Drug Detail:Balsalazide (Balsalazide [ bal-sal-a-zide ])
Drug Class: 5-aminosalicylates
Highlights of Prescribing Information
ASACOL (mesalamine) delayed-release tablets, for oral use
Initial U.S. Approval: 1987
Indications and Usage for Asacol
Asacol is an aminosalicylate indicated for:
- Treatment of mildly to moderately active ulcerative colitis (UC) in patients 5 years of age and older (1.1)
- Maintenance of remission of mildly to moderately active UC adults (1.2)
Asacol Dosage and Administration
Important Administration Instructions:
- Evaluate renal function prior to initiation of Asacol and periodically while on therapy (2.1, 5.1)
- Two Asacol 400 mg tablets are not interchangeable or substitutable with one mesalamine delayed-release 800 mg tablet (2.1)
Treatment of mildly to moderately active UC (2.2):
- Adults: 800 mg (two 400 mg tablets) three times daily for 6 weeks
- Pediatric Patients 5 years or older: Total daily dosage is weight-based up to a maximum of 2.4 grams/day divided into two daily doses (see Table 1)
Maintenance of remission of mildly to moderately active UC (2.3)
- Adults: 1.6 grams (four 400 mg tablets) daily in two to four divided doses.
Dosage Forms and Strengths
Delayed-release tablets: 400 mg (3)
Contraindications
Known or suspected hypersensitivity to salicylates or aminosalicylates or to any of the ingredients of Asacol tablets (4, 5.3)
Warnings and Precautions
-
Renal Impairment: Evaluate the risks and benefits in patients with known renal impairment or taking nephrotoxic drugs; monitor renal function (5.1, 7.1, 8.6, 13.2)
-
Mesalamine-induced Acute Intolerance Syndrome: Symptoms may be difficult to distinguish from a UC exacerbation; monitor for worsening symptoms; discontinue if acute intolerance syndrome suspected (5.2)
-
Hypersensitivity Reactions, including Myocarditis and Pericarditis: Evaluate patients immediately and discontinue if a hypersensitivity reaction is suspected (5.3)
- Hepatic Failure: Evaluate the risks and benefits in patients with known liver impairment (5.4)
Adverse Reactions/Side Effects
The most common adverse reactions (≥5%) for the treatment of mild to moderate UC are (6.1):
-
Adults: eructation, abdominal pain, constipation, dizziness, rhinitis, back pain, and rash
- Pediatric Patients 5 to 17 years of age: nasopharyngitis, headache, abdominal pain, dizziness, sinusitis, rash, cough and diarrhea
To report SUSPECTED ADVERSE REACTIONS, contact Warner Chilcott at 1-800-521-8813 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
Drug Interactions
-
Nephrotoxic Agents including NSAIDs: Increased risk of nephrotoxicity; monitor for changes in renal function and mesalamine-related adverse reactions. (7.1)
- Azathioprine or 6-Mercaptopurine: Increased risk of blood disorders; monitor complete blood cell counts and platelet counts (7.2)
Use In Specific Populations
-
Pregnancy: Contains dibutyl phthalate; may cause fetal harm (8.1)
- Geriatric Patients: Increased risk of blood dyscrasias; monitor complete blood cell counts and platelet counts (8.5)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 7/2015
Related/similar drugs
Lialda, Pentasa, Azulfidine, Apriso, Canasa, Delzicol, RowasaFull Prescribing Information
1. Indications and Usage for Asacol
2. Asacol Dosage and Administration
2.1 Important Administration Instructions
- Two Asacol 400 mg tablets are not interchangeable or substitutable with one mesalamine delayed-release 800 mg tablet.
- Evaluate renal function prior to initiation of Asacol
- Take Asacol tablets with or without food.
- Swallow Asacol tablets whole. Do not cut, break or chew the tablets.
- Intact, partially intact, and/or tablet shells have been reported in the stool; Instruct patients to contact their physician if this occurs repeatedly.
- Protect Asacol tablets from moisture. Close the container tightly and to leave any desiccant pouches present in the bottle along with the tablets.
2.2 Dosage for Treatment of Mildly to Moderately Active Ulcerative Colitis
Adults
For adults, the recommended dosage of Asacol is 800 mg (two 400 mg tablets) three times daily (total daily dosage of 2.4 grams) for a duration of 6 weeks.
