Drug Detail:Avastin (Bevacizumab [ bev-a-ciz-oo-mab ])
Drug Class: VEGF/VEGFR inhibitors
Highlights of Prescribing Information
AVASTIN® (bevacizumab) injection, for intravenous use
Initial U.S. Approval: 2004
Recent Major Changes
| Indications and Usage (1.1) | 9/2022 |
| Dosage and Administration (2.2) | 9/2022 |
| Warnings and Precautions, Infusion-Related Reactions (5.9) | 9/2022 |
Indications and Usage for Avastin
Avastin is a vascular endothelial growth factor inhibitor indicated for the treatment of:
- Metastatic colorectal cancer, in combination with intravenous fluorouracil-based chemotherapy for first- or second-line treatment. (1.1)
- Metastatic colorectal cancer, in combination with fluoropyrimidine-irinotecan- or fluoropyrimidine-oxaliplatin-based chemotherapy for second-line treatment in patients who have progressed on a first-line bevacizumab product-containing regimen. (1.1)
Limitations of Use: Avastin is not indicated for adjuvant treatment of colon cancer. (1.1)
- Unresectable, locally advanced, recurrent or metastatic non-squamous non-small cell lung cancer, in combination with carboplatin and paclitaxel for first-line treatment. (1.2)
- Recurrent glioblastoma in adults. (1.3)
- Metastatic renal cell carcinoma in combination with interferon alfa. (1.4)
- Persistent, recurrent, or metastatic cervical cancer, in combination with paclitaxel and cisplatin, or paclitaxel and topotecan. (1.5)
- Epithelial ovarian, fallopian tube, or primary peritoneal cancer:
- in combination with carboplatin and paclitaxel, followed by Avastin as a single agent, for stage III or IV disease following initial surgical resection (1.6)
- in combination with paclitaxel, pegylated liposomal doxorubicin, or topotecan for platinum-resistant recurrent disease who received no more than 2 prior chemotherapy regimens (1.6)
- in combination with carboplatin and paclitaxel or carboplatin and gemcitabine, followed by Avastin as a single agent, for platinum-sensitive recurrent disease (1.6)
- Hepatocellular Carcinoma (HCC)
- in combination with atezolizumab for the treatment of patients with unresectable or metastatic HCC who have not received prior systemic therapy (1.7)
Avastin Dosage and Administration
Withhold for at least 28 days prior to elective surgery. Do not administer Avastin for 28 days following major surgery and until adequate wound healing. (2.1)
Metastatic colorectal cancer (2.2)
- 5 mg/kg every 2 weeks with bolus-IFL
- 10 mg/kg every 2 weeks with FOLFOX4
- 5 mg/ kg every 2 weeks or 7.5 mg/kg every 3 weeks with fluoropyrimidine-irinotecan- or fluoropyrimidine-oxaliplatin-based chemotherapy after progression on a first-line bevacizumab product-containing regimen
First-line non–squamous non–small cell lung cancer (2.3)
- 15 mg/kg every 3 weeks with carboplatin and paclitaxel
Recurrent glioblastoma (2.4)
- 10 mg/kg every 2 weeks
Metastatic renal cell carcinoma (2.5)
- 10 mg/kg every 2 weeks with interferon alfa
Persistent, recurrent, or metastatic cervical cancer (2.6)
- 15 mg/kg every 3 weeks with paclitaxel and cisplatin, or paclitaxel and topotecan
Stage III or IV epithelial ovarian, fallopian tube or primary peritoneal cancer following initial surgical resection (2.7)
- 15 mg/kg every 3 weeks with carboplatin and paclitaxel for up to 6 cycles, followed by 15 mg/kg every 3 weeks as a single agent, for a total of up to 22 cycles
Platinum-resistant recurrent epithelial ovarian, fallopian tube or primary peritoneal cancer (2.7)
- 10 mg/kg every 2 weeks with paclitaxel, pegylated liposomal doxorubicin, or topotecan given every week
- 15 mg/kg every 3 weeks with topotecan given every 3 weeks
Platinum-sensitive recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer (2.7)
- 15 mg/kg every 3 weeks with carboplatin and paclitaxel for 6-8 cycles, followed by 15 mg/kg every 3 weeks as a single agent
- 15 mg/kg every 3 weeks with carboplatin and gemcitabine for 6-10 cycles, followed by 15 mg/kg every 3 weeks as a single agent
Hepatocellular Carcinoma (2.8)
- 15 mg/kg after administration of 1,200 mg of atezolizumab every 3 weeks
Administer as an intravenous infusion after dilution. See full Prescribing Information for preparation and administration instructions and dosage modifications for adverse reactions (2.9, 2.10)
Dosage Forms and Strengths
Injection: 100 mg/4 mL (25 mg/mL) or 400 mg/16 mL (25 mg/mL) in a single-dose vial (3)
Contraindications
None (4)
Warnings and Precautions
- Gastrointestinal Perforations and Fistula: Discontinue for gastrointestinal perforations, tracheoesophageal fistula, grade 4 fistula, or fistula formation involving any organ (5.1)
- Surgery and Wound Healing Complications: In patients who experience wound healing complications during Avastin treatment, withhold Avastin until adequate wound healing. Withhold for at least 28 days prior to elective surgery. Do not administer Avastin for at least 28 days following a major surgery, and until adequate wound healing. The safety of resumption of AVASTIN after resolution of wound healing complication has not been established. Discontinue for wound healing complication of necrotizing fasciitis. (5.2)
- Hemorrhage: Severe or fatal hemorrhages have occurred. Do not administer for recent hemoptysis. Discontinue for Grade 3-4 hemorrhage (5.3)
- Arterial Thromboembolic Events (ATE): Discontinue for severe ATE. (5.4)
- Venous Thromboembolic Events (VTE): Discontinue for Grade 4 VTE. (5.5)
- Hypertension: Monitor blood pressure and treat hypertension. Withhold if not medically controlled; resume once controlled. Discontinue for hypertensive crisis or hypertensive encephalopathy. (5.6)
- Posterior Reversible Encephalopathy Syndrome (PRES): Discontinue. (5.7)
- Renal Injury and Proteinuria: Monitor urine protein. Discontinue for nephrotic syndrome. Withhold until less than 2 grams of protein in urine. (5.8)
- Infusion-Related Reactions: Decrease rate for infusion-related reactions. Discontinue for severe infusion-related reactions and administer medical therapy. (5.9)
- Embryo-Fetal Toxicity: May cause fetal harm. Advise females of potential risk to fetus and need for use of effective contraception. (5.10, 8.1, 8.3)
- Ovarian Failure: Advise females of the potential risk. (5.11, 8.3)
- Congestive Heart Failure (CHF): Discontinue Avastin in patients who develop CHF. (5.12)
Adverse Reactions/Side Effects
Most common adverse reactions incidence (incidence > 10%) are epistaxis, headache, hypertension, rhinitis, proteinuria, taste alteration, dry skin, hemorrhage, lacrimation disorder, back pain and exfoliative dermatitis. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Genentech, Inc. at 1-888-835-2555 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Use In Specific Populations
Lactation: Advise not to breastfeed. (8.2)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 9/2022
Related/similar drugs
Retevmo, methotrexate, Keytruda, capecitabine, carboplatin, fluorouracil, pembrolizumabFull Prescribing Information
1. Indications and Usage for Avastin
1.1 Metastatic Colorectal Cancer
Avastin, in combination with intravenous fluorouracil-based chemotherapy, is indicated for the first-or second-line treatment of patients with metastatic colorectal cancer (mCRC).
Avastin, in combination with fluoropyrimidine-irinotecan- or fluoropyrimidine-oxaliplatin-based chemotherapy, is indicated for the second-line treatment of patients with mCRC who have progressed on a first-line bevacizumab product-containing regimen.
1.2 First-Line Non-Squamous Non–Small Cell Lung Cancer
Avastin, in combination with carboplatin and paclitaxel, is indicated for the first-line treatment of patients with unresectable, locally advanced, recurrent or metastatic non–squamous non–small cell lung cancer (NSCLC).
1.3 Recurrent Glioblastoma
Avastin is indicated for the treatment of recurrent glioblastoma (GBM) in adults.
1.4 Metastatic Renal Cell Carcinoma
Avastin, in combination with interferon alfa, is indicated for the treatment of metastatic renal cell carcinoma (mRCC).
1.5 Persistent, Recurrent, or Metastatic Cervical Cancer
Avastin, in combination with paclitaxel and cisplatin or paclitaxel and topotecan, is indicated for the treatment of patients with persistent, recurrent, or metastatic cervical cancer.
1.6 Epithelial Ovarian, Fallopian Tube, or Primary Peritoneal Cancer
Avastin, in combination with carboplatin and paclitaxel, followed by Avastin as a single agent, is indicated for the treatment of patients with stage III or IV epithelial ovarian, fallopian tube, or primary peritoneal cancer following initial surgical resection.
Avastin, in combination with paclitaxel, pegylated liposomal doxorubicin, or topotecan, is indicated for the treatment of patients with platinum-resistant recurrent epithelial ovarian, fallopian tube or primary peritoneal cancer who received no more than 2 prior chemotherapy regimens.
Avastin, in combination with carboplatin and paclitaxel, or with carboplatin and gemcitabine, followed by Avastin as a single agent, is indicated for the treatment of patients with platinum-sensitive recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer.
2. Avastin Dosage and Administration
2.1 Important Administration Information
Withhold for at least 28 days prior to elective surgery. Do not administer Avastin until at least 28 days following major surgery and until adequate wound healing.
2.2 Metastatic Colorectal Cancer
The recommended dosage when Avastin is administered in combination with intravenous fluorouracil-based chemotherapy is:
- 5 mg/kg intravenously every 2 weeks in combination with bolus-IFL.
- 10 mg/kg intravenously every 2 weeks in combination with FOLFOX4.
- 5 mg/kg intravenously every 2 weeks or 7.5 mg/kg intravenously every 3 weeks in combination with fluoropyrimidine-irinotecan- or fluoropyrimidine-oxaliplatin-based chemotherapy in patients who have progressed on a first-line bevacizumab product-containing regimen.
2.3 First-Line Non-Squamous Non-Small Cell Lung Cancer
The recommended dosage is 15 mg/kg intravenously every 3 weeks in combination with carboplatin and paclitaxel.
2.5 Metastatic Renal Cell Carcinoma
The recommended dosage is 10 mg/kg intravenously every 2 weeks in combination with interferon alfa.
2.6 Persistent, Recurrent, or Metastatic Cervical Cancer
The recommended dosage is 15 mg/kg intravenously every 3 weeks in combination with paclitaxel and cisplatin or in combination with paclitaxel and topotecan.
2.8 Hepatocellular Carcinoma
The recommended dosage is 15 mg/kg intravenously after administration of 1,200 mg of atezolizumab intravenously on the same day, every 3 weeks until disease progression or unacceptable toxicity.
Refer to the Prescribing Information for atezolizumab prior to initiation for recommended dosage information.
2.9 Dosage Modifications for Adverse Reactions
Table 1 describes dosage modifications for specific adverse reactions. No dose reductions for Avastin are recommended.
| Adverse Reaction | Severity | Dosage Modification |
|---|---|---|
| Gastrointestinal Perforations and Fistulae [see Warnings and Precautions (5.1)]. |
| Discontinue Avastin |
| Wound Healing Complications [see Warnings and Precautions (5.2)]. |
| Withhold AVASTIN until adequate wound healing.The safety of resumption of AVASTIN after resolution of wound healing complications has not been established. |
| Discontinue Avastin | |
| Hemorrhage [see Warnings and Precautions (5.3)]. |
| Discontinue Avastin |
| Withhold Avastin | |
| Thromboembolic Events [see Warnings and Precautions (5.4, 5.5)]. |
| Discontinue Avastin |
| Discontinue Avastin | |
| Hypertension [see Warnings and Precautions (5.6)]. |
| Discontinue Avastin |
| Withhold Avastin if not controlled with medical management; resume once controlled | |
| Posterior Reversible Encephalopathy Syndrome (PRES) [see Warnings and Precautions (5.7)]. |
| Discontinue Avastin |
| Renal Injury and Proteinuria [see Warnings and Precautions (5.8)]. |
| Discontinue Avastin |
| Withhold Avastin until proteinuria less than 2 grams per 24 hours | |
| Infusion-Related Reactions [see Warnings and Precautions (5.9)]. |
| Discontinue Avastin |
| Interrupt infusion; resume at a decreased rate of infusion after symptoms resolve | |
| Decrease infusion rate | |
| Congestive Heart Failure [see Warnings and Precautions (5.12)]. | Any | Discontinue Avastin |
3. Dosage Forms and Strengths
Injection: 100 mg/4 mL (25 mg/mL) or 400 mg/16 mL (25 mg/mL) clear to slightly opalescent, colorless to pale brown solution in a single-dose vial.
