to 114 anemic pediatric patients with CKD receiving or not receiving dialysis for initial treatment of anemia. In these studies, the most frequently reported serious adverse reactions with Aranesp were hypertension and convulsions. The most commonly reported adverse reactions were hypertension, injection site pain, rash, and convulsions. Aranesp administration was discontinued because of injection site pain in 2 patients and hypertension in 3 patients.
Patients with Cancer Receiving Chemotherapy
Adverse reactions were based on data from a randomized, double-blind, placebo-controlled study of Aranesp in 597 patients (Aranesp 301, placebo 296) with extensive stage small cell lung cancer (SCLC) receiving platinum-based chemotherapy. All patients were white, 64% were male, and the median age was 61 years (range: 28 to 82 years); 25% of the study population were from North America, Western Europe, and Australia. Patients received Aranesp at a dose of 300 mcg or placebo weekly for 4 weeks then every 3 weeks for a total of 24 weeks, and the median duration of exposure was 19 weeks (range: 1 to 26 weeks).
Adverse reactions were also based on data from 7 randomized, double-blind, placebo-controlled studies, including the SCLC study described above, that enrolled 2112 patients (Aranesp 1203, placebo 909) with non-myeloid malignancies. Most patients were white (95%), male (52%), and the median age was 63 years (range: 18 to 91 years); 73% of the study population were from North America, Western Europe, and Australia. Dosing and schedules varied by study from once weekly to once every 4 weeks, and the median duration of exposure was 12 weeks (range: 1 to 27 weeks).
| SCLC Study | All Placebo-controlled Studies | |||
| Adverse Reaction | Aranesp
(n = 301) | Placebo
(n = 296) | Aranesp
(n = 2888) | Placebo
(n = 1742) |
| Thromboembolic Adverse Reactions, n (%) | 25 (8.3%) | 13 (4.4%) | 147 (5.1%) | 64 (3.7%) |
| Arterial | 9 (3%) | 3 (1%) | 33 (1.1%) | 11 (0.6%) |
| Myocardial infarction | 5 (1.7%) | 0 | 18 (0.6%) | 5 (0.3%) |
| Venous | 16 (5.3%) | 10 (3.4%) | 118 (4.1%) | 55 (3.2%) |
| Pulmonary embolism | 5 (1.7%) | 3 (1%) | 43 (1.5%) | 14 (0.8%) |
| Cerebrovascular disorders* | 14 (4.7%) | 9 (3%) | 38 (1.3%) | 23 (1.3%) |
* “Cerebrovascular disorders” encompasses CNS hemorrhages and cerebrovascular accidents (ischemic and hemorrhagic). Events in this category may also be included under “thromboembolic adverse reactions.”
In addition to the thrombovascular adverse reactions, abdominal pain and edema occurred at a higher incidence in patients taking Aranesp compared to patients on placebo. Among all placebo-controlled studies, abdominal pain (13.2% vs. 9.4%) and edema (12.8% vs. 9.7%) were reported more frequently in patients receiving Aranesp compared to the placebo group. In the SCLC study the incidence of abdominal pain (10.3% vs. 3.4%) and edema (5.6% vs. 5.1%) in the Aranesp-treated patients compared to those receiving placebo.
12. Aranesp - Clinical Pharmacology
12.2 Pharmacodynamics
Increased hemoglobin levels are not generally observed until 2 to 6 weeks after initiating treatment with Aranesp.
12.3 Pharmacokinetics
Adult Patients with CKD
The pharmacokinetics of Aranesp were studied in patients with CKD receiving or not receiving dialysis and patients with cancer receiving chemotherapy.
Following intravenous administration of Aranesp to patients with CKD receiving dialysis, Aranesp serum concentration-time profiles were biphasic, with a distribution half-life of approximately 1.4 hours and a mean terminal half-life (t1/2) of 21 hours. The t1/2 of Aranesp was approximately 3-fold longer than that of epoetin alfa when administered intravenously.
Following subcutaneous administration of Aranesp to patients with CKD (receiving or not receiving dialysis), absorption was slow and Cmax occurred at 48 hours (range: 12 to 72 hours). In patients with CKD receiving dialysis, the average t1/2 was 46 hours (range: 12 to 89 hours), and in patients with CKD not receiving dialysis, the average t1/2 was 70 hours (range: 35 to 139 hours). Aranesp apparent clearance was approximately 1.4 times faster on average in patients receiving dialysis compared to patients not receiving dialysis. The bioavailability of Aranesp in patients with CKD receiving dialysis after subcutaneous administration was 37% (range: 30% to 50%).