Pediatrics
For pediatric patients 5 years of age and older, the recommended total daily dosage of Asacol is weight-based (up to maximum of 2.4 grams/day) divided into two daily doses for a duration of 6 weeks (see Table 1).
| Weight Group (kg) | Daily Dosage (mg/kg/day) | Maximum Daily Dosage (grams/day) | Morning Dosage | Afternoon Dosage |
| 17 to <33 | 36 to 71 | 1.2 | two 400 mg tablets | one 400 mg tablet |
| 33 to <54 | 37 to 61 | 2 | three 400 mg tablets | two 400 mg tablets |
| 54 to 90 | 27 to 44 | 2.4 | three 400 mg tablets | three 400 mg tablets |
3. Dosage Forms and Strengths
Asacol (mesalamine) Delayed-Release Tablets: 400 mg (red-brown, capsule-shaped and imprinted with “0752 DR” in black).
4. Contraindications
Asacol is contraindicated in patients with known or suspected hypersensitivity to salicylates or aminosalicylates or to any of the ingredients of Asacol [see Warnings and Precautions (5.3), Adverse Reactions (6.2), and Description (11)].
5. Warnings and Precautions
5.1 Renal Impairment
Renal impairment, including minimal change nephropathy, acute and chronic interstitial nephritis, and renal failure, has been reported in patients taking products such as Asacol that contain mesalamine or are converted to mesalamine. [see Adverse Reactions (6.2)].
Evaluate renal function prior to initiation of Asacol and periodically while on therapy.
Evaluate the risks and benefits of using Asacol in patients with known renal impairment or history of renal disease or taking concomitant nephrotoxic drugs [see Drug Interactions (7.1), Use in Specific Populations (8.6) and Nonclinical Toxicology (13.2)].
5.2 Mesalamine-Induced Acute Intolerance Syndrome
Mesalamine has been associated with an acute intolerance syndrome that may be difficult to distinguish from an exacerbation of ulcerative colitis. Although the exact frequency of occurrence has not been determined, it has occurred in 3% of controlled clinical trials of mesalamine or sulfasalazine. Symptoms include cramping, abdominal pain, bloody diarrhea, and sometimes fever, headache, and rash. Monitor patients for worsening of these symptoms while on treatment. If acute intolerance syndrome is suspected, promptly discontinue treatment with Asacol.
5.3 Hypersensitivity Reactions
Hypersensitivity reactions have been reported in patients taking sulfasalazine. Some patients may have a similar reaction to Asacol or to other compounds that contain or are converted to mesalamine.
As with sulfasalazine, mesalamine-induced hypersensitivity reactions may present as internal organ involvement, including myocarditis, pericarditis, nephritis, hepatitis, pneumonitis, and hematologic abnormalities. Evaluate patients immediately if signs or symptoms of a hypersensitivity reaction are present. Discontinue Asacol if an alternative etiology for the signs or symptoms cannot be established.
6. Adverse Reactions/Side Effects
The most serious adverse reactions seen in Asacol clinical trials or with other products that contain mesalamine or are metabolized to mesalamine are:
- Renal Impairment [see Warnings and Precautions (5.1)]
- Mesalamine-Induced Acute Intolerance Syndrome [see Warnings and Precautions (5.2)]
- Hypersensitivity Reactions [see Warnings and Precautions (5.3)]
- Hepatic Failure [see Warnings and Precautions (5.4)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug, and may not reflect the rates observed in practice.
In total, Asacol tablets have been evaluated in 2,690 patients with ulcerative colitis in controlled and open-label trials.
Clinical studies supporting Asacol use for the treatment of mildly to moderately active ulcerative colitis included two 6-week, placebo-controlled, randomized, double-blind studies in adults with mildly to moderately active ulcerative colitis (Studies 1 and 2), and one 6-week, randomized, double-blind, study of 2 dosage levels in children with mildly to moderately active ulcerative colitis (Study 3). Clinical studies supporting the use of Asacol tablets in the maintenance of remission of mildly to moderately active ulcerative colitis included a 6-month, randomized, double-blind, placebo-controlled, multi-center study (Study 4) and four active-controlled maintenance trials comparing Asacol tablets with sulfasalazine. Asacol has been evaluated in 427 adults and 82 children with ulcerative colitis in these controlled studies.