5. Warnings and Precautions
5.1 Gastrointestinal Perforations and Fistulae
Serious, and sometimes fatal, gastrointestinal perforation occurred at a higher incidence in patients receiving Avastin compared to patients receiving chemotherapy. The incidence ranged from 0.3% to 3% across clinical studies, with the highest incidence in patients with a history of prior pelvic radiation. Perforation can be complicated by intra-abdominal abscess, fistula formation, and the need for diverting ostomies. The majority of perforations occurred within 50 days of the first dose [see Adverse Reactions (6.1)].
Serious fistulae (including, tracheoesophageal, bronchopleural, biliary, vaginal, renal and bladder sites) occurred at a higher incidence in patients receiving Avastin compared to patients receiving chemotherapy. The incidence ranged from < 1% to 1.8% across clinical studies, with the highest incidence in patients with cervical cancer. The majority of fistulae occurred within 6 months of the first dose. Patients who develop a gastrointestinal vaginal fistula may also have a bowel obstruction and require surgical intervention, as well as a diverting ostomy.
Avoid Avastin in patients with ovarian cancer who have evidence of recto-sigmoid involvement by pelvic examination or bowel involvement on CT scan or clinical symptoms of bowel obstruction. Discontinue in patients who develop gastrointestinal perforation, tracheoesophageal fistula or any Grade 4 fistula. Discontinue in patients with fistula formation involving any internal organ.
5.2 Surgery and Wound Healing Complications
In a controlled clinical study in which Avastin was not administered within 28 days of major surgical procedures, the incidence of wound healing complications, including serious and fatal complications, was 15% in patients with mCRC who underwent surgery while receiving Avastin and 4% in patients who did not receive Avastin. In a controlled clinical study in patients with relapsed or recurrent GBM, the incidence of wound healing events was 5% in patients who received Avastin and 0.7% in patients who did not receive Avastin [see Adverse Reactions (6.1)].
In patients who experience wound healing complications during Avastin treatment, withhold Avastin until adequate wound healing. Withhold for at least 28 days prior to elective surgery. Do not administer for at least 28 days following major surgery and until adequate wound healing. The safety of resumption of AVASTIN after resolution of wound healing complications has not been established [see Dosage and Administration (2.9)].
Necrotizing fasciitis including fatal cases, has been reported in patients receiving Avastin, usually secondary to wound healing complications, gastrointestinal perforation or fistula formation. Discontinue Avastin in patients who develop necrotizing fasciitis.
5.3 Hemorrhage
Avastin can result in two distinct patterns of bleeding: minor hemorrhage, which is most commonly Grade 1 epistaxis, and serious hemorrhage, which in some cases has been fatal. Severe or fatal hemorrhage, including hemoptysis, gastrointestinal bleeding, hematemesis, CNS hemorrhage, epistaxis, and vaginal bleeding, occurred up to 5-fold more frequently in patients receiving Avastin compared to patients receiving chemotherapy alone. Across clinical studies, the incidence of Grades 3-5 hemorrhagic events ranged from 0.4% to 7% in patients receiving Avastin [see Adverse Reactions (6.1)].
Serious or fatal pulmonary hemorrhage occurred in 31% of patients with squamous NSCLC and 4% of patients with non-squamous NSCLC receiving Avastin with chemotherapy compared to none of the patients receiving chemotherapy alone.
An evaluation for the presence of varices is recommended within 6 months of initiation of Avastin in patients with HCC. There is lack of clinical data to support the safety of Avastin in patients with variceal bleeding within 6 months prior to treatment, untreated or incompletely treated varices with bleeding, or high risk of bleeding because these patients were excluded from clinical trials of Avastin in HCC [see Clinical Studies (14.10)].
Do not administer Avastin to patients with recent history of hemoptysis of 1/2 teaspoon or more of red blood. Discontinue in patients who develop a Grades 3-4 hemorrhage.
5.4 Arterial Thromboembolic Events
Serious, sometimes fatal, arterial thromboembolic events (ATE) including cerebral infarction, transient ischemic attacks, myocardial infarction, and angina, occurred at a higher incidence in patients receiving Avastin compared to patients receiving chemotherapy. Across clinical studies, the incidence of Grades 3-5 ATE was 5% in patients receiving Avastin with chemotherapy compared to ≤2% in patients receiving chemotherapy alone; the highest incidence occurred in patients with GBM. The risk of developing ATE was increased in patients with a history of arterial thromboembolism, diabetes, or >65 years [see Use in Specific Populations (8.5)].
Discontinue in patients who develop a severe ATE. The safety of reinitiating Avastin after an ATE is resolved is not known.
5.5 Venous Thromboembolic Events
An increased risk of venous thromboembolic events (VTE) was observed across clinical studies [see Adverse Reactions (6.1)]. In Study GOG-0240, Grades 3-4 VTE occurred in 11% of patients receiving Avastin with chemotherapy compared with 5% of patients receiving chemotherapy alone. In EORTC 26101, the incidence of Grades 3-4 VTE was 5% in patients receiving Avastin with chemotherapy compared to 2% in patients receiving chemotherapy alone.
Discontinue Avastin in patients with a Grade 4 VTE, including pulmonary embolism.
5.6 Hypertension
Severe hypertension occurred at a higher incidence in patients receiving Avastin as compared to patients receiving chemotherapy alone. Across clinical studies, the incidence of Grades 3-4 hypertension ranged from 5% to 18%.
Monitor blood pressure every two to three weeks during treatment with Avastin. Treat with appropriate anti-hypertensive therapy and monitor blood pressure regularly. Continue to monitor blood pressure at regular intervals in patients with Avastin-induced or -exacerbated hypertension after discontinuing Avastin. Withhold Avastin in patients with severe hypertension that is not controlled with medical management; resume once controlled with medical management. Discontinue in patients who develop hypertensive crisis or hypertensive encephalopathy.
5.7 Posterior Reversible Encephalopathy Syndrome
Posterior reversible encephalopathy syndrome (PRES) was reported in < 0.5% of patients across clinical studies. The onset of symptoms occurred from 16 hours to 1 year after the first dose. PRES is a neurological disorder which can present with headache, seizure, lethargy, confusion, blindness and other visual and neurologic disturbances. Mild to severe hypertension may be present. Magnetic resonance imaging is necessary to confirm the diagnosis of PRES.
Discontinue Avastin in patients who develop PRES. Symptoms usually resolve or improve within days after discontinuing Avastin, although some patients have experienced ongoing neurologic sequelae. The safety of reinitiating Avastin in patients who developed PRES is not known.
5.8 Renal Injury and Proteinuria
The incidence and severity of proteinuria was higher in patients receiving Avastin as compared to patients receiving chemotherapy. Grade 3 (defined as urine dipstick 4+ or > 3.5 grams of protein per 24 hours) to Grade 4 (defined as nephrotic syndrome) ranged from 0.7% to 7% in clinical studies. The overall incidence of proteinuria (all grades) was only adequately assessed in Study BO17705, in which the incidence was 20%. Median onset of proteinuria was 5.6 months (15 days to 37 months) after initiating Avastin. Median time to resolution was 6.1 months (95% CI: 2.8, 11.3). Proteinuria did not resolve in 40% of patients after median follow-up of 11.2 months and required discontinuation of Avastin in 30% of the patients who developed proteinuria [see Adverse Reactions (6.1)].
In an exploratory, pooled analysis of patients from seven randomized clinical studies, 5% of patients receiving Avastin with chemotherapy experienced Grades 2-4 (defined as urine dipstick 2+ or greater or > 1 gram of protein per 24 hours or nephrotic syndrome) proteinuria. Grades 2-4 proteinuria resolved in 74% of patients. Avastin was reinitiated in 42% of patients. Of the 113 patients who reinitiated Avastin, 48% experienced a second episode of Grades 2-4 proteinuria.
Nephrotic syndrome occurred in < 1% of patients receiving Avastin across clinical studies, in some instances with fatal outcome. In a published case series, kidney biopsy of 6 patients with proteinuria showed findings consistent with thrombotic microangiopathy. Results of a retrospective analysis of 5805 patients who received Avastin with chemotherapy and 3713 patients who received chemotherapy alone, showed higher rates of elevated serum creatinine levels (between 1.5 to 1.9 times baseline levels) in patients who received Avastin. Serum creatinine levels did not return to baseline in approximately one-third of patients who received Avastin.
Monitor proteinuria by dipstick urine analysis for the development or worsening of proteinuria with serial urinalyses during Avastin therapy. Patients with a 2+ or greater urine dipstick reading should undergo further assessment with a 24-hour urine collection. Withhold for proteinuria greater than or equal to 2 grams per 24 hours and resume when less than 2 grams per 24 hours. Discontinue in patients who develop nephrotic syndrome.
Data from a postmarketing safety study showed poor correlation between UPCR (Urine Protein/Creatinine Ratio) and 24-hour urine protein [Pearson Correlation 0.39 (95% CI: 0.17, 0.57)].
5.9 Infusion-Related Reactions
Infusion-related reactions reported across clinical studies and postmarketing experience include hypertension, hypertensive crises associated with neurologic signs and symptoms, wheezing, oxygen desaturation, Grade 3 hypersensitivity, anaphylactoid/anaphylactic reactions, chest pain, headaches, rigors, and diaphoresis. In clinical studies, infusion-related reactions with the first dose occurred in < 3% of patients and severe reactions occurred in 0.4% of patients.
Decrease the rate of infusion for mild, clinically insignificant infusion-related reactions. Interrupt the infusion in patients with clinically significant infusion-related reactions and consider resuming at a slower rate following resolution. Discontinue in patients who develop a severe infusion-related reaction and administer appropriate medical therapy (e.g., epinephrine, corticosteroids, intravenous antihistamines, bronchodilators and/or oxygen).
5.10 Embryo-Fetal Toxicity
Based on its mechanism of action and findings from animal studies, Avastin may cause fetal harm when administered to pregnant women. Congenital malformations were observed with the administration of bevacizumab to pregnant rabbits during organogenesis every 3 days at a dose as low as a clinical dose of 10 mg/kg. Furthermore, animal models link angiogenesis and VEGF and VEGFR2 to critical aspects of female reproduction, embryo-fetal development, and postnatal development. Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment with Avastin and for 6 months after the last dose [see Use in Specific Populations (8.1, 8.3)].
5.11 Ovarian Failure
The incidence of ovarian failure was 34% vs. 2% in premenopausal women receiving Avastin with chemotherapy as compared to those receiving chemotherapy alone for adjuvant treatment of a solid tumor. After discontinuing Avastin, recovery of ovarian function at all time points during the post-treatment period was demonstrated in 22% of women receiving Avastin. Recovery of ovarian function is defined as resumption of menses, a positive serum β-HCG pregnancy test, or an FSH level < 30 mIU/mL during the post-treatment period. Long-term effects of Avastin on fertility are unknown. Inform females of reproductive potential of the risk of ovarian failure prior to initiating Avastin [see Adverse Reactions (6.1), Use in Specific Populations (8.3)].
5.12 Congestive Heart Failure (CHF)
Avastin is not indicated for use with anthracycline-based chemotherapy. The incidence of Grade ≥ 3 left ventricular dysfunction was 1% in patients receiving Avastin compared to 0.6% of patients receiving chemotherapy alone. Among patients who received prior anthracycline treatment, the rate of CHF was 4% for patients receiving Avastin with chemotherapy as compared to 0.6% for patients receiving chemotherapy alone.
In previously untreated patients with a hematological malignancy, the incidence of CHF and decline in left ventricular ejection fraction (LVEF) were increased in patients receiving Avastin with anthracycline-based chemotherapy compared to patients receiving placebo with the same chemotherapy regimen. The proportion of patients with a decline in LVEF from baseline of ≥ 20% or a decline from baseline of 10% to < 50%, was 10% in patients receiving Avastin with chemotherapy compared to 5% in patients receiving chemotherapy alone. Time to onset of left-ventricular dysfunction or CHF was 1 to 6 months after the first dose in at least 85% of the patients and was resolved in 62% of the patients who developed CHF in the Avastin arm compared to 82% in the placebo arm. Discontinue Avastin in patients who develop CHF.
6. Adverse Reactions/Side Effects
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Gastrointestinal Perforations and Fistulae [see Warnings and Precautions (5.1)].
- Surgery and Wound Healing Complications [see Warnings and Precautions (5.2)].