Pediatric Patients with CKD
Aranesp pharmacokinetics was evaluated in 12 pediatric patients (age 3 to 16 years) with CKD receiving or not receiving dialysis in one study (n=12). In a phase 1 pharmacokinetic study, following a single intravenous or subcutaneous Aranesp dose, Cmax and t1/2 were similar to those obtained in adult patients with CKD on dialysis. Additionally, following a single subcutaneous dose, the average bioavailability was 54% (range: 32% to 70%), which was higher than that obtained in adult patients with CKD on dialysis.
Adult Patients with Cancer
Following the first subcutaneous dose of 6.75 mcg/kg (equivalent to 500 mcg for a 74-kg patient) in patients with cancer, the mean t1/2 was 74 hours (range: 24 to 144 hours) and Cmax was observed at 71 hours (range: 28 to 120 hours). When administered on a once every 3 week schedule, 48-hour postdose Aranesp levels after the fourth dose were similar to those after the first dose.
Over the dose range of 0.45 to 4.5 mcg/kg Aranesp administered intravenously or subcutaneously on a once weekly schedule and 4.5 to 15 mcg/kg administered subcutaneously on a once every 3 week schedule, systemic exposure was approximately proportional to dose. No evidence of accumulation was observed beyond an expected less than 2-fold increase in blood levels when compared to the initial dose.
14. Clinical Studies
Clinical studies in the nephrology and chemotherapy-induced anemia clinical programs are designated with the prefixes “N” and “C”, respectively.
14.1 Patients with Chronic Kidney Disease
Patients with chronic kidney disease on dialysis: ESA effects on rates of transfusion
In early clinical studies conducted in patients with CKD on dialysis, ESAs have been shown to reduce the use of RBC transfusions. These studies enrolled patients with mean baseline hemoglobin levels of approximately 7.5 g/dL and ESAs were generally titrated to achieve a hemoglobin level of approximately 12 g/dL. Fewer transfusions were given during the ESA treatment period when compared to a pre-treatment interval.
In the Normal Hematocrit Study, the yearly transfusion rate was 51.5% in the lower hemoglobin group (10 g/dL) and 32.4% in the higher hemoglobin group (14 g/dL).
Patients with chronic kidney disease not on dialysis: ESA effects on rates of transfusion
In TREAT, a randomized, double-blind trial of 4038 patients with CKD and type 2 diabetes not on dialysis, a post-hoc analysis showed that the proportion of patients receiving RBC transfusions was lower in patients administered Aranesp to target a hemoglobin of 13 g/dL compared to the control arm in which Aranesp was administered intermittently if hemoglobin concentration decreased to less than 9 g/dL (15% versus 25%, respectively). In CHOIR, a randomized open-label study of 1432 patients with CKD not on dialysis, use of an ESA to target a higher (13.5 g/dL) versus lower (11.3 g/dL) hemoglobin goal did not reduce the use of RBC transfusions. In each trial, no benefits occurred for the cardiovascular or end-stage renal disease outcomes. In each trial, the potential benefit of ESA therapy was offset by worse cardiovascular safety outcomes resulting in an unfavorable benefit-risk profile [see Warnings and Precautions (5.1)].
ESA Effects on quality of life
Aranesp use has not been demonstrated in controlled clinical trials to improve quality of life, fatigue, or patient well-being.
ESA Effects on rates of death and other serious cardiac adverse events
Three randomized outcome trials (Normal Hematocrit Study [NHS], Correction of Anemia with Epoetin Alfa in Chronic Kidney Disease [CHOIR], and Trial of Darbepoetin Alfa in Type 2 Diabetes and CKD [TREAT]) have been conducted in patients with CKD using Epogen/PROCRIT/Aranesp to target higher vs. lower hemoglobin levels. Though these trials were designed to establish a cardiovascular or renal benefit of targeting higher hemoglobin levels, in all 3 studies, patients randomized to the higher hemoglobin target experienced worse cardiovascular outcomes and showed no reduction in progression to ESRD. In each trial, the potential benefit of ESA therapy was offset by worse cardiovascular safety outcomes resulting in an unfavorable benefit-risk profile [see Warnings and Precautions (5.1)].
Other ESA trials
Three studies (2 in adults and 1 in pediatric patients) evaluated the safety and efficacy of the de novo use of Aranesp for the correction of anemia in patients with CKD, and 3 studies (2 in adults and 1 in pediatric patients) assessed the ability of Aranesp to maintain hemoglobin concentrations in patients with CKD who had been receiving other recombinant erythropoietins.
