Drug Detail:Bavencio (Avelumab [ a-vel-ue-mab ])
Drug Class: Anti-PD-1 and PD-L1 monoclonal antibodies (immune checkpoint inhibitors)
Highlights of Prescribing Information
BAVENCIO® (avelumab) injection, for intravenous use
Initial U.S. Approval: 2017
Indications and Usage for Bavencio
BAVENCIO is a programmed death ligand-1 (PD-L1) blocking antibody indicated for:
Merkel Cell Carcinoma (MCC)
- Adults and pediatric patients 12 years and older with metastatic MCC. This indication is approved under accelerated approval based on tumor response rate and duration of response. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials. (1.1, 14.1)
Urothelial Carcinoma (UC)
- Maintenance treatment of patients with locally advanced or metastatic UC that has not progressed with first-line platinum-containing chemotherapy. (1.2, 14.2)
- Patients with locally advanced or metastatic UC who:
- Have disease progression during or following platinum-containing chemotherapy. (1.2, 14.2)
- Have disease progression within 12 months of neoadjuvant or adjuvant treatment with platinum-containing chemotherapy. (1.2, 14.2)
Renal Cell Carcinoma (RCC)
- First-line treatment, in combination with axitinib, of patients with advanced RCC. (1.3, 14.3)
Bavencio Dosage and Administration
- Premedicate for the first 4 infusions and subsequently as needed. (2.1)
- Merkel Cell Carcinoma: 800 mg every 2 weeks. (2.2)
- Urothelial Carcinoma; 800 mg every 2 weeks. (2.3)
- Renal Cell Carcinoma: 800 mg every 2 weeks in combination with axitinib 5 mg orally twice daily. (2.4)
Administer BAVENCIO as an intravenous infusion over 60 minutes.
Dosage Forms and Strengths
Injection: 200 mg/10 mL (20 mg/mL) solution in single-dose vial. (3)
Contraindications
None. (4)
Warnings and Precautions
- Immune-Mediated Adverse Reactions (5.1)
- Immune-mediated adverse reactions, which may be severe or fatal, can occur in any organ system or tissue, including the following: immune-mediated pneumonitis, immune-mediated colitis, immune-mediated hepatitis, immune-mediated endocrinopathies, immune-mediated nephritis with renal dysfunction, immune-mediated dermatologic adverse reactions, and may result in solid organ transplant rejection.
- Monitor for early identification and management. Evaluate liver enzymes, creatinine, and thyroid function at baseline and periodically during treatment.
- Withhold or permanently discontinue based on severity and type of reaction.
- Infusion-related reactions: Interrupt, slow the rate of infusion, or permanently discontinue BAVENCIO based on severity of reaction. (5.2)
- Complications of allogeneic HSCT: Fatal and other serious complications can occur in patients who receive allogeneic HSCT before or after being treated with a PD-1/PD-L1 blocking antibody. (5.3)
- Major adverse cardiovascular events: Optimize management of cardiovascular risk factors. Discontinue BAVENCIO in combination with axitinib for Grade 3-4 events. (5.4)
- Embryo-fetal toxicity: BAVENCIO can cause fetal harm. Advise females of reproductive potential of the potential risk to a fetus and use of effective contraception. (5.5, 8.1, 8.3)
Adverse Reactions/Side Effects
Most common adverse reactions (≥ 20%) in patients were:
- MCC: fatigue, musculoskeletal pain, diarrhea, nausea, infusion-related reaction, rash, decreased appetite, and peripheral edema. (6.1)
-
UC:
- Maintenance treatment: fatigue, musculoskeletal pain, urinary tract infection, and rash. (6.1)
- Previously-treated: fatigue, infusion-related reaction, musculoskeletal pain, nausea, decreased appetite, and urinary tract infection. (6.1)
- RCC (with axitinib): diarrhea, fatigue, hypertension, musculoskeletal pain, nausea, mucositis, palmar-plantar erythrodysesthesia, dysphonia, decreased appetite, hypothyroidism, rash, hepatotoxicity, cough, dyspnea, abdominal pain, and headache. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact EMD Serono at 1-800-283-8088 ext. 5563 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Use In Specific Populations
Lactation: Advise not to breastfeed. (8.2)
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 7/2022
Related/similar drugs
Keytruda, Avastin, pembrolizumab, nivolumab, bevacizumab, Opdivo, AfinitorFull Prescribing Information
1. Indications and Usage for Bavencio
1.1 Metastatic Merkel Cell Carcinoma
BAVENCIO (avelumab) is indicated for the treatment of adults and pediatric patients 12 years and older with metastatic Merkel cell carcinoma (MCC). This indication is approved under accelerated approval based on tumor response rate and duration of response. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials [see Clinical Studies (14.1)].
2. Bavencio Dosage and Administration
2.1 Premedication
Premedicate patients with an antihistamine and with acetaminophen prior to the first 4 infusions of BAVENCIO. Premedication should be administered for subsequent BAVENCIO doses based upon clinical judgment and presence/severity of prior infusion reactions [see Dosage and Administration (2.5) and Warnings and Precautions (5.2)].