Treatment of Mildly to Moderately Active Ulcerative Colitis
Adults
In a 6-week placebo-controlled clinical study (Study 1) involving 105 patients, 53 of whom were randomized to Asacol 2.4 grams/day [see Clinical Studies (14.1)], 4% of the Asacol-treated patients in 2.4 grams/day group discontinued therapy because of adverse reactions as compared to 0% of the placebo-treated patients. The average age of patients was 41 years and 49% of patients were male. Adverse reactions leading to withdrawal from Asacol included (each in one patient): diarrhea and colitis flare; dizziness, nausea, joint pain, and headache.
The most common adverse reactions in patients treated with Asacol 2.4 grams/day in Study 1 are listed in Table 2 below.
| Adverse Reaction | % of Patients with Adverse Reactions | |
| Asacol 2.4 grams/day | Placebo | |
| (n = 53) | (n = 52) | |
| Eructation | 26 | 19 |
| Abdominal pain | 21 | 12 |
| Constipation | 11 | 0 |
| Dizziness | 9 | 8 |
| Rhinitis | 8 | 6 |
| Back pain | 6 | 4 |
| Rash | 6 | 4 |
| Dyspepsia | 4 | 0 |
| Flu syndrome | 4 | 2 |
* At Least 2% of Patients in the Asacol Group and at a Rate Greater than Placebo
Pediatric Patients 5 to 17 Years Old
A randomized, double-blind, 6-week study of 2 dosage levels of Asacol (Study 3) was conducted in 82 pediatric patients 5 to 17 years of age with mildly to moderately active ulcerative colitis. All patients were divided by body weight category (17 to less than 33 kg, 33 to less than 54 kg, and 54 to 90 kg) and randomly assigned to receive a low dosage (1.2, 2, and 2.4 grams/day for the respective body weight category) or a high dosage (2, 3.6, and 4.8 grams/day).
The high dosage regimen is not recommended because it was not found to be more effective than the recommended low dosage regimen [see Dosage and Administration (2.1) and Clinical Studies (14.1)].
Duration of exposure to Asacol among the 82 patients in the study ranged from 12 to 50 days (mean of 40 days in each dosage group). The majority (88%) of patients in each group were treated for more than 5 weeks. Table 3 provides a summary of the specific reported adverse reactions.
| Adverse Reaction | % of Patients with Adverse Reactions | |
| Asacol Low Dosage | Asacol High Dosage | |
| (n=41) | (n=41) | |
| Nasopharyngitis | 15 | 12 |
| Headache | 10 | 5 |
| Abdominal pain | 10 | 2 |
| Dizziness | 7 | 2 |
| Sinusitis | 7 | 0 |
| Rash | 5 | 5 |
| Cough | 5 | 0 |
| Diarrhea | 5 | 0 |
| Fatigue | 2 | 10 |
| Pyrexia | 0 | 7 |
| Increased Lipase | 0 | 5 |
| Low Dosage = Asacol 1.2 to 2.4 grams/day; High Dosage = Asacol 2.0 to 4.8 grams/day. Dosage was dependent on body weight. Adverse Reactions reported at the 1-week telephone follow-up visit are included. |
||
* At Least 5% of Patients in the low dosage or high dosage group
Twelve percent of the patients in the low dosage group (5 patients) and 2% of the patients in the high dosage group (1 patient) had serious adverse reactions. The serious adverse reactions consisted of sinusitis, adenovirus infection, and pancreatitis in one patient each in the low dosage group. Abdominal pain and decreased body mass index occurred in one patient and bloody diarrhea and sclerosing cholangitis also occurred in one patient in the low dosage group. Anemia and syncope occurred in one patient in the high dosage group.
Five patients were withdrawn from the study due to adverse reactions: 3 (7%) in the low dosage group (1 patient each with adenovirus infection, sclerosing cholangitis, and pancreatitis) and 2 patients (5%) in the high dosage group (1 patient with increased amylase and increased lipase, and 1 patient with upper abdominal pain).
In general, the nature and severity of reactions in the pediatric population was similar to those reported in adult populations of patients with ulcerative colitis.