De Novo Use of Aranesp
Adult Patients
Once Weekly Aranesp Starting Dose
In 2 randomized, open-label studies, Aranesp or epoetin alfa was administered for the correction of anemia in patients with CKD who had not been receiving prior treatment with exogenous erythropoietin. Study N1 evaluated patients with CKD receiving dialysis; Study N2 evaluated patients not requiring dialysis. In both studies, the starting dose of Aranesp was 0.45 mcg/kg administered once weekly. The starting dose of epoetin alfa was 50 Units/kg 3 times weekly in Study N1 and 50 Units/kg twice weekly in Study N2. When necessary, dosage adjustments were instituted to maintain hemoglobin in the study target range of 11 to 13 g/dL. (Note: The recommended hemoglobin target range is lower than the target range of these studies [see Dosage and Administration (2.2)]). The primary efficacy endpoint was the proportion of patients who experienced at least a 1 g/dL increase in hemoglobin concentration to a level of at least 11 g/dL by 20 weeks (Study N1) or 24 weeks (Study N2). The studies were designed to assess the safety and effectiveness of Aranesp but not to support conclusions regarding comparisons between the 2 products.
In Study N1, the primary efficacy endpoint was achieved by 72% (95% CI: 62%, 81%) of the 90 patients treated with Aranesp and 84% (95% CI: 66%, 95%) of the 31 patients treated with epoetin alfa. The mean increase in hemoglobin over the initial 4 weeks of Aranesp treatment was 1.1 g/dL (95% CI: 0.82 g/dL, 1.37 g/dL).
In Study N2, the primary efficacy endpoint was achieved by 93% (95% CI: 87%, 97%) of the 129 patients treated with Aranesp and 92% (95% CI: 78%, 98%) of the 37 patients treated with epoetin alfa. The mean increase in hemoglobin from baseline through the initial 4 weeks of Aranesp treatment was 1.38 g/dL (95% CI: 1.21 g/dL, 1.55 g/dL).
Once Every 2 Week Aranesp Starting Dose
In 2 single-arm studies (N3 and N4), Aranesp was administered for the correction of anemia in patients with CKD not receiving dialysis. In both studies, the starting dose of Aranesp was 0.75 mcg/kg administered once every 2 weeks.
In Study N3 (study duration of 18 weeks), the hemoglobin goal (hemoglobin concentration ≥ 11 g/dL) was achieved by 92% (95% CI: 86%, 96%) of the 128 patients treated with Aranesp.
In Study N4 (study duration of 24 weeks), the hemoglobin goal (hemoglobin concentration of 11 to 13 g/dL) was achieved by 85% (95% CI: 77%, 93%) of the 75 patients treated with Aranesp.
Pediatric Patients
Study N8 was a double-blind, randomized, controlled study in 114 pediatric patients from 1 to 18 years of age receiving darbepoetin alfa. In this study, pediatric patients with CKD receiving or not receiving dialysis who were anemic (hemoglobin [Hb] < 10.0 g/dL) and not being treated with an erythropoiesis stimulating agent (ESA) received darbepoetin alfa weekly or once every 2 weeks for the correction of anemia.
The primary efficacy endpoint was proportion of patients having hemoglobin corrected to ≥ 10.0 g/dL at any time point after the first dose without receiving any red blood cell transfusions after randomization and within 90 days prior to the Hb measurement. For pediatric patients receiving QW dosing, 98% (95% CI: 91%-100%), had hemoglobin concentrations corrected to ≥ 10 g/dL. For those receiving Q2W dosing, 84% (95% CI: 72%-92%), had hemoglobin concentrations corrected to ≥ 10 g/dL. The study was designed to assess the safety and effectiveness of Aranesp but not to support conclusions regarding comparisons between the 2 regimens.
Conversion from Other Recombinant Erythropoietins
Two studies of adults (N5 and N6) and 1 study in pediatric patients (N7) were conducted in patients who had been receiving other recombinant erythropoietins for treatment of the anemia due to CKD. The studies compared the abilities of Aranesp and other erythropoietins to maintain hemoglobin concentrations within a study target range of 9 to 13 g/dL in adults and 10 to 12.5 g/dL in pediatric patients. (Note: The recommended hemoglobin target is lower than the target range of these studies [see Dosage and Administration (2.2)]). Patients who had been receiving stable doses of other recombinant erythropoietins were randomized to Aranesp or continued with their prior erythropoietin at the previous dose and schedule. For patients randomized to Aranesp, the initial weekly dose was determined on the basis of the previous total weekly dose of recombinant erythropoietin.