2.2 Recommended Dosage for MCC
The recommended dose of BAVENCIO is 800 mg administered as an intravenous infusion over 60 minutes every 2 weeks until disease progression or unacceptable toxicity.
2.3 Recommended Dosage for UC
The recommended dose of BAVENCIO is 800 mg administered as an intravenous infusion over 60 minutes every 2 weeks until disease progression or unacceptable toxicity.
2.4 Recommended Dosage for RCC
The recommended dose of BAVENCIO is 800 mg administered as an intravenous infusion over 60 minutes every 2 weeks in combination with axitinib 5 mg orally taken twice daily (12 hours apart) with or without food until disease progression or unacceptable toxicity.
When axitinib is used in combination with BAVENCIO, dose escalation of axitinib above the initial 5 mg dose may be considered at intervals of two weeks or longer. Review the Full Prescribing Information for axitinib prior to initiation.
2.5 Dose Modifications
No dose reduction for BAVENCIO is recommended. In general, withhold BAVENCIO for severe (Grade 3) immune-mediated adverse reactions. Permanently discontinue BAVENCIO for life-threatening (Grade 4) immune-mediated adverse reactions, recurrent severe (Grade 3) immune-mediated reactions that require systemic immunosuppressive treatment, or an inability to reduce corticosteroid dose to 10 mg or less of prednisone or equivalent per day within 12 weeks of initiating corticosteroids.
Dosage modifications for BAVENCIO for adverse reactions that require management different from these general guidelines are summarized in Table 1.
| Adverse Reaction | Severity* | Dosage Modification |
|---|---|---|
| ALT = alanine aminotransferase, AST = aspartate aminotransferase, ULN = upper limit normal, SJS = Stevens-Johnson syndrome, TEN = toxic epidermal necrosis, DRESS = drug rash with eosinophilia and systemic symptoms | ||
|
||
| Immune-Mediated Adverse Reactions [see Warnings and Precautions (5.1)] | ||
| Pneumonitis | Grade 2 | Withhold† |
| Grade 3 or 4 | Permanently discontinue | |
| Colitis | Grade 2 or 3 | Withhold† |
| Grade 4 | Permanently discontinue | |
| Hepatitis with no tumor involvement of the liver For liver enzyme elevations in patients treated with combination therapy, see Table 2 | AST or ALT increases to more than 3 and up to 8 times ULN or Total bilirubin increases to more than 1.5 and up to 3 times ULN | Withhold† |
| AST or ALT increases to more than 8 times ULN or Total bilirubin increases to more than 3 times ULN | Permanently discontinue | |
| Hepatitis with tumor involvement of the liver‡ | Baseline AST or ALT is more than 1 and up to 3 times ULN and increases to more than 5 and up to 10 times ULN or Baseline AST or ALT is more than 3 and up to 5 times ULN and increases to more than 8 and up to 10 times ULN | Withhold† |
| AST or ALT increases to more than 10 times ULN or Total bilirubin increases to more than 3 times ULN | Permanently discontinue | |
| Endocrinopathies | Grade 3 or 4 | Withhold until clinically stable or permanently discontinue depending on severity |
| Nephritis with Renal Dysfunction | Grade 2 or 3 increased blood creatinine | Withhold† |
| Grade 4 increased blood creatinine | Permanently discontinue | |
| Exfoliative Dermatologic Conditions | Suspected SJS, TEN, or DRESS | Withhold† |
| Confirmed SJS, TEN, or DRESS | Permanently discontinue | |
| Myocarditis | Grade 2, 3 or 4 | Permanently discontinue |
| Neurological Toxicities | Grade 2 | Withhold† |
| Grade 3 or 4 | Permanently discontinue | |
| Other Adverse Reactions | ||
| Infusion-related reactions [see Warnings and Precautions (5.2)] | Grade 1 or 2 | Interrupt or slow the rate of infusion |
| Grade 3 or 4 | Permanently discontinue | |
Table 2 presents dosage modifications that are different from those described above in Table 1 for BAVENCIO used as monotherapy or in the Full Prescribing Information for the drug administered in combination.
| Treatment | Adverse Reaction | Severity* | Dosage Modification |
|---|---|---|---|
|
|||
| BAVENCIO in combination with axitinib | Liver enzyme elevations | ALT or AST at least 3 times ULN but less than 10 times ULN without concurrent total bilirubin at least 2 times ULN | Withhold both BAVENCIO and axitinib until adverse reactions recover to Grades 0-1†
Consider rechallenge with BAVENCIO or axitinib or sequential rechallenge with both BAVENCIO and axitinib after recovery‡ |
| ALT or AST at least 10 times ULN or more than 3 times ULN with concurrent total bilirubin at least 2 times ULN | Permanently discontinue both BAVENCIO and axitinib† | ||
3. Dosage Forms and Strengths
Injection: 200 mg/10 mL (20 mg/mL), clear, colorless to slightly yellow solution in a single-dose vial.