- Hemorrhage [see Warnings and Precautions (5.3)].
- Arterial Thromboembolic Events [see Warnings and Precautions (5.4)].
- Venous Thromboembolic Events [see Warnings and Precautions (5.5)].
- Hypertension [see Warnings and Precautions (5.6)].
- Posterior Reversible Encephalopathy Syndrome [see Warnings and Precautions (5.7)].
- Renal Injury and Proteinuria [see Warnings and Precautions (5.8)].
- Infusion-Related Reactions [see Warnings and Precautions (5.9)].
- Ovarian Failure [see Warnings and Precautions (5.11)].
- Congestive Heart Failure [see Warnings and Precautions (5.12)].
6.1 Clinical Trials Experience
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to rates in the clinical studies of another drug and may not reflect the rates observed in practice.
The safety data in Warnings and Precautions and described below reflect exposure to Avastin in 4463 patients including those with mCRC (AVF2107g, E3200), non-squamous NSCLC (E4599), GBM (EORTC 26101), mRCC (BO17705), cervical cancer (GOG-0240), epithelial ovarian, fallopian tube, or primary peritoneal cancer (MO22224, AVF4095, GOG-0213, and GOG-0218), or HCC (IMbrave150) at the recommended dose and schedule for a median of 6 to 23 doses. The most common adverse reactions observed in patients receiving Avastin as a single agent or in combination with other anti-cancer therapies at a rate > 10% were epistaxis, headache, hypertension, rhinitis, proteinuria, taste alteration, dry skin, hemorrhage, lacrimation disorder, back pain, and exfoliative dermatitis.
Across clinical studies, Avastin was discontinued in 8% to 22% of patients because of adverse reactions [see Clinical Studies (14)].
Metastatic Colorectal Cancer
In Combination with bolus-IFL
The safety of Avastin was evaluated in 392 patients who received at least one dose of Avastin in a double-blind, active-controlled study (AVF2107g), which compared Avastin (5 mg/kg every 2 weeks) with bolus-IFL to placebo with bolus-IFL in patients with mCRC [see Clinical Studies (14.1)]. Patients were randomized (1:1:1) to placebo with bolus-IFL, Avastin with bolus-IFL, or Avastin with fluorouracil and leucovorin. The demographics of the safety population were similar to the demographics of the efficacy population. All Grades 3–4 adverse reactions and selected Grades 1–2 adverse reactions (i.e., hypertension, proteinuria, thromboembolic events) were collected in the entire study population. Adverse reactions are presented in Table 2.
| Adverse Reaction* | Avastin with IFL (N=392) | Placebo with IFL (N=396) |
|---|---|---|
|
||
| Hematology | ||
| Leukopenia | 37% | 31% |
| Neutropenia | 21% | 14% |
| Gastrointestinal | ||
| Diarrhea | 34% | 25% |
| Abdominal pain | 8% | 5% |
| Constipation | 4% | 2% |
| Vascular | ||
| Hypertension | 12% | 2% |
| Deep vein thrombosis | 9% | 5% |
| Intra-abdominal thrombosis | 3% | 1% |
| Syncope | 3% | 1% |
| General | ||
| Asthenia | 10% | 7% |
| Pain | 8% | 5% |
Metastatic Renal Cell Carcinoma
The safety of Avastin was evaluated in 337 patients who received at least one dose of Avastin in a multicenter, double-blind study (BO17705) in patients with mRCC. Patients who had undergone a nephrectomy were randomized (1:1) to receive either Avastin (10 mg/kg every 2 weeks) or placebo with interferon alfa [see Clinical Studies (14.5)]. Patients were treated until disease progression or unacceptable toxicity. The demographics of the safety population were similar to the demographics of the efficacy population.
Grades 3-5 adverse reactions occurring at a higher incidence ( >2%) were fatigue (13% vs. 8%), asthenia (10% vs. 7%), proteinuria (7% vs. 0%), hypertension (6% vs. 1%; including hypertension and hypertensive crisis), and hemorrhage (3% vs. 0.3%; including epistaxis, small intestinal hemorrhage, aneurysm ruptured, gastric ulcer hemorrhage, gingival bleeding, hemoptysis, hemorrhage intracranial, large intestinal hemorrhage, respiratory tract hemorrhage, and traumatic hematoma). Adverse reactions are presented in Table 3.
| Adverse Reaction* | Avastin with Interferon Alfa (N=337) | Placebo with Interferon Alfa (N=304) |
|---|---|---|
|
||
| Metabolism and nutrition | ||
| Decreased appetite | 36% | 31% |
| Weight loss | 20% | 15% |
| General | ||
| Fatigue | 33% | 27% |
| Vascular | ||
| Hypertension | 28% | 9% |
| Respiratory, thoracic and mediastinal | ||
| Epistaxis | 27% | 4% |
| Dysphonia | 5% | 0% |
| Nervous system | ||
| Headache | 24% | 16% |
| Gastrointestinal | ||
| Diarrhea | 21% | 16% |
| Renal and urinary | ||
| Proteinuria | 20% | 3% |
| Musculoskeletal and connective tissue | ||
| Myalgia | 19% | 14% |
| Back pain | 12% | 6% |
The following adverse reactions were reported at a 5-fold greater incidence in patients receiving Avastin with interferon-alfa compared to patients receiving placebo with interferon-alfa and not represented in Table 3: gingival bleeding (13 patients vs. 1 patient); rhinitis (9 vs. 0); blurred vision (8 vs. 0); gingivitis (8 vs. 1); gastroesophageal reflux disease (8 vs. 1); tinnitus (7 vs. 1); tooth abscess (7 vs. 0); mouth ulceration (6 vs. 0); acne (5 vs. 0); deafness (5 vs. 0); gastritis (5 vs. 0); gingival pain (5 vs. 0) and pulmonary embolism (5 vs. 1).
Persistent, Recurrent, or Metastatic Cervical Cancer
The safety of Avastin was evaluated in 218 patients who received at least one dose of Avastin in a multicenter study (GOG-0240) in patients with persistent, recurrent, or metastatic cervical cancer[see Clinical Studies (14.6)]. Patients were randomized (1:1:1:1) to receive paclitaxel and cisplatin with or without Avastin (15 mg/kg every 3 weeks), or paclitaxel and topotecan with or without Avastin (15 mg/kg every 3 weeks). The demographics of the safety population were similar to the demographics of the efficacy population.
Grades 3-4 adverse reactions occurring at a higher incidence (≥ 2%) in 218 patients receiving Avastin with chemotherapy compared to 222 patients receiving chemotherapy alone were abdominal pain (12% vs. 10%), hypertension (11% vs. 0.5%), thrombosis (8% vs. 3%), diarrhea (6% vs. 3%), anal fistula (4% vs. 0%), proctalgia (3% vs. 0%), urinary tract infection (8% vs. 6%), cellulitis (3% vs. 0.5%), fatigue (14% vs. 10%), hypokalemia (7% vs. 4%), hyponatremia (4% vs. 1%), dehydration (4% vs. 0.5%), neutropenia (8% vs. 4%), lymphopenia (6% vs. 3%), back pain (6% vs. 3%), and pelvic pain (6% vs. 1%). Adverse reactions are presented in Table 4.
| Adverse Reaction* | Avastin with Chemotherapy (N=218) | Chemotherapy (N=222) |
|---|---|---|
|
||
| General | ||
| Fatigue | 80% | 75% |
| Peripheral edema | 15% | 22% |
| Metabolism and nutrition | ||
| Decreased appetite | 34% | 26% |
| Hyperglycemia | 26% | 19% |
| Hypomagnesemia | 24% | 15% |
| Weight loss | 21% | 7% |
| Hyponatremia | 19% | 10% |
| Hypoalbuminemia | 16% | 11% |
| Vascular | ||
| Hypertension | 29% | 6% |
| Thrombosis | 10% | 3% |
| Infections | ||
| Urinary tract infection | 22% | 14% |
| Infection | 10% | 5% |
| Nervous system | ||
| Headache | 22% | 13% |
| Dysarthria | 8% | 1% |
| Psychiatric | ||
| Anxiety | 17% | 10% |
| Respiratory, thoracic and mediastinal | ||
| Epistaxis | 17% | 1% |
| Renal and urinary | ||
| Increased blood creatinine | 16% | 10% |
| Proteinuria | 10% | 3% |
| Gastrointestinal | ||
| Stomatitis | 15% | 10% |
| Proctalgia | 6% | 1% |
| Anal fistula | 6% | 0% |
| Reproductive system and breast | ||
| Pelvic pain | 14% | 8% |
| Hematology | ||
| Neutropenia | 12% | 6% |
| Lymphopenia | 12% | 5% |
Epithelial Ovarian, Fallopian Tube or Primary Peritoneal Cancer
Stage III or IV Following Initial Surgical Resection
The safety of Avastin was evaluated in GOG-0218, a multicenter, randomized, double-blind, placebo controlled, three arm study, which evaluated the addition of Avastin to carboplatin and paclitaxel for the treatment of patients with stage III or IV epithelial ovarian, fallopian tube or primary peritoneal cancer following initial surgical resection [see Clinical Studies (14.7)]. Patients were randomized (1:1:1) to carboplatin and paclitaxel without Avastin (CPP), carboplatin and paclitaxel with Avastin for up to six cycles (CPB15), or carboplatin and paclitaxel with Avastin for six cycles followed by Avastin as a single agent for up to 16 additional doses (CPB15+). Avastin was given at 15 mg/kg every three weeks. On this trial, 1215 patients received at least one dose of Avastin. The demographics of the safety population were similar to the demographics of the efficacy population.
Grades 3-4 adverse reactions occurring at a higher incidence (≥2%) in either of the Avastin arms versus the control arm were fatigue (CPB15+ - 9%, CPB15 - 6%, CPP - 6%), hypertension (CPB15+ - 10%, CPB15 - 6%, CPP - 2%), thrombocytopenia (CPB15+ - 21%, CPB15 - 20%, CPP - 15%) and leukopenia (CPB15+ - 51%, CPB15 - 53%, CPP - 50%). Adverse reactions are presented in Table 5.
| Adverse Reaction* | Avastin with carboplatin and paclitaxel followed by Avastin alone†
(N=608) | Avastin with carboplatin and paclitaxel‡
(N= 607) | Carboplatin and paclitaxel§
(N= 602) |
|---|---|---|---|
|
|||
| General | |||
| Fatigue | 80% | 72% | 73% |
| Gastrointestinal | |||
| Nausea | 58% | 53% | 51% |
| Diarrhea | 38% | 40% | 34% |
| Stomatitis | 25% | 19% | 14% |
| Musculoskeletal and connective tissue | |||
| Arthralgia | 41% | 33% | 35% |
| Pain in extremity | 25% | 19% | 17% |
| Muscular weakness | 15% | 13% | 9% |
| Nervous system | |||
| Headache | 34% | 26% | 21% |
| Dysarthria | 12% | 10% | 2% |
| Vascular | |||
| Hypertension | 32% | 24% | 14% |
| Respiratory, thoracic and mediastinal | |||
| Epistaxis | 31% | 30% | 9% |
| Dyspnea | 26% | 28% | 20% |
| Nasal mucosal disorder | 10% | 7% | 4% |
Platinum-Resistant Recurrent Epithelial Ovarian, Fallopian Tube, or Primary Peritoneal Cancer
The safety of Avastin was evaluated in 179 patients who received at least one dose of Avastin in a multicenter, open-label study (MO22224) in which patients were randomized (1:1) to Avastin with chemotherapy or chemotherapy alone in patients with platinum resistant, recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer that recurred within < 6 months from the most recent platinum based therapy [see Clinical Studies (14.8)]. Patients were randomized to receive Avastin 10 mg/kg every 2 weeks or 15 mg/kg every 3 weeks. Patients had received no more than 2 prior chemotherapy regimens. The trial excluded patients with evidence of recto-sigmoid involvement by pelvic examination or bowel involvement on CT scan or clinical symptoms of bowel obstruction. Patients were treated until disease progression or unacceptable toxicity. Forty percent of patients on the chemotherapy alone arm received Avastin alone upon progression. The demographics of the safety population were similar to the demographics of the efficacy population.
Grades 3-4 adverse reactions occurring at a higher incidence ( ≥ 2%) in 179 patients receiving Avastin with chemotherapy compared to 181 patients receiving chemotherapy alone were hypertension (6.7% vs. 1.1%) and palmar-plantar erythrodysaesthesia syndrome (4.5% vs. 1.7%).