Adult Patients
Study N5 was a double-blind study in which 169 hemodialysis patients were randomized to treatment with Aranesp and 338 patients continued on epoetin alfa. Study N6 was an open-label study in which 347 patients were randomized to treatment with Aranesp and 175 patients were randomized to continue on epoetin alfa or epoetin beta. Of the patients randomized to Aranesp, 92% were receiving hemodialysis and 8% were receiving peritoneal dialysis.
In Study N5, a median weekly dose of 0.53 mcg/kg Aranesp (25th, 75th percentiles: 0.30, 0.93 mcg/kg) was required to maintain hemoglobin in the study target range. In Study N6, a median weekly dose of 0.41 mcg/kg Aranesp (25th, 75th percentiles: 0.26, 0.65 mcg/kg) was required to maintain hemoglobin in the study target range.
Pediatric Patients
Study N7 was an open-label, randomized study conducted in the United States in pediatric patients from 1 to 18 years of age with CKD receiving or not receiving dialysis. Eighty-one patients with hemoglobin concentrations that were stable on epoetin alfa received Aranesp (subcutaneously or intravenously), and 42 patients continued to receive epoetin alfa at the current dose, schedule, and route of administration. Patients received Aranesp once weekly if previously receiving epoetin alfa 2 or 3 times weekly or once every other week if previously receiving epoetin alfa weekly. A median weekly dose of 0.41 mcg/kg Aranesp (25th, 75th percentiles: 0.25, 0.82 mcg/kg) was required to maintain hemoglobin in the study target range.
17. Patient Counseling Information
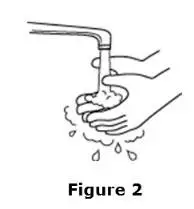
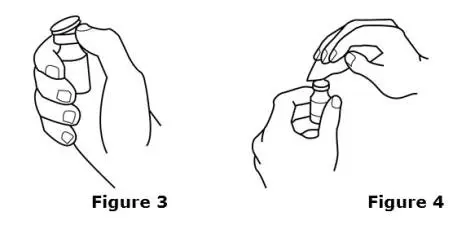