5. Warnings and Precautions
5.1 Severe and Fatal Immune-Mediated Adverse Reactions
BAVENCIO is a monoclonal antibody that belongs to a class of drugs that bind to either the programmed death-receptor 1 (PD-1) or the PD-ligand 1 (PD-L1), blocking the PD-1/PD-L1 pathway, thereby removing inhibition of the immune response, potentially breaking peripheral tolerance and inducing immune-mediated adverse reactions. Important immune-mediated adverse reactions listed under Warnings and Precautions may not include all possible severe and fatal immune-mediated reactions.
Immune-mediated adverse reactions, which may be severe or fatal, can occur in any organ system or tissue. Immune-mediated adverse reactions can occur at any time after starting treatment with a PD-1/PD-L1 blocking antibody. While immune-mediated adverse reactions usually manifest during treatment with PD-1/PD-L1 blocking antibodies, immune-mediated adverse reactions can also manifest after discontinuation of PD-1/PD-L1 blocking antibodies.
Early identification and management of immune-mediated adverse reactions are essential to ensure safe use of PD-1/PD-L1 blocking antibodies. Monitor patients closely for symptoms and signs that may be clinical manifestations of underlying immune-mediated adverse reactions. Evaluate liver enzymes, creatinine, and thyroid function at baseline and periodically during treatment. In cases of suspected immune-mediated adverse reactions, initiate appropriate workup to exclude alternative etiologies, including infection. Institute medical management promptly, including specialty consultation as appropriate.
Withhold or permanently discontinue BAVENCIO depending on severity [see Dosage and Administration (2.5)]. In general, if BAVENCIO requires interruption or discontinuation, administer systemic corticosteroid therapy (1 to 2 mg/kg/day prednisone or equivalent) until improvement to Grade 1 or less. Upon improvement to Grade 1 or less, initiate corticosteroid taper and continue to taper over at least 1 month. Consider administration of other systemic immunosuppressants in patients whose immune-mediated adverse reactions are not controlled with corticosteroid therapy.
Toxicity management guidelines for adverse reactions that do not necessarily require systemic corticosteroids (e.g., endocrinopathies and dermatologic reactions) are discussed below.
Immune-Mediated Endocrinopathies
5.2 Infusion-Related Reactions
BAVENCIO can cause severe or life-threatening infusion-related reactions [see Adverse Reactions (6.1)]. Premedicate with antihistamine and acetaminophen prior to the first 4 infusions. Monitor patients for signs and symptoms of infusion-related reactions including pyrexia, chills, flushing, hypotension, dyspnea, wheezing, back pain, abdominal pain, and urticaria. Interrupt or slow the rate of infusion for mild or moderate infusion-related reactions. Stop the infusion and permanently discontinue BAVENCIO for severe (Grade 3) or life-threatening (Grade 4) infusion-related reactions [see Dosage and Administration (2.5) and Adverse Reactions (6.1)].
Infusion-related reactions occurred in 25% of patients treated with BAVENCIO including three (0.2%) Grade 4 and nine (0.5%) Grade 3 infusion-related reactions. Ninety-three percent of patients received premedication with antihistamine and acetaminophen. Eleven (92%) of the 12 patients with Grade ≥ 3 reactions were treated with intravenous corticosteroids. Fourteen percent of patients had infusion-related reactions that occurred after the BAVENCIO infusion was completed.
5.3 Complications of Allogeneic HSCT
Fatal and other serious complications can occur in patients who receive allogeneic hematopoietic stem cell transplantation (HSCT) before or after being treated with a PD-1/PD-L1 blocking antibody. Transplant-related complications include hyperacute graft-versus-host-disease (GVHD), acute GVHD, chronic GVHD, hepatic veno-occlusive disease (VOD) after reduced intensity conditioning, and steroid-requiring febrile syndrome (without an identified infectious cause). These complications may occur despite intervening therapy between PD-1/PD-L1 blockade and allogeneic HSCT.
Follow patients closely for evidence of transplant-related complications and intervene promptly. Consider the benefit versus risks of treatment with a PD-1/PD-L1 blocking antibody prior to or after an allogeneic HSCT.
5.4 Major Adverse Cardiovascular Events (MACE)
BAVENCIO in combination with axitinib can cause severe and fatal cardiovascular events. Consider baseline and periodic evaluations of left ventricular ejection fraction. Monitor for signs and symptoms of cardiovascular events. Optimize management of cardiovascular risk factors, such as hypertension, diabetes, or dyslipidemia. Discontinue BAVENCIO and axitinib for Grade 3-4 cardiovascular events.
MACE occurred in 7% of patients with advanced RCC treated with BAVENCIO in combination with axitinib compared to 3.4% treated with sunitinib in a randomized trial, JAVELIN Renal 101. These events included death due to cardiac events (1.4%), Grade 3-4 myocardial infarction (2.8%), and Grade 3-4 congestive heart failure (1.8%). Median time to onset of MACE was 4.2 months (range: 2 days to 24.5 months).