Maintenance of Remission of Mildly to Moderately Active Ulcerative Colitis
Clinical studies supporting the use of Asacol tablets in the maintenance of remission of mildly to moderately active ulcerative colitis in adults included a randomized, double-blind, multi-center, placebo-controlled clinical trial of 6 months’ duration in 264 patients (Study 4 [see Clinical Studies (14.2)].
In Study 4, a randomized, double-blind, multi-center, placebo-controlled clinical trial of 6 months’ duration, 87 patients were randomized to receive Asacol 1.6 grams/day compared to 87 patients randomized to placebo. The average age of patients in Study 4 was 42 years and 55 % of patients were male. Adverse reactions leading to study withdrawal in patients using Asacol included (each in one patient): anxiety, stomatitis and asthenia.
In addition to the adverse reactions listed in Table 2, the following occurred at a frequency of 2% or greater in patients who received Asacol in Study 4: abdominal enlargement, gastroenteritis, gastrointestinal hemorrhage, infection, joint disorder, nervousness, paresthesia, hemorrhoids, tenesmus, urinary frequency, and vision abnormalities.
6.2 Postmarketing Experience
In addition to the adverse reactions reported above in clinical trials involving Asacol, the adverse reactions listed below have been identified during post-approval use of Asacol and other mesalamine-containing products. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Body as a Whole: Neck pain, facial edema, edema, lupus-like syndrome, drug fever.
Cardiovascular: Pericarditis, myocarditis [see Warnings and Precautions (5.3)].
Gastrointestinal: Anorexia, pancreatitis, gastritis, increased appetite, cholecystitis, dry mouth, oral ulcers, perforated peptic ulcer, bloody diarrhea.
Hematologic: Agranulocytosis aplastic anemia, thrombocytopenia, eosinophilia, leukopenia, anemia, lymphadenopathy.
Musculoskeletal: Gout.
Nervous: Depression, somnolence, emotional lability, hyperesthesia, vertigo, confusion, tremor, peripheral neuropathy, transverse myelitis, Guillain-Barré syndrome.
Renal: Renal failure, interstitial nephritis, minimal change nephropathy [see Warnings and Precautions (5.1)].
Respiratory/Pulmonary: Eosinophilic pneumonia, interstitial pneumonitis, asthma exacerbation, pleuritis.
Skin: Alopecia, psoriasis, pyoderma gangrenosus, dry skin, erythema nodosum, urticaria.
Special Senses: Eye pain, taste perversion, blurred vision, tinnitus.
Urogenital: Dysuria, urinary urgency, hematuria, epididymitis, menorrhagia, reversible oligospermia.
Laboratory Abnormalities: Elevated AST (SGOT) or ALT (SGPT), elevated alkaline phosphatase, elevated GGT, elevated LDH, elevated bilirubin, elevated serum creatinine and BUN.
7. Drug Interactions
7.1 Nephrotoxic Agents, Including Non-Steroidal Anti-Inflammatory Drugs
The concurrent use of mesalamine with known nephrotoxic agents, including nonsteroidal anti-inflammatory drugs (NSAIDs) may increase the risk of nephrotoxicity. Monitor patients taking nephrotoxic drugs for changes in renal function and mesalamine-related adverse reactions. [see Warnings and Precautions (5.1)].
7.2 Azathioprine or 6-Mercaptopurine
The concurrent use of mesalamine with azathioprine or 6-mercaptopurine may increase the risk for blood disorders. If concomitant use of Asacol and azathioprine or 6-mercaptopurine cannot be avoided, monitor blood tests, including complete blood cell counts and platelet counts.
8. Use In Specific Populations
8.1 Pregnancy
Risk Summary
Limited published data on mesalamine use in pregnant women are insufficient to inform a drug-associated risk. No fetal harm was observed in animal reproduction studies of mesalamine in rats and rabbits at oral doses approximately 1.9 times (rat) and 3.9 times (rabbit) the recommended human dose [see Data]. However, dibutyl phthalate (DBP) is an inactive ingredient in Asacol’s enteric coating, and in animal studies in rats at doses greater than 190 times the human dose, maternal DBP was associated with external and skeletal malformations and adverse effects on the reproductive system of male offspring. Advise pregnant women of the potential risk to the fetus.