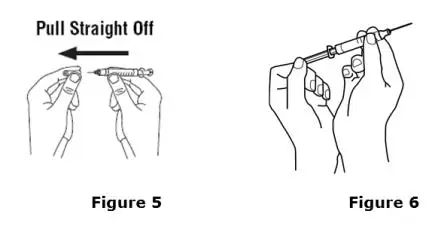
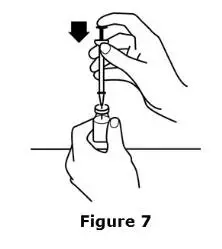
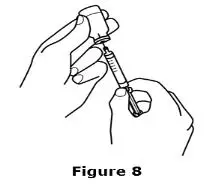
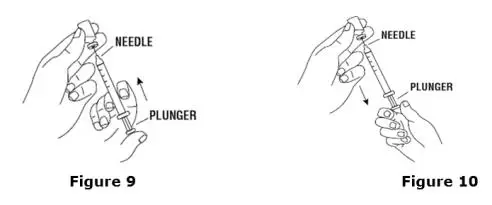
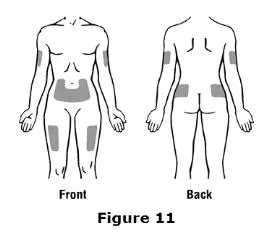
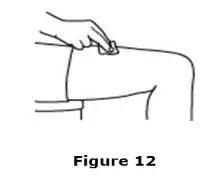
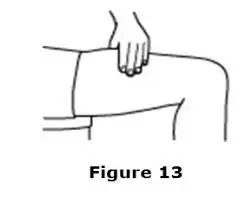
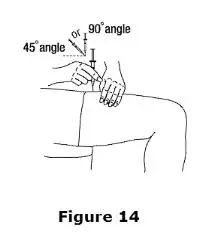
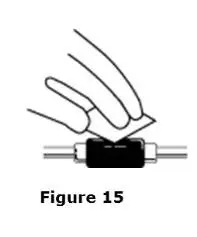
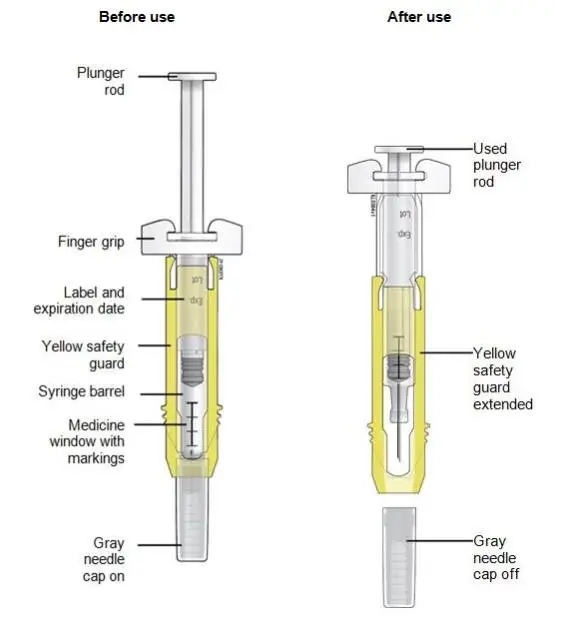
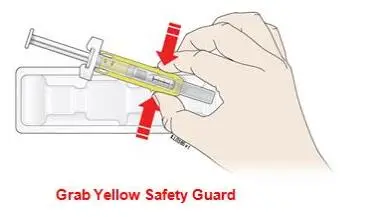
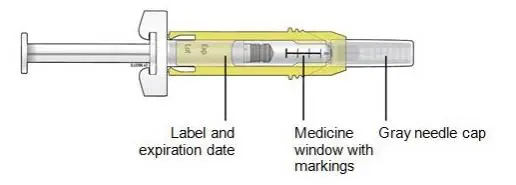
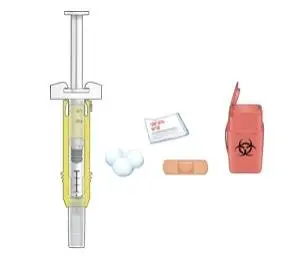
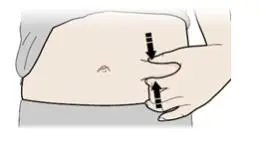
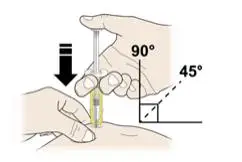
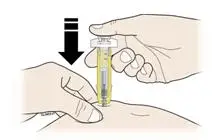
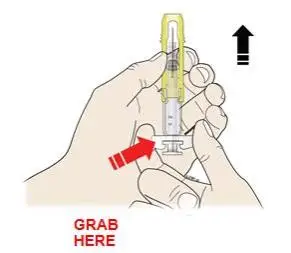
Advise the patient to read the FDA-approved patient labeling (Medication Guide and Instructions for Use).
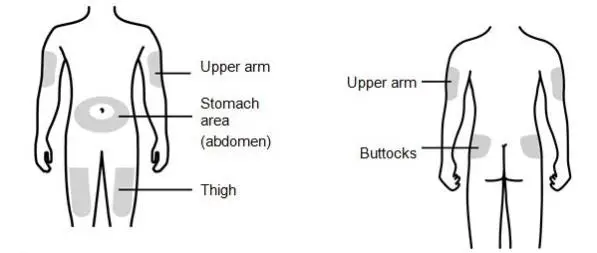
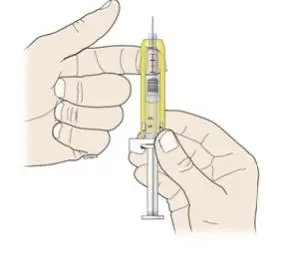
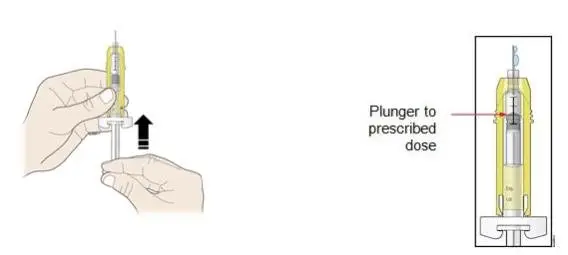
Review the steps for direct patient administration with patients and caregivers. Training should aim to ensure that patients and caregivers can successfully perform all of the steps in the Instructions for Use of Aranesp prefilled syringe, including showing the patient or caregiver how to measure the required dose, particularly if a patient is on a dose other than the entire prefilled syringe. If a patient or caregiver is not able to demonstrate that they can measure the dose and administer the product successfully, you should consider whether the patient is an appropriate candidate for self-administration of Aranesp or whether the patient would benefit from a different Aranesp presentation.