Adverse reactions are presented in Table 6.
| Adverse Reaction* | Avastin with Chemotherapy (N=179) | Chemotherapy (N=181) |
|---|---|---|
|
||
| Hematology | ||
| Neutropenia | 31% | 25% |
| Vascular | ||
| Hypertension | 19% | 6% |
| Nervous system | ||
| Peripheral sensory neuropathy | 18% | 7% |
| General | ||
| Mucosal inflammation | 13% | 6% |
| Renal and urinary | ||
| Proteinuria | 12% | 0.6% |
| Skin and subcutaneous tissue | ||
| Palmar-plantar erythrodysaesthesia | 11% | 5% |
| Infections | ||
| Infection | 11% | 4% |
| Respiratory, thoracic and mediastinal | ||
| Epistaxis | 5% | 0% |
Platinum-Sensitive Recurrent Epithelial Ovarian, Fallopian Tube, or Primary Peritoneal Cancer
Study AVF4095g
The safety of Avastin was evaluated in 247 patients who received at least one dose of Avastin in a double-blind study (AVF4095g) in patients with platinum sensitive recurrent epithelial ovarian, fallopian tube or primary peritoneal cancer [see Clinical Studies (14.9]. Patients were randomized (1:1) to receive Avastin (15 mg/kg) or placebo every 3 weeks with carboplatin and gemcitabine for 6 to 10 cycles followed by Avastin or placebo alone until disease progression or unacceptable toxicity. The demographics of the safety population were similar to the demographics of the efficacy population.
Grades 3-4 adverse reactions occurring at a higher incidence (≥ 2%) in patients receiving Avastin with chemotherapy compared to placebo with chemotherapy were: thrombocytopenia (40% vs. 34%), nausea (4% vs. 1.3%), fatigue (6% vs. 4%), headache (4% vs. 0.9%), proteinuria (10% vs. 0.4%), dyspnea (4% vs. 1.7%), epistaxis (5% vs. 0.4%), and hypertension (17% vs. 0.9%). Adverse reactions are presented in Table 7.
| Adverse Reaction* | Avastin with Carboplatin and Gemcitabine (N=247) | Placebo with Carboplatin and Gemcitabine (N=233) |
|---|---|---|
|
||
| General | ||
| Fatigue | 82% | 75% |
| Mucosal inflammation | 15% | 10% |
| Gastrointestinal | ||
| Nausea | 72% | 66% |
| Diarrhea | 38% | 29% |
| Stomatitis | 15% | 7% |
| Hemorrhoids | 8% | 3% |
| Gingival bleeding | 7% | 0% |
| Hematology | ||
| Thrombocytopenia | 58% | 51% |
| Respiratory, thoracic and mediastinal | ||
| Epistaxis | 55% | 14% |
| Dyspnea | 30% | 24% |
| Cough | 26% | 18% |
| Oropharyngeal pain | 16% | 10% |
| Dysphonia | 13% | 3% |
| Rhinorrhea | 10% | 4% |
| Sinus congestion | 8% | 2% |
| Nervous system | ||
| Headache | 49% | 30% |
| Dizziness | 23% | 17% |
| Vascular | ||
| Hypertension | 42% | 9% |
| Musculoskeletal and connective tissue | ||
| Arthralgia | 28% | 19% |
| Back pain | 21% | 13% |
| Psychiatric | ||
| Insomnia | 21% | 15% |
| Renal and urinary | ||
| Proteinuria | 20% | 3% |
| Injury and procedural | ||
| Contusion | 17% | 9% |
| Infections | ||
| Sinusitis | 15% | 9% |
Study GOG-0213
The safety of Avastin was evaluated in an open-label, controlled study (GOG-0213) in 325 patients with platinum-sensitive recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer, who have not received more than one previous regimen of chemotherapy[see Clinical Studies (14.9)]. Patients were randomized (1:1) to receive carboplatin and paclitaxel for 6 to 8 cycles or Avastin (15 mg/kg every 3 weeks) with carboplatin and paclitaxel for 6 to 8 cycles followed by Avastin as a single agent until disease progression or unacceptable toxicity. The demographics of the safety population were similar to the demographics of the efficacy population.
Grades 3-4 adverse reactions occurring at a higher incidence (≥ 2%) in patients receiving Avastin with chemotherapy compared to chemotherapy alone were: hypertension (11% vs. 0.6%), fatigue (8% vs. 3%), febrile neutropenia (6% vs. 3%), proteinuria (8% vs. 0%), abdominal pain (6% vs. 0.9%), hyponatremia (4% vs. 0.9%), headache (3% vs. 0.9%), and pain in extremity (3% vs. 0%).
Adverse reactions are presented in Table 8.
| Adverse Reaction* | Avastin with Carboplatin and Paclitaxel (N=325) | Carboplatin and Paclitaxel (N=332) |
|---|---|---|
|
||
| Musculoskeletal and connective tissue | ||
| Arthralgia | 45% | 30% |
| Myalgia | 29% | 18% |
| Pain in extremity | 25% | 14% |
| Back pain | 17% | 10% |
| Muscular weakness | 13% | 8% |
| Neck pain | 9% | 0% |
| Vascular | ||
| Hypertension | 42% | 3% |
| Gastrointestinal | ||
| Diarrhea | 39% | 32% |
| Abdominal pain | 33% | 28% |
| Vomiting | 33% | 25% |
| Stomatitis | 33% | 16% |
| Nervous system | ||
| Headache | 38% | 20% |
| Dysarthria | 14% | 2% |
| Dizziness | 13% | 8% |
| Metabolism and nutrition | ||
| Decreased appetite | 35% | 25% |
| Hyperglycemia | 31% | 24% |
| Hypomagnesemia | 27% | 17% |
| Hyponatremia | 17% | 6% |
| Weight loss | 15% | 4% |
| Hypocalcemia | 12% | 5% |
| Hypoalbuminemia | 11% | 6% |
| Hyperkalemia | 9% | 3% |
| Respiratory, thoracic and mediastinal | ||
| Epistaxis | 33% | 2% |
| Dyspnea | 30% | 25% |
| Cough | 30% | 17% |
| Rhinitis allergic | 17% | 4% |
| Nasal mucosal disorder | 14% | 3% |
| Skin and subcutaneous tissue | ||
| Exfoliative rash | 23% | 16% |
| Nail disorder | 10% | 2% |
| Dry skin | 7% | 2% |
| Renal and urinary | ||
| Proteinuria | 17% | 1% |
| Increased blood creatinine | 13% | 5% |
| Hepatic | ||
| Increased aspartate aminotransferase | 15% | 9% |
| General | ||
| Chest pain | 8% | 2% |
| Infections | ||
| Sinusitis | 7% | 2% |
Hepatocellular Carcinoma (HCC)
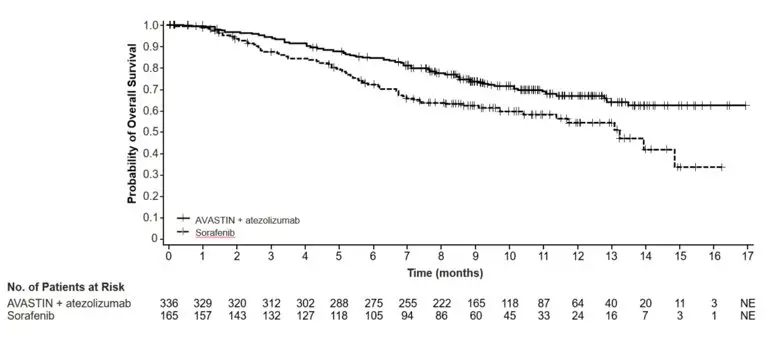
The safety of Avastin in combination with atezolizumab was evaluated in IMbrave150, a multicenter, international, randomized, open-label trial in patients with locally advanced or metastatic or unresectable hepatocellular carcinoma who have not received prior systemic treatment [see Clinical Studies (14.10)]. Patients received 1,200 mg of atezolizumab intravenously followed by 15 mg/kg Avastin (n=329) every 3 weeks, or 400 mg of sorafenib (n=156) given orally twice daily, until disease progression or unacceptable toxicity. The median duration of exposure to Avastin was 6.9 months (range: 0-16 months) and to atezolizumab was 7.4 months (range: 0-16 months).
Fatal adverse reactions occurred in 4.6% of patients in the Avastin and atezolizumab arm. The most common adverse reactions leading to death were gastrointestinal and esophageal varices hemorrhage (1.2%) and infections (1.2%).
Serious adverse reactions occurred in 38% of patients in the Avastin and atezolizumab arm. The most frequent serious adverse reactions (≥ 2%) were gastrointestinal hemorrhage (7%), infections (6%), and pyrexia (2.1%).
Adverse reactions leading to discontinuation of Avastin occurred in 15% of patients in the Avastin and atezolizumab arm. The most common adverse reactions leading to Avastin discontinuation were hemorrhages (4.9%), including bleeding varicose vein, hemorrhage and gastrointestinal, subarachnoid, and pulmonary hemorrhages; and increased transaminases or bilirubin (0.9%).
Adverse reactions leading to interruption of Avastin occurred in 46% of patients in the Avastin and atezolizumab arm; the most common (≥ 2%) were proteinuria (6%); infections (6%); hypertension (6%); liver function laboratory abnormalities including increased transaminases, bilirubin, or alkaline phosphatase (4.6%); gastrointestinal hemorrhages (3%); thrombocytopenia/decreased platelet count (4.3%); and pyrexia (2.4%).
Tables 9 and 10 summarize adverse reactions and laboratory abnormalities, respectively, in patients who received Avastin and atezolizumab in IMbrave150.
| Adverse Reaction | Avastin in combination with atezolizumab (n = 329) | Sorafenib (n=156) |
||
|---|---|---|---|---|
| All Grades*
(%) | Grades 3–4*
(%) | All Grades*
(%) | Grades 3–4*
(%) |
|
| 2 Graded per NCI CTCAE v4.0 | ||||
|
||||
| Vascular Disorders | ||||
| Hypertension | 30 | 15 | 24 | 12 |
| General Disorders and Administration Site Conditions | ||||
| Fatigue/asthenia* | 26 | 2 | 32 | 6 |
| Pyrexia | 18 | 0 | 10 | 0 |
| Renal and Urinary Disorders | ||||
| Proteinuria | 20 | 3 | 7 | 0.6 |
| Investigations | ||||
| Weight Decreased | 11 | 0 | 10 | 0 |
| Skin and Subcutaneous Tissue Disorders | ||||
| Pruritus | 19 | 0 | 10 | 0 |
| Rash | 12 | 0 | 17 | 2.6 |
| Gastrointestinal Disorders | ||||
| Diarrhea | 19 | 1.8 | 49 | 5 |
| Constipation | 13 | 0 | 14 | 0 |
| Abdominal Pain | 12 | 0 | 17 | 0 |
| Nausea | 12 | 0 | 16 | 0 |
| Vomiting | 10 | 0 | 8 | 0 |
| Metabolism and Nutrition Disorders | ||||
| Decreased Appetite | 18 | 1.2 | 24 | 3.8 |
| Respiratory, Thoracic and Mediastinal Disorders | ||||
| Cough | 12 | 0 | 10 | 0 |
| Epistaxis | 10 | 0 | 4.5 | 0 |
| Injury, Poisoning and Procedural Complications | ||||
| Infusion Related Reaction | 11 | 2.4 | 0 | 0 |
| Laboratory Abnormality | Avastin in combination with atezolizumab (n=329) | Sorafenib (n=156) |
||
|---|---|---|---|---|
| All Grades*
(%) | Grades 3–4*
(%) | All Grades*
(%) | Grades 3–4*
(%) |
|
| Each test incidence is based on the number of patients who had both baseline and at least one on-study laboratory measurement available: Avastin plus atezolizumab (222-323) and sorafenib (90-153) NA = Not applicable. | ||||
|
||||
| Chemistry | ||||
| Increased AST | 86 | 16 | 90 | 16 |
| Increased Alkaline Phosphatase | 70 | 4 | 76 | 4.6 |
| Increased ALT | 62 | 8 | 70 | 4.6 |
| Decreased Albumin | 60 | 1.5 | 54 | 0.7 |
| Decreased Sodium | 54 | 13 | 49 | 9 |
| Increased Glucose | 48 | 9 | 43 | 4.6 |
| Decreased Calcium | 30 | 0.3 | 35 | 1.3 |
| Decreased Phosphorus | 26 | 4.7 | 58 | 16 |
| Increased Potassium | 23 | 1.9 | 16 | 2 |
| Hypomagnesemia | 22 | 0 | 22 | 0 |
| Hematology | ||||
| Decreased Platelet | 68 | 7 | 63 | 4.6 |
| Decreased Lymphocytes | 62 | 13 | 58 | 11 |
| Decreased Hemoglobin | 58 | 3.1 | 62 | 3.9 |
| Increased Bilirubin | 57 | 8 | 59 | 14 |
| Decreased Leukocyte | 32 | 3.4 | 29 | 1.3 |
| Decreased Neutrophil | 23 | 2.3 | 16 | 1.1 |
6.2 Immunogenicity
As with all therapeutic proteins, there is a potential for immunogenicity. The detection of antibody formation is highly dependent on the sensitivity and the specificity of the assay. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors, including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies to bevacizumab in the studies described below with the incidence of antibodies in other studies or to other bevacizumab products may be misleading.