The estimated background risk of major birth defects and miscarriage for the indicated populations is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Data
Animal Data
Reproduction studies with mesalamine were performed during organogenesis in rats and rabbits at oral doses up to 480 mg/kg/day. There was no evidence of impaired fertility or harm to the fetus. These mesalamine doses were about 1.9 times (rat) and 3.9 times (rabbit) the recommended human dose, based on body surface area.
DBP is an inactive ingredient in Asacol’s enteric coating. The human daily intake of DBP from the maximum recommended dose of Asacol tablets is about 21 mg. Published reports in rats show that male rat offspring exposed in utero to DBP (greater than or equal to 100 mg/kg/day, approximately 39 times the human dose based on body surface area), display reproductive system aberrations compatible with disruption of androgenic dependent development. The clinical significance of this finding in rats is unknown. At higher dosages (greater than or equal to 500 mg/kg/day, approximately 194 times the human dose based on body surface area), additional effects, including cryptorchidism, hypospadias, atrophy or agenesis of sex accessory organs, testicular injury, reduced daily sperm production, permanent retention of nipples, and decreased anogenital distance are noted. Female offspring are unaffected. High doses of DBP, administered to pregnant rats was associated with increased incidences of developmental abnormalities, such as cleft palate (greater than or equal to 630 mg/kg/day, about 244 times the human dose, based on body surface area) and skeletal abnormalities (greater than or equal to 750 mg/kg/day, about 290 times the human dose based on body surface area) in the offspring.
8.5 Geriatric Use
Clinical studies of Asacol did not include sufficient numbers of patients aged 65 and over to determine whether they respond differently than younger patients. Reports from uncontrolled clinical studies and postmarketing experience suggest a higher incidence of blood dyscrasias (agranulocytosis, neutropenia, pancytopenia) in patients receiving Asacol who are 65 years or older compared to younger patients. Monitor complete blood cell counts and platelet counts in elderly patients during treatment with Asacol. In general, the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy in elderly patients should be considered when prescribing Asacol [see Use in Specific Populations (8.6)].
8.6 Renal Impairment
Mesalamine is known to be substantially excreted by the kidney, and the risk of adverse reactions may be greater in patients with impaired renal function. Evaluate renal function in all patients prior to initiation and periodically while on Asacol therapy. Monitor patients with known renal impairment or history of renal disease or taking nephrotoxic drugs for decreased renal function and mesalamine-related adverse reactions [see Warnings and Precautions (5.1), Drug Interactions (7.1) and Adverse Reactions (6.2)].
11. Asacol Description
Each Asacol (mesalamine) delayed-release tablet for oral administration contains 400 mg of mesalamine, an aminosalicylate. Asacol (mesalamine) Delayed-Release Tablets contain acrylic based resin, Eudragit S (methacrylic acid and methyl methancrylate copolymer), which dissolves at pH 7 or greater and releases mesalamine in the terminal ileum and beyond for topical anti-inflammatory action in the colon. Mesalamine (also referred to as 5-aminosalicylic acid or 5-ASA) has the chemical name 5-amino-2-hydroxybenzoic acid. Its structural formula is:
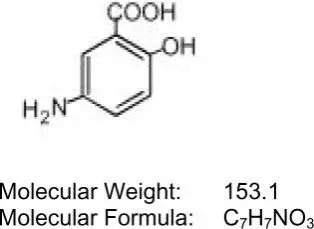
Inactive Ingredients: Each tablet contains colloidal silicon dioxide, dibutyl phthalate, edible black ink, ferric oxide red, ferric oxide yellow, lactose monohydrate, magnesium stearate, methacrylic acid and methyl methancrylate copolymer (Eudragit S), polyethylene glycol, povidone, sodium starch glycolate, and talc.
12. Asacol - Clinical Pharmacology
12.3 Pharmacokinetics
Absorption
Approximately 28% of mesalamine in Asacol tablets is absorbed after oral ingestion. Absorption of mesalamine is similar in fasted and fed subjects. The Tmax for mesalamine and its metabolite, is usually delayed, reflecting the delayed-release, and ranges from 4 to 16 hours.
Elimination
Metabolism
The absorbed mesalamine is rapidly acetylated in the gut mucosal wall and by the liver to N-acetyl-5-aminosalicylic acid.
Excretion
Absorbed mesalamine is excreted mainly by the kidney as N-acetyl-5-aminosalicylic acid. Unabsorbed mesalamine is excreted in feces.