5.5 Embryo-Fetal Toxicity
Based on its mechanism of action, BAVENCIO can cause fetal harm when administered to a pregnant woman. Animal studies have demonstrated that inhibition of the PD-1/PD-L1 pathway can lead to increased risk of immune-mediated rejection of the developing fetus resulting in fetal death. If this drug is used during pregnancy, or if the patient becomes pregnant while taking BAVENCIO, inform the patient of the potential risk to a fetus. Advise females of childbearing potential to use effective contraception during treatment with BAVENCIO and for at least one month after the last dose of BAVENCIO [see Use in Specific Populations (8.1, 8.3)].
6. Adverse Reactions/Side Effects
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Severe and fatal immune-mediated adverse reactions [see Warnings and Precautions (5.1)]
- Infusion-related reactions [see Warnings and Precautions (5.2)]
- Complications of allogeneic HSCT [see Warnings and Precautions (5.3)]
- Major adverse cardiovascular events [see Warnings and Precautions (5.4)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The data described in the WARNINGS AND PRECAUTIONS section reflect exposure to BAVENCIO 10 mg/kg intravenously every 2 weeks as a single agent in 1738 patients enrolled in the JAVELIN Merkel 200 and JAVELIN Solid Tumor trials and to BAVENCIO 10 mg/kg intravenously every 2 weeks in combination with axitinib 5 mg orally twice daily in 489 patients enrolled in the JAVELIN Renal 100 and JAVELIN Renal 101 trials. In the BAVENCIO monotherapy population, 24% of patients were exposed for ≥ 6 months and 7% were exposed for ≥ 12 months. The population characteristics of BAVENCIO in combination with axitinib are shown below. When BAVENCIO was used in combination with axitinib, 70% of patients were exposed for ≥ 6 months and 31% were exposed for ≥ 12 months. The following criteria were used to classify an adverse reaction as immune-mediated: onset within 90 days after last dose of BAVENCIO, no spontaneous resolution within 7 days of onset, treatment with corticosteroids or other immunosuppressant or hormone replacement therapy, biopsy consistent with immune-mediated reaction, and no other clear etiology.
Metastatic Merkel Cell Carcinoma
The data described below reflect exposure to BAVENCIO 10 mg/kg intravenously every 2 weeks in 88 patients with metastatic MCC enrolled in the JAVELIN Merkel 200 trial. Patients with any of the following were excluded: autoimmune disease; medical conditions requiring systemic immunosuppression; prior organ or allogeneic stem cell transplantation; prior treatment with anti-PD-1, anti-PD-L1, or anti-CTLA-4 antibodies; central nervous system (CNS) metastases; infection with HIV, hepatitis B, or hepatitis C; or ECOG performance score ≥ 2.
The median duration of exposure to BAVENCIO was 4 months (range: 2 weeks to 21 months). Forty percent of patients received BAVENCIO for more than 6 months and 14% were treated for more than one year [see Clinical Studies (14.1)]. The study population characteristics were: median age of 73 years (range: 33 to 88), 74% male, 92% White, ECOG performance score of 0 (56%) or 1 (44%), and 65% of patients had one prior anti-cancer therapy for metastatic MCC and 35% had two or more prior therapies.
BAVENCIO was permanently discontinued for adverse reactions in six (7%) patients; adverse reactions resulting in permanent discontinuation were ileus, Grade 3 transaminitis, Grade 3 creatine kinase elevation, tubulointerstitial nephritis, and Grade 3 pericardial effusion. BAVENCIO was temporarily discontinued in 21 (24%) patients for adverse events, excluding temporary dose interruption for infusion-related reactions where infusion was restarted the same day. The most common adverse reaction requiring dose interruption was anemia. Serious adverse reactions that occurred in more than one patient were acute kidney injury, anemia, abdominal pain, ileus, asthenia, and cellulitis. The most common adverse reactions (≥ 20%) were fatigue, musculoskeletal pain, diarrhea, nausea, infusion-related reaction, rash, decreased appetite, and peripheral edema.
Table 3 and Table 4 summarize the incidence of adverse reactions and laboratory abnormalities, respectively, that occurred in patients receiving BAVENCIO.