In clinical studies for adjuvant treatment of a solid tumor, 0.6% (14/2233) of patients tested positive for treatment-emergent anti-bevacizumab antibodies as detected by an electrochemiluminescent (ECL) based assay. Among these 14 patients, three tested positive for neutralizing antibodies against bevacizumab using an enzyme-linked immunosorbent assay (ELISA). The clinical significance of these anti-bevacizumab antibodies is not known.
6.3 Postmarketing Experience
The following adverse reactions have been identified during postapproval use of Avastin. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
General: Polyserositis
Cardiovascular: Pulmonary hypertension, Mesenteric venous occlusion
Gastrointestinal: Gastrointestinal ulcer, Intestinal necrosis, Anastomotic ulceration
Hemic and lymphatic: Pancytopenia
Hepatobiliary disorders: Gallbladder perforation
Musculoskeletal and Connective Tissue Disorders: Osteonecrosis of the jaw
Renal: Renal thrombotic microangiopathy (manifested as severe proteinuria)
Respiratory: Nasal septum perforation
Vascular: Arterial (including aortic) aneurysms, dissections, and rupture
8. Use In Specific Populations
8.4 Pediatric Use
The safety and effectiveness of Avastin in pediatric patients have not been established.
In published literature reports, cases of non-mandibular osteonecrosis have been observed in patients under the age of 18 years who received Avastin. Avastin is not approved for use in patients under the age of 18 years.
Antitumor activity was not observed among eight pediatric patients with relapsed GBM who received bevacizumab and irinotecan. Addition of Avastin to standard of care did not result in improved event-free survival in pediatric patients enrolled in two randomized clinical studies, one in high grade glioma (n= 121) and one in metastatic rhabdomyosarcoma or non-rhabdomyosarcoma soft tissue sarcoma (n= 154).
Based on the population pharmacokinetics analysis of data from 152 pediatric and young adult patients with cancer (7 months to 21 years of age), bevacizumab clearance normalized by body weight in pediatrics was comparable to that in adults.
8.5 Geriatric Use
In an exploratory pooled analysis of 1745 patients from five randomized, controlled studies, 35% of patients were ≥ 65 years old. The overall incidence of ATE was increased in all patients receiving Avastin with chemotherapy as compared to those receiving chemotherapy alone, regardless of age; however, the increase in the incidence of ATE was greater in patients ≥ 65 years (8% vs. 3%) as compared to patients < 65 years (2% vs. 1%) [see Warnings and Precautions (5.4)].
11. Avastin Description
Bevacizumab is a vascular endothelial growth factor inhibitor. Bevacizumab is a recombinant humanized monoclonal IgG1 antibody that contains human framework regions and murine complementarity-determining regions. Bevacizumab has an approximate molecular weight of 149 kDa. Bevacizumab is produced in a mammalian cell (Chinese Hamster Ovary) expression system.
Avastin (bevacizumab) injection is a sterile, preservative-free, clear to slightly opalescent, colorless to pale brown solution in a single-dose vial for intravenous use. Avastin contains bevacizumab at a concentration of 25 mg/mL in either a 100 mg/4 mL or 400 mg/16 mL single-dose vial.
Each mL of solution contains 25 mg bevacizumab, α,α-trehalose dihydrate (60 mg), polysorbate 20 (0.4 mg), sodium phosphate dibasic, anhydrous (1.2 mg), sodium phosphate monobasic, monohydrate (5.8 mg), and Water for Injection, USP. The pH is 6.2.
12. Avastin - Clinical Pharmacology
12.1 Mechanism of Action
Bevacizumab binds VEGF and prevents the interaction of VEGF to its receptors (Flt-1 and KDR) on the surface of endothelial cells. The interaction of VEGF with its receptors leads to endothelial cell proliferation and new blood vessel formation in in vitro models of angiogenesis. Administration of bevacizumab to xenotransplant models of colon cancer in nude (athymic) mice caused reduction of microvascular growth and inhibition of metastatic disease progression.
12.3 Pharmacokinetics
The pharmacokinetic profile of bevacizumab was assessed using an assay that measures total serum bevacizumab concentrations (i.e., the assay did not distinguish between free bevacizumab and bevacizumab bound to VEGF ligand). Based on a population pharmacokinetic analysis of 491 patients who received 1 to 20 mg/kg of Avastin every week, every 2 weeks, or every 3 weeks, bevacizumab pharmacokinetics are linear and the predicted time to reach more than 90% of steady state concentration is 84 days. The accumulation ratio following a dose of 10 mg/kg once every 2 weeks is 2.8.
Population simulations of bevacizumab exposures provide a median trough concentration of 80.3 mcg/mL on Day 84 (10th, 90th percentile: 45, 128) following a dose of 5 mg/kg once every two weeks.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
No studies have been conducted to assess potential of bevacizumab for carcinogenicity or mutagenicity.
Bevacizumab may impair fertility. Female cynomolgus monkeys treated with 0.4 to 20 times the recommended human dose of bevacizumab exhibited arrested follicular development or absent corpora lutea, as well as dose-related decreases in ovarian and uterine weights, endometrial proliferation, and the number of menstrual cycles. Following a 4- or 12-week recovery period, there was a trend suggestive of reversibility. After the 12-week recovery period, follicular maturation arrest was no longer observed, but ovarian weights were still moderately decreased. Reduced endometrial proliferation was no longer observed at the 12-week recovery time point; however, decreased uterine weight, absent corpora lutea, and reduced number of menstrual cycles remained evident.
13.2 Animal Toxicology and/or Pharmacology
Rabbits dosed with bevacizumab exhibited reduced wound healing capacity. Using full-thickness skin incision and partial thickness circular dermal wound models, bevacizumab dosing resulted in reductions in wound tensile strength, decreased granulation and re-epithelialization, and delayed time to wound closure.
14. Clinical Studies
14.1 Metastatic Colorectal Cancer
Study AVF2107g
The safety and efficacy of Avastin was evaluated in a double-blind, active-controlled study [AVF2107g (NCT00109070)] in 923 patients with previously untreated mCRC who were randomized (1:1:1) to placebo with bolus-IFL (irinotecan 125 mg/m2, fluorouracil 500 mg/m2, and leucovorin 20 mg/m2 given once weekly for 4 weeks every 6 weeks), Avastin (5 mg/kg every 2 weeks) with bolus-IFL, or Avastin (5 mg/kg every 2 weeks) with fluorouracil and leucovorin. Enrollment to the Avastin with fluorouracil and leucovorin arm was discontinued after enrollment of 110 patients in accordance with the protocol-specified adaptive design. Avastin was continued until disease progression or unacceptable toxicity or for a maximum of 96 weeks. The main outcome measure was overall survival (OS).
The median age was 60 years; 60% were male, 79% were White, 57% had an ECOG performance status of 0, 21% had a rectal primary and 28% received prior adjuvant chemotherapy. The dominant site of disease was extra-abdominal in 56% of patients and was the liver in 38% of patients.
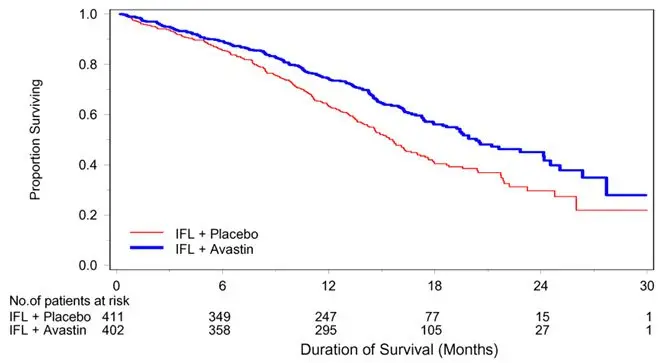
The addition of Avastin improved survival across subgroups defined by age (< 65 years, ≥ 65 years) and sex. Results are presented in Table 11 and Figure 1.
| Efficacy Parameter | Avastin with bolus-IFL (N=402) | Placebo with bolus-IFL (N=411) |
|---|---|---|
|
||
| Overall Survival | ||
| Median, in months | 20.3 | 15.6 |
| Hazard ratio (95% CI) | 0.66 (0.54, 0.81) |
|
| p-value* | < 0.001 | |
| Progression-Free Survival | ||
| Median, in months | 10.6 | 6.2 |
| Hazard ratio (95% CI) | 0.54 (0.45, 0.66) |
|
| p-value* | < 0.001 | |
| Overall Response Rate | ||
| Rate (%) | 45% | 35% |
| p-value† | < 0.01 | |
| Duration of Response | ||
| Median, in months | 10.4 | 7.1 |
| Figure 1: Kaplan-Meier Curves for Duration of Survival in Metastatic Colorectal Cancer in Study AVF2107g |
 |
Among the 110 patients randomized to Avastin with fluorouracil and leucovorin, median OS was 18.3 months, median progression-free survival (PFS) was 8.8 months, overall response rate (ORR) was 39%, and median duration of response was 8.5 months.
Study ML18147
The safety and efficacy of Avastin were evaluated in a prospective, randomized, open-label, multinational, controlled study [ML18147 (NCT00700102)] in 820 patients with histologically confirmed mCRC who had progressed on a first-line Avastin containing regimen. Patients were excluded if they progressed within 3 months of initiating first-line chemotherapy and if they received Avastin for less than 3 consecutive months in the first-line setting. Patients were randomized (1:1) within 3 months after discontinuing Avastin as first-line treatment to receive fluoropyrimidine-irinotecan- or fluoropyrimidine-oxaliplatin-based chemotherapy with or without Avastin (5 mg/kg every 2 weeks or 7.5 mg/kg every 3 weeks). The choice of second-line treatment was contingent upon first-line chemotherapy. Second-line treatment was administered until progressive disease or unacceptable toxicity. The main outcome measure was OS. A secondary outcome measure was ORR.
The median age was 63 years (21 to 84 years); 64% were male, 52% had an ECOG performance status of 1, 44% had an ECOG performance status of 0, 58% received irinotecan-based therapy as first-line treatment, 55% progressed on first-line treatment within 9 months, and 77% received their last dose of Avastin as first-line treatment within 42 days of being randomized. Second-line chemotherapy regimens were generally balanced between each arm.
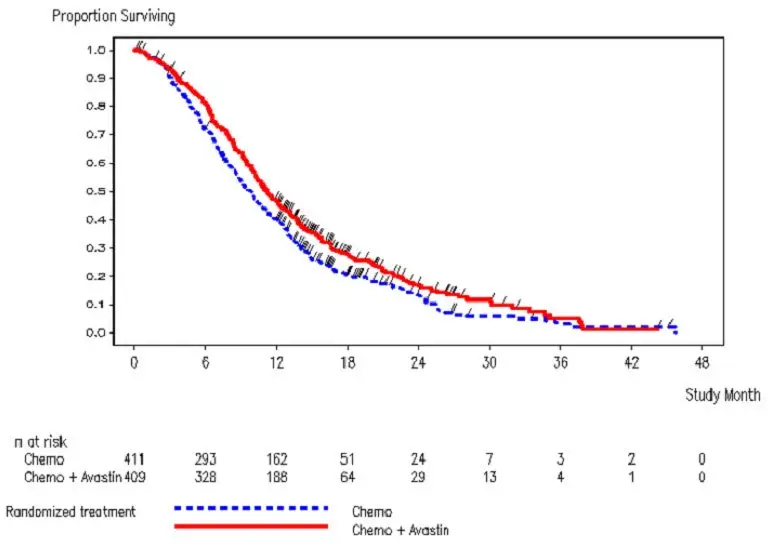
The addition of Avastin to fluoropyrimidine-based chemotherapy resulted in a statistically significant prolongation of OS and PFS. There was no significant difference in ORR. Results are presented in Table 12 and Figure 2.
| Efficacy Parameter | Avastin with Chemotherapy (N=409) | Chemotherapy (N=411) |
|---|---|---|
|
||
| Overall Survival* | ||
| Median, in months | 11.2 | 9.8 |
| Hazard ratio (95% CI) | 0.81 (0.69, 0.94) | |
| Progression-Free Survival† | ||
| Median, in months | 5.7 | 4.0 |
| Hazard ratio (95% CI) | 0.68 (0.59, 0.78) | |
| Figure 2: Kaplan-Meier Curves for Duration of Survival in Metastatic Colorectal Cancer in Study ML18147 |
 |
14.2 Lack of Efficacy in Adjuvant Treatment of Colon Cancer
Lack of efficacy of Avastin as an adjunct to standard chemotherapy for the adjuvant treatment of colon cancer was determined in two randomized, open-label, multicenter clinical studies.