After intravenous administration, the elimination half-life of mesalamine is reported to be approximately 40 minutes. After oral dosing, the terminal t1/2 values for mesalamine and N-acetyl-5-aminosalicylic acid are usually about 12 hours, but are variable, ranging from 2 to 15 hours. There is a large inter-subject and intra-subject variability in the plasma concentrations of mesalamine and N-acetyl-5-aminosalicylic acid and in their elimination half-lives following administration of Asacol.
Specific Populations
Pediatric Patients
In a dose-ranging pharmacokinetic study evaluating 30, 60 and 90 mg/kg/day doses of Asacol administered twice daily for four weeks, the mean average concentration (Cavg) values of mesalamine in pediatric ulcerative colitis patients ranged from approximately 400 ng/mL to 2100 ng/mL based on data from all dose levels.
In a study in pediatric ulcerative colitis patients (Study 3), mean plasma concentrations of mesalamine (based on sparse sampling) were 820 to 988 ng/mL at the low dosage level (that is, 1.2, 2 or 2.4 grams/day based on body weight strata of 17 to less than 33 kg, 33 to less than 54 kg, and 54 to 90 kg, respectively).
13. Nonclinical Toxicology
13.2 Animal Toxicology and/or Pharmacology
In animal studies (rats, mice, dogs), the kidney was the principal organ for toxicity. (In the following, comparisons of animal dosing to recommended human dosing are based on body surface area and a 2.4 grams/day dose for a 60 kg person.)
Mesalamine causes renal papillary necrosis in rats at single doses of approximately 750 mg/kg to 1000 mg/kg (approximately 3 to 4 times the recommended human dose based on body surface area). Doses of 170 and 360 mg/kg/day (about 0.7 and 1.5 times the recommended human dose based on body surface area) given to rats for six months produced papillary necrosis, papillary edema, tubular degeneration, tubular mineralization, and urothelial hyperplasia.
In mice, oral doses of 4000 mg/kg/day mesalamine (approximately 8 times the recommended human dose based on body surface area) for three months produced tubular nephrosis, multifocal/diffuse tubulo-interstitial inflammation, and multifocal/diffuse papillary necrosis.
In dogs, single doses of 6000 mg (approximately 8 times the recommended human dose based on body surface area) of delayed-release mesalamine tablets resulted in renal papillary necrosis but were not fatal. Renal changes have occurred in dogs given chronic administration of mesalamine at doses of 80 mg/kg/day (1.1 times the recommended human dose based on body surface area).
14. Clinical Studies
14.1 Treatment of Mildly to Moderately Active Ulcerative Colitis
Adults
Two placebo-controlled studies (Studies 1 and 2) have demonstrated the efficacy of Asacol in patients with mildly to moderately active ulcerative colitis.
In one randomized, double-blind, multi-center, placebo-controlled clinical trial of 6 weeks’ duration in 158 patients (Study 1), patients received Asacol dosages of 1.6 grams/day (800 mg twice a day; n=53) and 2.4 grams/day (800 mg three times a day; n=53), compared to placebo (n=52). The scoring system for determination of treatment efficacy included assessment of stool frequency, rectal bleeding, sigmoidoscopic findings, patient’s functional assessment, and physician global assessment. At the dosage of 2.4 grams/day, 21 of 43 (49%) patients using Asacol showed an improvement in sigmoidoscopic appearance of the bowel compared to 12 of 44 (27%) patients using placebo (p = 0.048). In addition, significantly more patients in the Asacol 2.4 grams/day group showed improvement in rectal bleeding and stool frequency. The 1.6 grams/day dosage regimen is not recommended because it did not produce consistent evidence of effectiveness [see Dosage and Administration (2.2)].
In a second randomized, double-blind, placebo-controlled clinical trial of 6 weeks’ duration in 87 patients (Study 2), patients received Asacol dosages of 1.6 grams/day (400 mg four times a day; n=11) and 4.8 grams/day (1.2 g four times a day; n=38), compared to placebo four times a day (n=38). Asacol 4.8 grams/day for 6 weeks resulted in sigmoidoscopic improvement in 28 of 38 (74 %) patients compared to 10 of 38 (26 %) placebo patients (p less than 0.001). Also, more patients in the Asacol 4.8 grams/day group than the placebo group showed improvement in overall symptoms. The 4.8 grams/day dosage regimen is not recommended because greater efficacy was not demonstrated with this dosage compared to the 2.4 grams/day dosage [see Dosage and Administration (2.2).