| Adverse Reactions | BAVENCIO (N=88) |
|
|---|---|---|
| All Grades % | Grade 3-4 % |
|
|
||
| General Disorders | ||
| Fatigue* | 50 | 2 |
| Infusion-related reaction† | 22 | 0 |
| Peripheral edema‡ | 20 | 0 |
| Musculoskeletal and Connective Tissue Disorders | ||
| Musculoskeletal pain§ | 32 | 2 |
| Arthralgia | 16 | 1 |
| Gastrointestinal Disorders | ||
| Diarrhea | 23 | 0 |
| Nausea | 22 | 0 |
| Constipation | 17 | 1 |
| Abdominal pain¶ | 16 | 2 |
| Vomiting | 13 | 0 |
| Skin and Subcutaneous Tissue Disorders | ||
| Rash# | 22 | 0 |
| PruritusÞ | 10 | 0 |
| Metabolism and Nutrition Disorders | ||
| Decreased appetite | 20 | 2 |
| Decreased weight | 15 | 0 |
| Respiratory, Thoracic and Mediastinal Disorders | ||
| Cough | 18 | 0 |
| Dyspneaß | 11 | 1 |
| Nervous System Disorders | ||
| Dizziness | 14 | 0 |
| Headache | 10 | 0 |
| Vascular Disorders | ||
| Hypertension | 13 | 6 |
| Laboratory Tests | Any Grade (N=88) % | Grade 3-4 (N=88) % |
|---|---|---|
|
||
| Chemistry | ||
| Increased aspartate aminotransferase (AST) | 34 | 1 |
| Increased alanine aminotransferase (ALT) | 20 | 5 |
| Increased lipase | 14 | 4 |
| Increased amylase | 8 | 1 |
| Increased bilirubin | 6 | 1 |
| Hyperglycemia† | - | 7 |
| Hematology | ||
| Anemia | 35 | 9 |
| Lymphopenia | 49 | 19 |
| Thrombocytopenia | 27 | 1 |
| Neutropenia | 6 | 1 |
Advanced Renal Cell Carcinoma
The safety of BAVENCIO was evaluated in JAVELIN Renal 101. Patients with autoimmune disease other than type I diabetes mellitus, vitiligo, psoriasis, or thyroid disorders not requiring immunosuppressive treatment were excluded. Patients received BAVENCIO 10 mg/kg every 2 weeks administered in combination with axitinib 5 mg twice daily (N=434) or sunitinib 50 mg once daily for 4 weeks followed by 2 weeks off (N=439).
In the BAVENCIO plus axitinib arm, 70% were exposed to BAVENCIO for ≥ 6 months and 29% were exposed for ≥ 1 year in JAVELIN Renal 101 [see Clinical Studies (14.3)].
The median age of patients treated with BAVENCIO in combination with axitinib was 62 years (range: 29 to 83), 38% of patients were 65 years or older, 71% were male, 75% were White, and the ECOG performance score was 0 (64%) or 1 (36%).
Fatal adverse reactions occurred in 1.8% of patients receiving BAVENCIO in combination with axitinib. These included sudden cardiac death (1.2%), stroke (0.2%), myocarditis (0.2%), and necrotizing pancreatitis (0.2%).
Serious adverse reactions occurred in 35% of patients receiving BAVENCIO in combination with axitinib. Serious adverse reactions in ≥ 1% of patients included diarrhea (2.5%), dyspnea (1.8%), hepatotoxicity (1.8%), venous thromboembolic disease (1.6%), acute kidney injury (1.4%), and pneumonia (1.2%).
Permanent discontinuation due to an adverse reaction of either BAVENCIO or axitinib occurred in 22% of patients: 19% BAVENCIO only, 13% axitinib only, and 8% both drugs. The most common adverse reactions (> 1%) resulting in permanent discontinuation of BAVENCIO or the combination were hepatotoxicity (6%) and infusion-related reaction (1.8%).
Dose interruptions or reductions due to an adverse reaction, excluding temporary interruptions of BAVENCIO infusions due to infusion-related reactions, occurred in 76% of patients receiving BAVENCIO in combination with axitinib. This includes interruption of BAVENCIO in 50% of patients. Axitinib was interrupted in 66% and dose reduced in 19% of patients. The most common adverse reaction (> 10%) resulting in interruption of BAVENCIO was diarrhea (10%) and the most common adverse reactions resulting in either interruption or dose reduction of axitinib were diarrhea (19%), hypertension (18%), palmar-plantar erythrodysesthesia (18%), and hepatotoxicity (10%).
The most common adverse reactions (≥ 20%) in patients receiving BAVENCIO in combination with axitinib were diarrhea, fatigue, hypertension, musculoskeletal pain, nausea, mucositis, palmar-plantar erythrodysesthesia, dysphonia, decreased appetite, hypothyroidism, rash, hepatotoxicity, cough, dyspnea, abdominal pain, and headache.
Forty-eight (11%) patients treated with BAVENCIO in combination with axitinib received an oral prednisone dose equivalent to ≥ 40 mg daily for an immune-mediated adverse reaction [see Warnings and Precautions (5)].
Table 7 summarizes adverse reactions that occurred in ≥ 20% of BAVENCIO in combination with axitinib-treated patients.