The first study [BO17920 (NCT00112918)] was conducted in 3451 patients with high-risk stage II and III colon cancer, who had undergone surgery for colon cancer with curative intent. Patients were randomized to receive Avastin at a dose equivalent to 2.5 mg/kg/week on either a 2-weekly schedule with FOLFOX4 (N=1155) or on a 3-weekly schedule with XELOX (N=1145) or FOLFOX4 alone (N=1151). The main outcome measure was disease free survival (DFS) in patients with stage III colon cancer.
The median age was 58 years; 54% were male, 84% were White and 29% were ≥ 65 years. Eighty-three percent had stage III disease.
The addition of Avastin to chemotherapy did not improve DFS. As compared to FOLFOX4 alone, the proportion of stage III patients with disease recurrence or with death due to disease progression were numerically higher for patients receiving Avastin with FOLFOX4 or with XELOX. The hazard ratios for DFS were 1.17 (95% CI: 0.98,1.39) for Avastin with FOLFOX4 versus FOLFOX4 alone and 1.07 (95% CI: 0.90, 1.28) for Avastin with XELOX versus FOLFOX4 alone. The hazard ratios for OS were 1.31 (95% CI: 1.03, 1.67) and 1.27 (95% CI: 1, 1.62) for the comparison of Avastin with FOLFOX4 versus FOLFOX4 alone and Avastin with XELOX versus FOLFOX4 alone, respectively. Similar lack of efficacy for DFS was observed in the Avastin-containing arms compared to FOLFOX4 alone in the high-risk stage II cohort.
In a second study [NSABP-C-08 (NCT00096278)], patients with stage II and III colon cancer who had undergone surgery with curative intent, were randomized to receive either Avastin administered at a dose equivalent to 2.5 mg/kg/week with mFOLFOX6 (N=1354) or mFOLFOX6 alone (N=1356). The median age was 57 years, 50% were male and 87% White. Seventy-five percent had stage III disease. The main outcome was DFS among stage III patients. The HR for DFS was 0.92 (95% CI: 0.77, 1.10). OS was not significantly improved with the addition of Avastin to mFOLFOX6 [HR 0.96 (95% CI: 0.75,1.22)].
14.3 First-Line Non–Squamous Non–Small Cell Lung Cancer
Study E4599
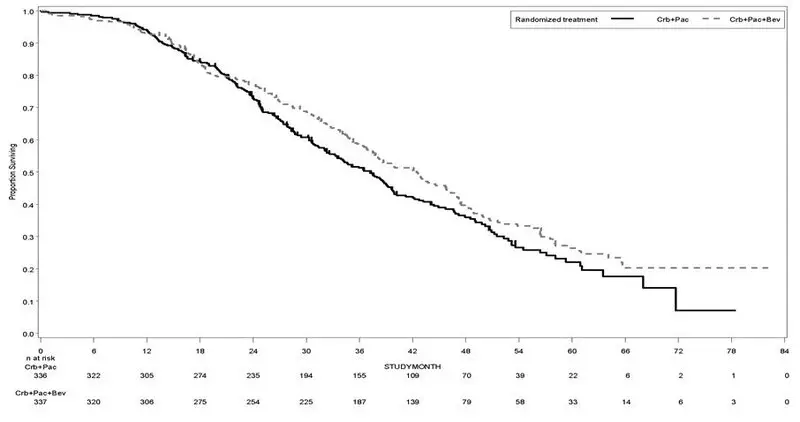
The safety and efficacy of Avastin as first-line treatment of patients with locally advanced, metastatic, or recurrent non–squamous NSCLC was studied in a single, large, randomized, active-controlled, open-label, multicenter study [E4599 (NCT00021060)]. A total of 878 chemotherapy-naïve patients with locally advanced, metastatic or recurrent non–squamous NSCLC were randomized (1:1) to receive six 21-day cycles of paclitaxel (200 mg/m2) and carboplatin (AUC 6) with or without Avastin 15 mg/kg. After completing or discontinuing chemotherapy, patients randomized to receive Avastin continued to receive Avastin alone until disease progression or until unacceptable toxicity. The trial excluded patients with predominant squamous histology (mixed cell type tumors only), CNS metastasis, gross hemoptysis (1/2 teaspoon or more of red blood), unstable angina, or receiving therapeutic anticoagulation. The main outcome measure was duration of survival.
The median age was 63 years; 54% were male, 43% were ≥ 65 years, and 28% had ≥ 5% weight loss at study entry. Eleven percent had recurrent disease. Of the 89% with newly diagnosed NSCLC, 12% had Stage IIIB with malignant pleural effusion and 76% had Stage IV disease.
OS was statistically significantly longer for patients receiving Avastin with paclitaxel and carboplatin compared with those receiving chemotherapy alone. Median OS was 12.3 months vs. 10.3 months [HR 0.80 (95% CI: 0.68, 0.94), final p-value of 0.013, stratified log-rank test]. Based on investigator assessment which was not independently verified, patients were reported to have longer PFS with Avastin with paclitaxel and carboplatin compared to chemotherapy alone. Results are presented in Figure 3.
| Figure 3: Kaplan-Meier Curves for Duration of Survival in First-Line Non-Squamous Non-Small Cell Lung Cancer in Study E4599 |
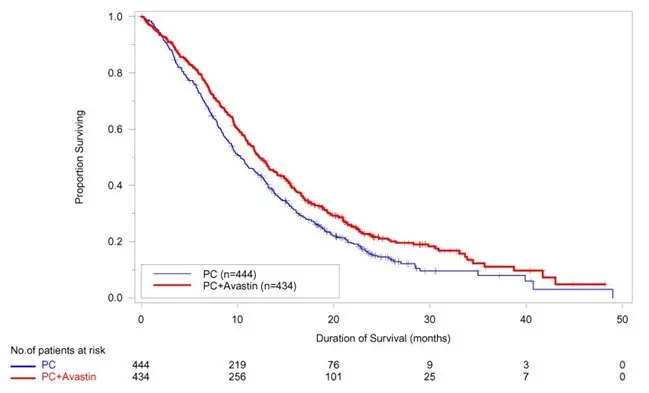 |
In an exploratory analysis across patient subgroups, the impact of Avastin on OS was less robust in the following subgroups: women [HR 0.99 (95% CI: 0.79, 1.25)], patients ≥ 65 years [HR 0.91 (95% CI: 0.72, 1.14)] and patients with ≥ 5% weight loss at study entry [HR 0.96 (95% CI: 0.73, 1.26)].
14.5 Metastatic Renal Cell Carcinoma
Study BO17705
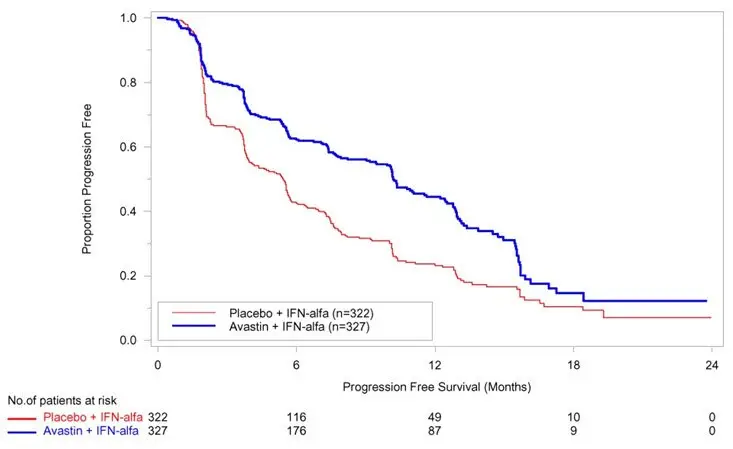
The safety and efficacy of Avastin were evaluated in patients with treatment-naïve mRCC in a multicenter, randomized, double-blind, international study [BO17705 (NCT00738530)] comparing interferon alfa and Avastin versus interferon alfa and placebo. A total of 649 patients who had undergone a nephrectomy were randomized (1:1) to receive either Avastin (10 mg/kg every 2 weeks; N = 327) or placebo (every 2 weeks; N = 322) with interferon alfa (9 MIU subcutaneously three times weekly for a maximum of 52 weeks). Patients were treated until disease progression or unacceptable toxicity. The main outcome measure was investigator-assessed PFS. Secondary outcome measures were ORR and OS.
The median age was 60 years (18 to 82 years); 70% were male and 96% were White. The study population was characterized by Motzer scores as follows: 28% favorable (0), 56% intermediate (1-2), 8% poor (3–5), and 7% missing.
PFS was statistically significantly prolonged among patients receiving Avastin compared to placebo; median PFS was 10.2 months vs. 5.4 months [HR 0.60 (95% CI: 0.49, 0.72), p-value < 0.0001, stratified log-rank test]. Among the 595 patients with measurable disease, ORR was also significantly higher (30% vs. 12%, p-value < 0.0001, stratified CMH test). There was no improvement in OS based on the final analysis conducted after 444 deaths, with a median OS of 23 months in the patients receiving Avastin with interferon alfa and 21 months in patients receiving interferon alone [HR 0.86, (95% CI: 0.72, 1.04)]. Results are presented in Figure 4.
| Figure 4: Kaplan-Meier Curves for Progression-Free Survival in Metastatic Renal Cell Carcinoma in Study BO17705 |
 |
14.6 Persistent, Recurrent, or Metastatic Cervical Cancer
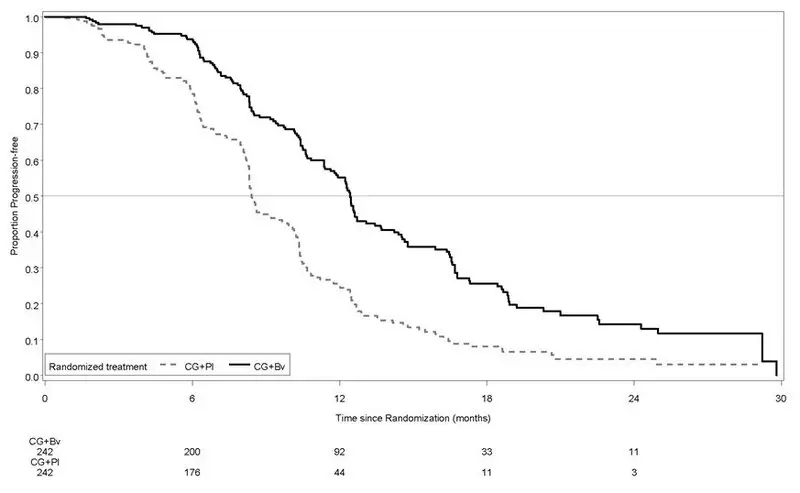
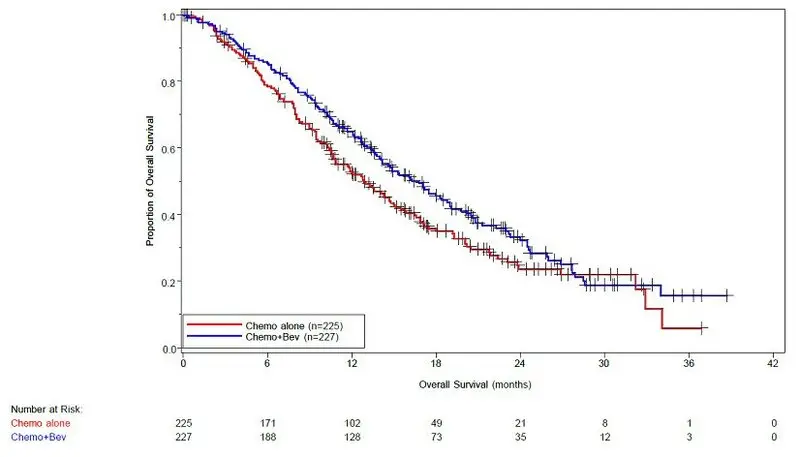
Study GOG-0240
The safety and efficacy of Avastin were evaluated in patients with persistent, recurrent, or metastatic cervical cancer in a randomized, four-arm, multicenter study comparing Avastin with chemotherapy versus chemotherapy alone [GOG-0240 (NCT00803062)]. A total of 452 patients were randomized (1:1:1:1) to receive paclitaxel and cisplatin with or without Avastin, or paclitaxel and topotecan with or without Avastin.