Inform patients:
- Of the increased risks of mortality, serious cardiovascular reactions, thromboembolic reactions, stroke, and tumor progression [see Warnings and Precautions (5.1 and 5.2)].
- To undergo regular blood pressure monitoring, adhere to prescribed anti-hypertensive regimen and follow recommended dietary restrictions.
- To contact their healthcare provider for new-onset neurologic symptoms or change in seizure frequency.
- Of the need to have regular laboratory tests for hemoglobin.
Instruct patients who self-administer Aranesp of the:
- Importance of following the Instructions for Use.
- Dangers of reusing needles, syringes, or unused portions of single-dose vials.
- Proper disposal of used syringes, needles, and unused vials, and of the full container.
- Importance of informing healthcare provider if difficulty occurs when measuring or administering partial doses from the Aranesp prefilled syringe. If difficulty occurs, use of other syringes or Aranesp vial may be considered.
Aranesp® (darbepoetin alfa)
Manufactured by:
Amgen Inc.
One Amgen Center Drive
Thousand Oaks, CA 91320-1799 U.S.A.
U.S. License Number 1080
Patent: http://pat.amgen.com/aranesp/
© 2001-2019 Amgen Inc. All rights reserved.
*UltraSafe® is a registered trademark of Safety Syringes, Inc.
1xxxxxx- v36
Medication Guide
| MEDICATION GUIDE
Aranesp® (Air-uh-nesp) (darbepoetin alfa) |
Read this Medication Guide:
|
| What is the most important information I should know about Aranesp?
Aranesp may cause serious side effects that can lead to death, including: For people with cancer:
If you decide to take Aranesp, your healthcare provider should prescribe the smallest dose of Aranesp that is necessary to reduce your chance of needing red blood cell transfusions. |
| What is Aranesp?
Aranesp is a prescription medicine used to treat anemia. People with anemia have a lower-than-normal number of RBCs. Aranesp works like the human protein called erythropoietin to help your body make more RBCs. Aranesp is used to reduce or avoid the need for RBC transfusions. Aranesp may be used to treat anemia if it is caused by:
Aranesp has not been proven to improve the quality of life, fatigue, or well-being. Aranesp should not be used for the treatment of anemia:
|
| Who should not take Aranesp?
Do not take Aranesp if you:
|
Before taking Aranesp, tell your healthcare provider about all of your medical conditions, including if you:
|
How should I take Aranesp?
|
| What are the possible side effects of Aranesp?
Aranesp may cause serious side effects, including:
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
How should I store Aranesp?
|
| General information about Aranesp.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use Aranesp for a condition for which it was not prescribed. Do not give Aranesp to other people even if they have the same symptoms that you have. It may harm them. You can ask your healthcare provider or pharmacist for information about Aranesp that is written for healthcare professionals. |
| What are the ingredients in Aranesp?
Active ingredient: darbepoetin alfa Inactive ingredients: polysorbate 80, sodium chloride, sodium phosphate dibasic anhydrous, and sodium phosphate monobasic monohydrate in Water for Injection, USP. Manufactured by: Amgen Inc. One Amgen Center Drive Thousand Oaks, CA 91320-1799 U.S.A. U.S. License Number 1080 © 2001-2018 Amgen Inc. All rights reserved. 1xxxxxx – v8 PMV 5 For more information, go to the following website: www.aranesp.com or call 1-800-77-AMGEN. |
This Medication Guide has been approved by the U.S. Food and Drug Administration Revised: 12/2018
| ARANESP
darbepoetin alfa injection, solution |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| ARANESP
darbepoetin alfa solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| ARANESP
darbepoetin alfa solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| ARANESP
darbepoetin alfa solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| ARANESP
darbepoetin alfa solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| ARANESP
darbepoetin alfa solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| ARANESP
darbepoetin alfa solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| ARANESP
darbepoetin alfa injection, solution |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| ARANESP
darbepoetin alfa injection, solution |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| ARANESP
darbepoetin alfa injection, solution |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| ARANESP
darbepoetin alfa injection, solution |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| ARANESP
darbepoetin alfa injection, solution |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| ARANESP
darbepoetin alfa injection, solution |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| ARANESP
darbepoetin alfa injection, solution |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| ARANESP
darbepoetin alfa injection, solution |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - Amgen Inc (039976196) |