Pediatrics
The safety and effectiveness of Asacol in pediatric patients 5 to 17 years of age for treatment of mildly to moderately active ulcerative colitis are supported by evidence from adequate and well controlled studies of Asacol in adults and a single study in pediatric patients.
A randomized, double-blind, 6-week study of two dosage levels of Asacol (Study 3) was conducted in 82 pediatric patients 5 to 17 years of age with mildly or moderately active ulcerative colitis defined as a score of 10 to 55 on the Pediatric Ulcerative Colitis Activity Index (PUCAI) (which includes assessment of abdominal pain, rectal bleeding, stool consistency, number of stools per 24 hours, presence of nocturnal bowel movement and activity level, and has a total maximum score of 85; each of the subscales are scored from 0 to 10 except rectal bleeding which is scored from 0 to 30, and number of stools per 24 hours which is scored from 0 to 15) and rectal bleeding and stool frequency Mayo subscale scores of ≥1 (each of these subscales are scored from zero (normal) to three (most severe)).1,2
All patients were divided by weight category (17 to less than 33 kg, 33 to less than 54 kg, and 54 to 90 kg) and randomly assigned to receive a low dosage (1.2, 2, and 2.4 grams/day for the respective weight category) or a high dosage (2, 3.6, and 4.8 grams/day). Doses were administered every 12 hours.
The proportion of patients who achieved success based on the Truncated Mayo Score (TM-Mayo) (based on the stool frequency and rectal bleeding subscales of the Mayo Score) and based on the PUCAI was measured after 6 weeks of treatment. Success based on TM-Mayo was defined as either partial response (improvement from baseline in stool frequency or rectal bleeding subscores with no worsening in the other) or complete response (both stool frequency and rectal bleeding subscores equal 0). Success based on PUCAI was defined as either partial response (PUCAI reduction of greater than or equal to 20 points from Baseline to Week 6 with Week 6 score greater than or equal to 10) or complete response (PUCAI less than 10 at Week 6).
There were 41 patients in the low dosage group and 41 patients in the high dosage group who received at least one dose of Asacol; 36 patients in each dosage group completed the study. Patients were considered treatment failures if they did not achieve success or dropped out due to adverse reaction or lack of efficacy.
At Week 6, 73% of the patients in the low dosage group, and 70% of the patients in the high dosage group achieved success based on the TM-Mayo; 34% of the patients in the low dosage group and 43% of the patients in the high dosage group achieved complete response. At Week 6, 56% of the patients in the low dosage group, and 55% of the patients in the high dosage group achieved success based on the PUCAI; 46% of the patients in the low dosage group and 43% of the patients in the high dosage group achieved complete response.
The high dosage regimen is not recommended because it was not more effective than the low dosage regimen [see Dosage and Administration (2.2)].
14.2 Maintenance of Remission of Mildly to Moderately Active Ulcerative Colitis
Adults
In a randomized, double-blind, multi-center, placebo-controlled clinical trial of 6 months’ duration in 264 patients (Study 4), patients received Asacol dosages of 0.8 grams/day (400 mg twice a day; n = 90) and 1.6 grams/day (400 mg four times a day; n = 87), compared to placebo four times a day (n = 87). The proportion of patients treated with 0.8 grams/day who maintained endoscopic remission was not statistically significant compared to placebo; the 0.8 grams/day dosage regimen is not recommended [see Dosage and Administration (2.2)]. The number of patients using Asacol 1.6 grams/day who maintained endoscopic remission of ulcerative colitis was 61 of 87 (70%) compared with 42 of 87 (48%) of placebo patients (p = 0.005).
A pooled efficacy analysis of 4 maintenance trials compared Asacol at dosages of 0.8 to 2.8 grams/day, in divided doses ranging from twice daily to four times per day, with sulfasalazine, at dosages of 2 to 4 grams/day. Treatment success was seen in 59 of 98 (59%) patients using Asacol and 70 of 102 (69%) patients using sulfasalazine, a non-significant difference.
| ASACOL
mesalamine tablet, delayed release |
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| Labeler - Warner Chilcott (US), LLC (957203177) |