The dosing regimens for Avastin, paclitaxel, cisplatin and topotecan were as follows:
- Day 1: Paclitaxel 135 mg/m2 over 24 hours, Day 2: cisplatin 50 mg/m2 with Avastin;
- Day 1: Paclitaxel 175 mg/m2 over 3 hours, Day 2: cisplatin 50 mg/m2 with Avastin;
- Day 1: Paclitaxel 175 mg/m2 over 3 hours with cisplatin 50 mg/m2 with Avastin;
- Day 1: Paclitaxel 175 mg/m2 over 3 hours with Avastin, Days 1-3: topotecan IV 0.75 mg/m2 over 30 minutes
Patients were treated until disease progression or unacceptable adverse reactions. The main outcome measure was OS. Secondary outcome measures included ORR.
The median age was 48 years (20 to 85 years). Of the 452 patients randomized at baseline, 78% of patients were White, 80% had received prior radiation, 74% had received prior chemotherapy concurrent with radiation, and 32% had a platinum-free interval (PFI) of less than 6 months. Patients had a GOG performance status of 0 (58%) or 1 (42%). Demographic and disease characteristics were balanced across arms.
Results are presented in Figure 5 and Table 13.
| Figure 5: Kaplan-Meier Curves for Overall Survival in Persistent, Recurrent, or Metastatic Cervical Cancer in Study GOG-0240 |
 |
| Efficacy Parameter | Avastin with Chemotherapy (N=227) | Chemotherapy (N=225) |
|---|---|---|
|
||
| Overall Survival | ||
| Median, in months* | 16.8 | 12.9 |
| Hazard ratio (95% CI) | 0.74 (0.58, 0.94) | |
| p-value† | 0.0132 | |
The ORR was higher in patients who received Avastin with chemotherapy [45% (95% CI: 39, 52)] compared to patients who received chemotherapy alone [34% (95% CI: 28,40)].
| Efficacy Parameter | Topotecan and Paclitaxel with or without Avastin (N=223) | Cisplatin and Paclitaxel with or without Avastin (N=229) |
|---|---|---|
|
||
| Overall Survival | ||
| Median, in months* | 13.3 | 15.5 |
| Hazard ratio (95% CI) | 1.15 (0.91, 1.46) | |
| p-value | 0.23 | |
The HR for OS with Avastin with cisplatin and paclitaxel as compared to cisplatin and paclitaxel alone was 0.72 (95% CI: 0.51,1.02). The HR for OS with Avastin with topotecan and paclitaxel as compared to topotecan and paclitaxel alone was 0.76 (95% CI: 0.55, 1.06).
14.7 Stage III or IV Epithelial Ovarian, Fallopian Tube, or Primary Peritoneal Cancer Following Initial Surgical Resection
Study GOG-0218
The safety and efficacy of Avastin were evaluated in a multicenter, randomized, double-blind, placebo controlled, three arm study [Study GOG-0218 (NCT00262847)] evaluating the effect of adding Avastin to carboplatin and paclitaxel for the treatment of patients with stage III or IV epithelial ovarian, fallopian tube or primary peritoneal cancer (N=1873) following initial surgical resection. Patients were randomized (1:1:1) to one of the following arms:
- CPP: carboplatin (AUC 6) and paclitaxel (175 mg/m2) for six cycles, with concurrent placebo started at cycle 2, followed by placebo alone every three weeks for a total of up to 22 cycles of therapy (n=625) or
- CPB15: carboplatin (AUC 6) and paclitaxel (175 mg/m2) for six cycles, with concurrent Avastin started at cycle 2, followed by placebo alone every three weeks for a total of up to 22 cycles of therapy (n=625) or
- CPB15+: carboplatin (AUC 6) and paclitaxel (175 mg/m2) for six cycles, with concurrent Avastin started at cycle 2, followed by Avastin as a single agent every three weeks for a total of up to 22 cycles of therapy (n=623).
The main outcome measure was investigator-assessed PFS. OS was a secondary outcome measure.
The median age was 60 years (range 22-89 years) and 28% of patients were >65 years of age.
Overall, approximately 50% of patients had a GOG PS of 0 at baseline, and 43% a GOG PS score of 1. Patients had either epithelial ovarian cancer (83%), primary peritoneal cancer (15%), or fallopian tube cancer (2%). Serous adenocarcinoma was the most common histologic type (85% in CPP and CPB15 arms, 86% in CPB15+ arm). Overall, approximately 34% of patients had resected FIGO Stage III with residual disease < 1 cm, 40% had resected Stage III with residual disease >1 cm, and 26% had resected Stage IV disease.
The majority of patients in all three treatment arms received subsequent antineoplastic treatment, 78.1% in the CPP arm, 78.6% in the CPB15 arm, and 73.2% in the CPB15+ arm. A higher proportion of patients in the CPP arm (25.3%) and CPB15 arm (26.6%) received at least one anti-angiogenic (including bevacizumab) treatment after discontinuing from study compared with the CPB15+ arm (15.6%).
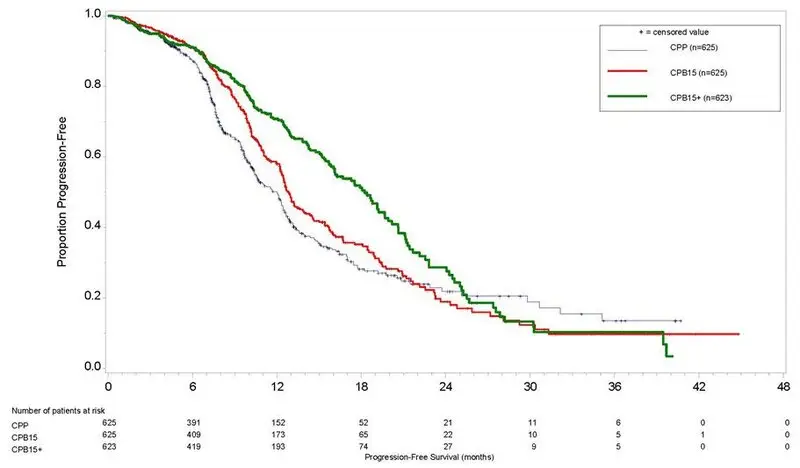
Study results are presented in Table 15 and Figure 6.
| Efficacy Parameter | Avastin with carboplatin and paclitaxel followed by Avastin alone (N=623) | Avastin with carboplatin and paclitaxel (N=625) | Carboplatin and paclitaxel (N= 625) |
|---|---|---|---|
| NS=not significant | |||
|
|||
| Progression-Free Survival per Investigator | |||
| Median, in months | 18.2 | 12.8 | 12.0 |
| Hazard ratio (95% CI)* | 0.62 (0.52, 0.75) | 0.83 (0.70, 0.98) | |
| p –value† | < 0.0001 | NS | |
| Overall Survival‡ | |||
| Median, in months | 43.8 | 38.8 | 40.6 |
| Hazard ratio (95% CI)* | 0.89 (0.76, 1.05) | 1.06 (0.90, 1.24) | |
| Figure 6: Kaplan-Meier Curves for Investigator-Assessed Progression-Free Survival in Stage III or IV Epithelial Ovarian, Fallopian Tube, or Primary Peritoneal Cancer Following Initial Surgical Resection in Study GOG-0218 |
|
|
14.8 Platinum-Resistant Recurrent Epithelial Ovarian, Fallopian Tube, or Primary Peritoneal Cancer
Study MO22224
The safety and efficacy of Avastin were evaluated in a multicenter, open-label, randomized study [MO22224 (NCT00976911)] comparing Avastin with chemotherapy versus chemotherapy alone in patients with platinum-resistant, recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer that recurred within <6 months from the most recent platinum-based therapy (N=361). Patients had received no more than 2 prior chemotherapy regimens. Patients received one of the following chemotherapy regimens at the discretion of the investigator: paclitaxel (80 mg/m2 on days 1, 8, 15 and 22 every 4 weeks; pegylated liposomal doxorubicin 40 mg/m2 on day 1 every 4 weeks; or topotecan 4 mg/m2 on days 1, 8 and 15 every 4 weeks or 1.25 mg/m2 on days 1-5 every 3 weeks). Patients were treated until disease progression, unacceptable toxicity, or withdrawal. Forty percent of patients on the chemotherapy alone arm received Avastin alone upon progression. The main outcome measure was investigator-assessed PFS. Secondary outcome measures were ORR and OS.
The median age was 61 years (25 to 84 years) and 37% of patients were ≥65 years. Seventy-nine percent had measurable disease at baseline, 87% had baseline CA-125 levels ≥2 times ULN and 31% had ascites at baseline. Seventy-three percent had a PFI of 3 months to 6 months and 27% had PFI of <3 months. ECOG performance status was 0 for 59%, 1 for 34% and 2 for 7% of the patients.
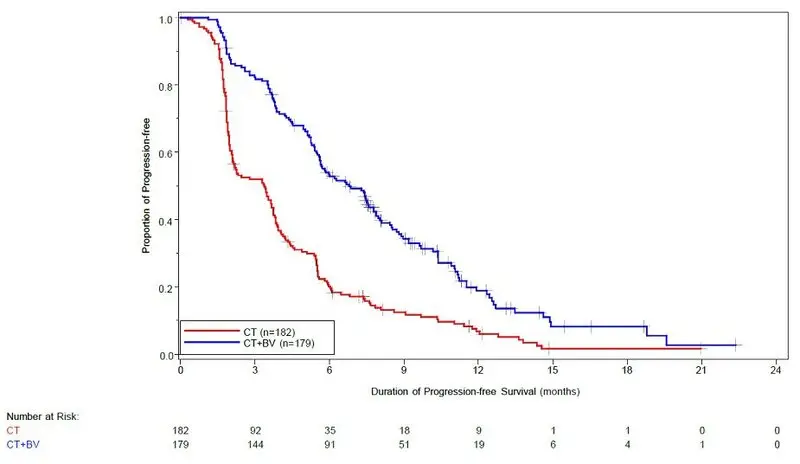
The addition of Avastin to chemotherapy demonstrated a statistically significant improvement in investigator-assessed PFS, which was supported by a retrospective independent review analysis. Results for the ITT population are presented in Table 16 and Figure 7. Results for the separate chemotherapy cohorts are presented in Table 17.
| Efficacy Parameter | Avastin with Chemotherapy (N=179) | Chemotherapy (N=182) |
|---|---|---|
|
||
| Progression-Free Survival per Investigator | ||
| Median (95% CI), in months | 6.8 (5.6, 7.8) | 3.4 (2.1, 3.8) |
| HR (95% CI)* | 0.38 (0.30, 0.49) | |
| p-value † | <0.0001 | |
| Overall Survival | ||
| Median (95% CI), in months | 16.6 (13.7, 19.0) | 13.3 (11.9, 16.4) |
| HR (95% CI)* | 0.89 (0.69, 1.14) | |
| Overall Response Rate | ||
| Number of Patients with Measurable Disease at Baseline | 142 | 144 |
| Rate, % (95% CI) | 28% (21%, 36%) | 13% (7%, 18%) |
| Duration of Response | ||
| Median, in months | 9.4 | 5.4 |
| Figure 7: Kaplan-Meier Curves for Investigator-Assessed Progression-Free Survival in Platinum-Resistant Recurrent Epithelial Ovarian, Fallopian Tube, or Primary Peritoneal Cancer in Study MO22224 |
|
|
| Efficacy Parameter | Paclitaxel | Topotecan | Pegylated Liposomal Doxorubicin | |||
|---|---|---|---|---|---|---|
| Avastin with Chemotherapy | Chemotherapy | Avastin with Chemotherapy | Chemotherapy | Avastin with Chemotherapy | Chemotherapy | |
| (N=60) | (N=55) | (N=57) | (N=63) | (N=62) | (N=64) | |
| NE=Not Estimable | ||||||
|
||||||
| Progression-Free Survival per Investigator | ||||||
| Median, in months (95% CI) | 9.6 (7.8, 11.5) | 3.9 (3.5, 5.5) | 6.2 (5.3, 7.6) | 2.1 (1.9, 2.3) | 5.1 (3.9, 6.3) | 3.5 (1.9, 3.9) |
| Hazard ratio*
(95% CI) | 0.47 (0.31, 0.72) | 0.24 (0.15, 0.38) | 0.47 (0.32, 0.71) |
|||
| Overall Survival | ||||||
| Median, in months (95% CI) | 22.4 (16.7, 26.7) | 13.2 (8.2, 19.7) | 13.8 (11.0, 18.3) | 13.3 (10.4, 18.3) | 13.7 (11.0, 18.3) | 14.1 (9.9, 17.8) |
| Hazard ratio *
(95% CI) | 0.64 (0.41, 1.01) | 1.12 (0.73, 1.73) | 0.94 (0.63, 1.42) |
|||
| Overall Response Rate | ||||||
| Number of patients with measurable disease at baseline | 45 | 43 | 46 | 50 | 51 | 51 |
| Rate, % (95% CI) | 53 (39, 68) | 30 (17, 44) | 17 (6, 28) | 2 (0, 6) | 16 (6, 26) | 8 (0, 15) |
| Duration of Response | ||||||
| Median, in months | 11.6 | 6.8 | 5.2 | NE | 8.0 | 4.6 |
14.9 Platinum-Sensitive Recurrent Epithelial Ovarian, Fallopian Tube, or Primary Peritoneal Cancer
Study AVF4095g
The safety and efficacy of Avastin were evaluated in a randomized, double-blind, placebo-controlled study [AVF4095g (NCT00434642)] studying Avastin with chemotherapy versus chemotherapy alone in the treatment of patients with platinum-sensitive recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer who have not received prior chemotherapy in the recurrent setting or prior bevacizumab treatment (N=484). Patients were randomized (1:1) to receive Avastin (15 mg/kg day 1) or placebo every 3 weeks with carboplatin (AUC 4, day 1) and gemcitabine (1000 mg/m2 on days 1 and 8) a for 6 to 10 cycles followed by Avastin or placebo alone until disease progression or unacceptable toxicity. The main outcome measures were investigator-assessed PFS. Secondary outcome measures were ORR and OS.