| Adverse Reactions | BAVENCIO plus Axitinib (N=434) | Sunitinib (N=439) |
||
|---|---|---|---|---|
| All Grades % | Grade 3-4 % | All Grades % | Grade 3-4 % |
|
|
||||
| Gastrointestinal Disorders | ||||
| Diarrhea* | 62 | 8 | 48 | 2.7 |
| Nausea | 34 | 1.4 | 39 | 1.6 |
| Mucositis† | 34 | 2.8 | 35 | 2.1 |
| Hepatotoxicity‡ | 24 | 9 | 18 | 3.6 |
| Abdominal pain§ | 22 | 1.4 | 19 | 2.1 |
| General Disorders and Administration Site Conditions | ||||
| Fatigue¶ | 53 | 6 | 54 | 6 |
| Vascular Disorders | ||||
| Hypertension# | 50 | 26 | 36 | 17 |
| Musculoskeletal and Connective Tissue Disorders | ||||
| Musculoskeletal painÞ | 40 | 3.2 | 33 | 2.7 |
| Skin and Subcutaneous Tissue Disorders | ||||
| Palmar-plantar erythrodysesthesia | 33 | 6 | 34 | 4 |
| Rashß | 25 | 0.9 | 16 | 0.5 |
| Respiratory, Thoracic and Mediastinal Disorders | ||||
| Dysphonia | 31 | 0.5 | 3.2 | 0 |
| Dyspneaà | 23 | 3 | 16 | 1.8 |
| Cough | 23 | 0.2 | 19 | 0 |
| Metabolism and Nutrition Disorders | ||||
| Decreased appetite | 26 | 2.1 | 29 | 0.9 |
| Endocrine Disorders | ||||
| Hypothyroidism | 25 | 0.2 | 14 | 0.2 |
| Nervous System Disorders | ||||
| Headache | 21 | 0.2 | 16 | 0.2 |
Other clinically important adverse reactions that occurred in less than 20% of patients in JAVELIN Renal 101 included arthralgia, weight decreased, and chills.
Patients received pre-medication with an anti-histamine and acetaminophen prior to each infusion. Infusion-related reactions occurred in 12% (Grade 3: 1.6%; no Grade 4) of patients treated with BAVENCIO in combination with axitinib.
Table 8 summarizes selected laboratory abnormalities that occurred in ≥ 20% of BAVENCIO in combination with axitinib-treated patients.
| Laboratory Abnormality | BAVENCIO plus Axitinib* | Sunitinib* | ||
|---|---|---|---|---|
| Any Grade % | Grade 3-4 % | Any Grade % | Grade 3-4 % |
|
|
||||
| Chemistry | ||||
| Blood triglycerides increased | 71 | 13 | 48 | 5 |
| Blood creatinine increased | 62 | 2.3 | 68 | 1.4 |
| Blood cholesterol increased | 57 | 1.9 | 22 | 0.7 |
| Alanine aminotransferase increased (ALT) | 50 | 9 | 46 | 3.2 |
| Aspartate aminotransferase increased (AST) | 47 | 7 | 57 | 3.2 |
| Blood sodium decreased | 38 | 9 | 37 | 10 |
| Lipase increased | 37 | 14 | 25 | 7 |
| Blood potassium increased | 35 | 3 | 28 | 3.9 |
| Blood bilirubin increased | 21 | 1.4 | 23 | 1.4 |
| Hematology | ||||
| Platelet count decreased | 27 | 0.7 | 80 | 15 |
| Hemoglobin decreased | 21 | 2.1 | 65 | 8 |
6.2 Immunogenicity
As with all therapeutic proteins, there is potential for immunogenicity. The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies to avelumab in the studies described below with the incidence of antibodies in other studies or to other products may be misleading.
Of the 344 patients treated with BAVENCIO 10 mg/kg as an intravenous infusion every 2 weeks plus BSC, 325 were evaluable for treatment-emergent anti-drug antibodies (ADA) and 62 (19.1%) tested positive in the JAVELIN Bladder 100 trial.
Of the 480 patients treated with BAVENCIO 10 mg/kg as an intravenous infusion every 2 weeks in combination with axitinib 5 mg twice daily, 453 were evaluable for treatment-emergent ADA and 66 (15%) tested positive in the JAVELIN Renal 100 and JAVELIN Renal 101 trials.
Patients who tested positive for treatment-emergent ADA had decreased systemic BAVENCIO exposure [see Clinical Pharmacology (12.3)]. In exploratory analyses, the effect of ADA on the efficacy or safety could not be determined due to insufficient numbers of patients in the ADA-positive subgroup and confounding variables.
8. Use In Specific Populations
8.4 Pediatric Use
The safety and effectiveness of BAVENCIO have been established in pediatric patients aged 12 years and older for metastatic MCC. Use of BAVENCIO in this age group is supported by evidence from adequate and well-controlled studies of BAVENCIO in adults with additional population pharmacokinetic data demonstrating that age and body weight had no clinically meaningful effect on the steady state exposure of avelumab, that drug exposure is generally similar between adults and pediatric patients age 12 years and older for monoclonal antibodies, and that the course of MCC is sufficiently similar in adult and pediatric patients to allow extrapolation of data in adults to pediatric patients. The recommended dose in pediatric patients 12 years of age or greater is the same as that in adults [see Dosage and Administration (2.2), Clinical Pharmacology (12.3), and Clinical Studies (14)].
Safety and effectiveness of BAVENCIO have not been established in pediatric patients less than 12 years of age.
11. Bavencio Description
Avelumab is a programmed death ligand1 (PD-L1) blocking antibody. Avelumab- is a human IgG1 lambda monoclonal antibody produced in Chinese hamster ovary cells and has a molecular weight of approximately 147 kDa.