The median age was 61 years (28 to 87 years) and 37% of patients were ≥65 years. All patients had measurable disease at baseline, 74% had baseline CA-125 levels >ULN (35 U/mL). The platinum-free interval (PFI) was 6 months to 12 months in 42 % of patients and >12 months in 58% of patients. The ECOG performance status was 0 or 1 for 99.8% of patients.
A statistically significant prolongation in PFS was demonstrated among patients receiving Avastin with chemotherapy compared to those receiving placebo with chemotherapy (Table 18 and Figure 8). Independent radiology review of PFS was consistent with investigator assessment [HR 0.45 (95% CI: 0.35, 0.58)]. OS was not significantly improved with the addition of Avastin to chemotherapy [HR 0.95 (95% CI: 0.77, 1.17)].
| Efficacy Parameter | Avastin with Gemcitabine and Carboplatin (N=242) | Placebo with Gemcitabine and Carboplatin (N=242) |
|---|---|---|
| Progression-Free Survival | ||
| Median, in months | 12.4 | 8.4 |
| Hazard ratio (95% CI) | 0.46 (0.37, 0.58) |
|
| p-value | < 0.0001 | |
| Overall Response Rate | ||
| % patients with overall response | 78% | 57% |
| p-value | < 0.0001 | |
| Figure 8: Kaplan-Meier Curves for Progression-Free Survival in Platinum-Sensitive Recurrent Epithelial Ovarian, Fallopian Tube, or Primary Peritoneal Cancer in Study AVF4095g |
|
|
Study GOG-0213
The safety and efficacy of Avastin were evaluated in a randomized, controlled, open-label study [Study GOG-0213 (NCT00565851)] of Avastin with chemotherapy versus chemotherapy alone in the treatment of patients with platinum-sensitive recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer, who have not received more than one previous regimen of chemotherapy (N=673). Patients were randomized (1:1) to receive carboplatin (AUC 5) and paclitaxel (175 mg/m2 IV over 3 hours) every 3 weeks for 6 to 8 cycles (N=336) or Avastin (15 mg/kg) every 3 weeks with carboplatin (AUC 5) and paclitaxel (175 mg/m2 IV over 3 hours) for 6 to 8 cycles followed by Avastin (15 mg/kg every 3 weeks) as a single agent until disease progression or unacceptable toxicity. The main outcome measure was OS. Other outcome measures were investigator-assessed PFS, and ORR.
The median age was 60 years (23 to 85 years) and 33% of patients were ≥ 65 years. Eighty-three percent had measurable disease at baseline and 74% had abnormal CA-125 levels at baseline. Ten percent of patients had received prior bevacizumab. Twenty-six percent had a PFI of 6 months to 12 months and 74% had a PFI of >12 months. GOG performance status was 0 or 1 for 99% of patients.
Results are presented in Table 19 and Figure 9.
| Efficacy Parameter | Avastin with Carboplatin and Paclitaxel (N=337) | Carboplatin and Paclitaxel (N=336) |
|---|---|---|
|
||
| Overall Survival | ||
| Median, in months | 42.6 | 37.3 |
| Hazard ratio (95% CI) (IVRS)* | 0.84 (0.69, 1.01) | |
| Hazard ratio (95% CI) (eCRF)† | 0.82 (0.68, 0.996) | |
| Progression-Free Survival | ||
| Median, in months | 13.8 | 10.4 |
| Hazard ratio (95% CI) (IVRS)* | 0.61 (0.51, 0.72) | |
| Overall Response Rate | ||
| Number of patients with measurable disease at baseline | 274 | 286 |
| Rate, % | 213 (78%) | 159 (56%) |
| Figure 9: Kaplan Meier Curves for Overall Survival in Platinum-Sensitive Recurrent Epithelial Ovarian, Fallopian Tube, or Primary Peritoneal Cancer in Study GOG-0213 |
|
|
14.10 Hepatocellular Carcinoma
The efficacy of Avastin in combination with atezolizumab was investigated in IMbrave150 (NCT03434379), a multicenter, international, open-label, randomized trial in patients with locally advanced unresectable and/or metastatic hepatocellular carcinoma who have not received prior systemic therapy. Randomization was stratified by geographic region (Asia excluding Japan vs. rest of world), macrovascular invasion and/or extrahepatic spread (presence vs. absence), baseline AFP (<400 vs. ≥400 ng/mL), and by ECOG performance status (0 vs. 1).
A total of 501 patients were randomized (2:1) to receive either atezolizumab as an intravenous infusion of 1200 mg, followed by 15 mg/kg Avastin, on the same day every 3 weeks or sorafenib 400 mg given orally twice daily, until disease progression or unacceptable toxicity. Patients could discontinue either atezolizumab or Avastin (e.g., due to adverse events) and continue on single-agent therapy until disease progression or unacceptable toxicity associated with the single-agent.
The study enrolled patients who were ECOG performance score 0 or 1 and who had not received prior systemic treatment. Patients were required to be evaluated for the presence of varices within 6 months prior to treatment, and were excluded if they had variceal bleeding within 6 months prior to treatment, untreated or incompletely treated varices with bleeding, or high risk of bleeding. Patients with Child-Pugh B or C cirrhosis, moderate or severe ascites; history of hepatic encephalopathy; a history of autoimmune disease; administration of a live, attenuated vaccine within 4 weeks prior to randomization; administration of systemic immunostimulatory agents within 4 weeks or systemic immunosuppressive medications within 2 weeks prior to randomization; or untreated or corticosteroid-dependent brain metastases were excluded. Tumor assessments were performed every 6 weeks for the first 54 weeks and every 9 weeks thereafter.
The demographics and baseline disease characteristics of the study population were balanced between the treatment arms. The median age was 65 years (range: 26 to 88) and 83% of patients were male. The majority of patients were Asian (57%) or White (35%); 40% were from Asia (excluding Japan). Approximately 75% of patients presented with macrovascular invasion and/or extrahepatic spread and 37% had a baseline AFP ≥400 ng/mL. Baseline ECOG performance status was 0 (62%) or 1 (38%). HCC risk factors were Hepatitis B in 48% of patients, Hepatitis C in 22% and 31% of patients had non-viral liver disease. The majority of patients had BCLC stage C (82%) disease at baseline, while 16% had stage B and 3% had stage A.
The major efficacy outcome measures were overall survival (OS) and independent review facility (IRF)-assessed progression free survival (PFS) per RECIST v1.1. Additional efficacy outcome measures were IRF-assessed overall response rate (ORR) per RECIST and mRECIST.
Efficacy results are presented in Table 20 and Figure 10.
| Avastin in combination with Atezolizumab (N= 336) | Sorafenib (N=165) |
|
|---|---|---|
| CI=confidence interval; HCC mRECIST= Modified RECIST Assessment for Hepatocellular Carcinoma; NE=not estimable; N/A=not applicable; RECIST 1.1= Response Evaluation Criteria in Solid Tumors v1.1 | ||
|
||
| Overall Survival | ||
| Number of deaths (%) | 96 (29) | 65 (39) |
| Median OS in months (95% CI) | NE (NE, NE) | 13.2 (10.4, NE) |
| Hazard ratio* (95% CI) | 0.58 (0.42, 0.79) | |
| p-value† | 0.0006† | |
| Progression-Free Survival‡ | ||
| Number of events(%) | 197 (59) | 109 (66) |
| Median PFS in months (95% CI) | 6.8 (5.8, 8.3) | 4.3 (4.0, 5.6) |
| Hazard ratio* (95% CI) | 0.59 (0.47, 0.76) | |
| p-value | <0.0001 | |
| Overall Response Rate‡,§ (ORR), RECIST 1.1 | ||
| Number of responders (%) | 93 (28) | 19 (12) |
| (95% CI) | (23, 33) | (7,17) |
| p-value¶ | <0.0001 | |
| Complete responses, n (%) | 22 (7) | 0 |
| Partial responses, n (%) | 71 (21) | 19 (12) |
| Duration of Response‡,§ (DOR) RECIST 1.1 | ||
| (n=93) | (n=19) | |
| Median DOR in months (95% CI) | NE (NE, NE) | 6.3 (4.7, NE) |
| Range (months) | (1.3#, 13.4#) | (1.4#, 9.1#) |
| Overall Response Rate‡,§ (ORR), HCC mRECIST | ||
| Number of responders (%) | 112 (33) | 21 (13) |
| (95% CI) | (28, 39) | (8, 19) |
| p-value¶ | <0.0001 | |
| Complete responses, n (%) | 37 (11) | 3 (1.8) |
| Partial responses, n (%) | 75 (22) | 18 (11) |
| Duration of Response‡,§ (DOR) HCC mRECIST | ||
| (n=112) | (n=21) | |
| Median DOR in months (95% CI) | NE (NE, NE) | 6.3 (4.9, NE) |
| Range (months) | (1.3#, 13.4#) | (1.4#, 9.1#) |
Figure 10: Kaplan-Meier Plot of Overall Survival in IMbrave150
16. How is Avastin supplied
Avastin (bevacizumab) injection is a clear to slightly opalescent, colorless to pale brown, sterile solution for intravenous infusion supplied as single-dose vials in the following strengths:
- 100 mg/4 mL (25 mg/mL): carton of one vial (NDC 50242-060-01)
- 400 mg/16 mL (25 mg/mL): carton of one vial (NDC 50242-061-01)
| AVASTIN
bevacizumab injection, solution |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| AVASTIN
bevacizumab injection, solution |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Genentech, Inc. (080129000) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Roche Singapore Technical Operations Pte. Ltd. | 937189173 | ANALYSIS(50242-060, 50242-061) , API MANUFACTURE(50242-060, 50242-061) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| F. Hoffmann-La Roche Ltd | 485244961 | MANUFACTURE(50242-060, 50242-061) , ANALYSIS(50242-060, 50242-061) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Genentech, Inc. | 080129000 | ANALYSIS(50242-060, 50242-061) , MANUFACTURE(50242-060, 50242-061) , API MANUFACTURE(50242-060, 50242-061) , PACK(50242-060, 50242-061) , LABEL(50242-060, 50242-061) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Genentech, Inc. | 004074162 | ANALYSIS(50242-060, 50242-061) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Genentech, Inc. | 833220176 | MANUFACTURE(50242-060, 50242-061) , PACK(50242-060, 50242-061) , LABEL(50242-060, 50242-061) , ANALYSIS(50242-060, 50242-061) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Genentech, Inc. | 146373191 | ANALYSIS(50242-060, 50242-061) , API MANUFACTURE(50242-060, 50242-061) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| F. Hoffmann-La Roche AG | 482242971 | API MANUFACTURE(50242-060, 50242-061) , ANALYSIS(50242-060, 50242-061) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Roche Diagnostics GmbH | 323105205 | ANALYSIS(50242-060, 50242-061) | |