BAVENCIO (avelumab) Injection for intravenous use is a sterile, preservative-free, non-pyrogenic, clear, colorless to slightly yellow solution. Each single-dose vial contains 200 mg avelumab in 10 mL (20 mg/mL). Each mL contains 20 mg avelumab, D-mannitol (51 mg), glacial acetic acid (0.6 mg), polysorbate 20 (0.5 mg), sodium hydroxide (0.3 mg), and Water for Injection. The pH range of the solution is 5.0 – 5.6.
12. Bavencio - Clinical Pharmacology
12.1 Mechanism of Action
PD-L1 may be expressed on tumor cells and tumor-infiltrating immune cells and can contribute to the inhibition of the anti-tumor immune response in the tumor microenvironment. Binding of PD-L1 to the PD-1 and B7.1 receptors found on T cells and antigen presenting cells suppresses cytotoxic T-cell activity, T-cell proliferation, and cytokine production. Avelumab binds PD-L1 and blocks the interaction between PD-L1 and its receptors PD-1 and B7.1. This interaction releases the inhibitory effects of PD-L1 on the immune response resulting in the restoration of immune responses, including anti-tumor immune responses. Avelumab has also been shown to induce antibody-dependent cell-mediated cytotoxicity (ADCC) in vitro. In syngeneic mouse tumor models, blocking PD-L1 activity resulted in decreased tumor growth.
12.2 Pharmacodynamics
Based on exposure efficacy and exposure safety relationships, there are no expected clinically meaningful differences in the safety or efficacy of BAVENCIO administered every 2 weeks at 800 mg or 10 mg/kg in patients with metastatic Merkel cell carcinoma, in patients with urothelial carcinoma and in patients with advanced renal cell carcinoma.
12.3 Pharmacokinetics
Avelumab pharmacokinetics (PK) was assessed using a population PK approach for both single-agent BAVENCIO and BAVENCIO in combination with axitinib. There are no expected clinically meaningful differences in exposure of avelumab administered every 2 weeks at 800 mg or 10 mg/kg in both settings.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
No studies have been conducted to assess the potential of avelumab for genotoxicity or carcinogenicity.
Fertility studies have not been conducted with avelumab; however, an assessment of male and female reproductive organs was included in 3-month repeat-dose toxicity study in Cynomolgus monkeys. Weekly administration of avelumab did not result in any notable effects in the male and female reproductive organs.
13.2 Animal Toxicology and/or Pharmacology
In animal models, inhibition of PD-L1/PD-1 signaling increased the severity of some infections and enhanced inflammatory responses. M. tuberculosis-infected PD-1 knockout mice exhibit markedly decreased survival compared with wild-type controls, which correlated with increased bacterial proliferation and inflammatory responses in these animals. PD-L1 and PD-1 knockout mice and mice receiving PD-L1 blocking antibody have also shown decreased survival following infection with lymphocytic choriomeningitis virus.
14. Clinical Studies
14.1 Metastatic Merkel Cell Carcinoma
The efficacy and safety of BAVENCIO was demonstrated in the JAVELIN Merkel 200 trial (NCT02155647), an open-label, single-arm, multi-center study conducted in patients with histologically confirmed metastatic MCC whose disease had progressed on or after chemotherapy administered for distant metastatic disease. The trial excluded patients with autoimmune disease; medical conditions requiring systemic immunosuppression; prior organ or allogeneic stem cell transplantation; prior treatment with anti-PD-1, anti-PD-L1, or anti-CTLA-4 antibodies; CNS metastases; infection with HIV, hepatitis B, or hepatitis C; or ECOG performance score ≥ 2.
Patients received BAVENCIO 10 mg/kg as an intravenous infusion over 60 minutes every 2 weeks until disease progression or unacceptable toxicity. Patients with radiological disease progression not associated with significant clinical deterioration, defined as no new or worsening symptoms, no change in performance status for greater than 2 weeks, and no need for salvage therapy, could continue treatment. Tumor response assessments were performed every 6 weeks. The major efficacy outcome measures were confirmed overall response rate (ORR) according to Response Evaluation Criteria in Solid Tumors (RECIST) v1.1 as assessed by a blinded independent central review committee (IRC) and IRC-assessed duration of response. The efficacy analysis was conducted when the last patient enrolled had completed 12 months of follow-up.
A total of 88 patients were enrolled. Baseline patient characteristics were a median age of 73 years (range: 33 to 88), 74% of patients were male, 92% were White, and the ECOG performance score was 0 (56%) or 1 (44%). Seventy-five percent of patients were 65 years or older, 35% were 75 or older, and 3% were 85 or older. Sixty-five percent of patients were reported to have had one prior anti-cancer therapy for metastatic MCC and 35% had two or more prior therapies. Fifty-three percent of patients had visceral metastases. All patients had tumor samples evaluated for PD-L1 expression; of these, 66% were PD-L1-positive (≥ 1% of tumor cells), 18% were PD-L1 negative, and 16% had non-evaluable results by an investigational immunohistochemistry assay. Archival tumor samples were evaluated for Merkel cell polyomavirus (MCV) using an investigational assay; of the 77 patients with evaluable results, 52% had evidence of MCV.
Efficacy results are presented in Table 9. Responses were observed in patients regardless of tumor PD-L1 expression or presence of MCV.
| Efficacy Endpoints | Results (N=88) |
|---|---|
| CI: Confidence interval. | |
| Overall Response Rate (ORR) | |
| Overall response rate, (95% CI) | 33.0% (23.3%, 43.8%) |
| Complete response (CR) rate, (95% CI) | 11.4% (6.6%, 19.9%) |
| Partial response (PR) rate, (95% CI) | 21.6% (13.5%, 31.7%) |
| Duration of Response (DOR) | N=29 |
| Range in months | 2.8 to 23.3+ |
| Patients with DOR ≥ 6 months, n (%) | 25 (86%) |
| Patients with DOR ≥ 12 months, n (%) | 13 (45%) |
14.2 Locally Advanced or Metastatic Urothelial Carcinoma
First-Line Maintenance Treatment of Urothelial Carcinoma
The efficacy and safety of BAVENCIO was demonstrated in the JAVELIN Bladder 100 trial (NCT02603432), a randomized, multi-center, open-label study conducted in 700 patients with unresectable, locally advanced or metastatic urothelial carcinoma that did not progress with first-line platinum-containing chemotherapy. Patients with autoimmune disease or a medical condition that required immunosuppression were excluded.
Randomization was stratified by best response to chemotherapy (CR/PR vs. stable disease [SD]) and site of metastasis (visceral vs. non-visceral) at the time of initiating first-line chemotherapy. Patients were randomized (1:1) to receive either BAVENCIO 10 mg/kg intravenous infusion every 2 weeks plus best supportive care (BSC) or BSC alone. Treatment was initiated within 4-10 weeks after the last dose of chemotherapy.
Treatment with BAVENCIO continued until RECIST v1.1-defined progression of disease by Blinded Independent Central Review (BICR) assessment or unacceptable toxicity. Administration of BAVENCIO was permitted beyond RECIST-defined disease progression if the patient was clinically stable and was considered to be deriving clinical benefit by the investigator. Assessment of tumor status was performed at baseline, 8 weeks after randomization, then every 8 weeks up to 12 months after randomization, and every 12 weeks thereafter until documented confirmed disease progression based on BICR assessment per RECIST v1.1.
Baseline characteristics were well-balanced between arms. Overall, the median age was 69 years (range: 32 to 90), with 66% of patients ≥ 65 years of age and 24% of patients ≥ 75 years of age. Most patients were male (77%). The majority of patients were White (67%) and 22% were Asian. Baseline ECOG PS was 0 (61%) or 1 (39%).
Fifty-six percent (56%) of patients received prior gemcitabine plus cisplatin, 38% of patients received prior gemcitabine plus carboplatin, and 6% of patients received prior gemcitabine plus cisplatin and gemcitabine plus carboplatin. Best response to first-line chemotherapy was CR or PR (72%) or SD (28%). Sites of metastasis prior to chemotherapy were visceral (55%) or non-visceral (45%). Fifty-one (51%) of patients had PD-L1-positive-tumors, 39% of patients had PD-L1-negative tumors, and 10% of patients had unknown PD-L1 tumor status. Six percent (6%) of patients received another PD-1/PD-L1 checkpoint inhibitor after discontinuation of treatment in the BAVENCIO plus BSC arm and 44% of patients in the BSC arm.
The major efficacy outcome measure was overall survival (OS) in all randomized patients and patients with PD-L1-positive tumors. The results from a pre-specified interim analysis demonstrated a statistically significant improvement in OS for patients randomized to BAVENCIO plus BSC as compared with BSC alone. An updated OS analysis was conducted when 452 deaths were observed. Consistent results were observed across the pre-specified subgroups of CR/PR versus SD to first-line chemotherapy.
| Efficacy Endpoints | BAVENCIO plus BSC | BSC |
|---|---|---|
| (N=350) | (N=350) | |
| BSC: Best supportive care; CI: Confidence interval; OS: overall survival. | ||
|
||
| Primary OS | ||
| Events (%) | 145 (41.4) | 179 (51.1) |
| Median in months | 21.4 | 14.3 |
| (95% CI) | (18.9, 26.1) | (12.9, 17.9) |
| Hazard ratio (95% CI) | 0.69 (0.56, 0.86) | |
| 2-sided p-value* | 0.001 | |
| Updated OS | ||
| Events (%) | 215 (61.4) | 237 (67.7) |
| Median in months | 23.8 | 15.0 |
| (95% CI) | (19.9, 28.8) | (13.5, 18.2) |
| Hazard ratio (95% CI) | 0.76 (0.63, 0.92) | |
Figure 1: K-M Estimates for Updated OS from the JAVELIN Bladder 100 Trial
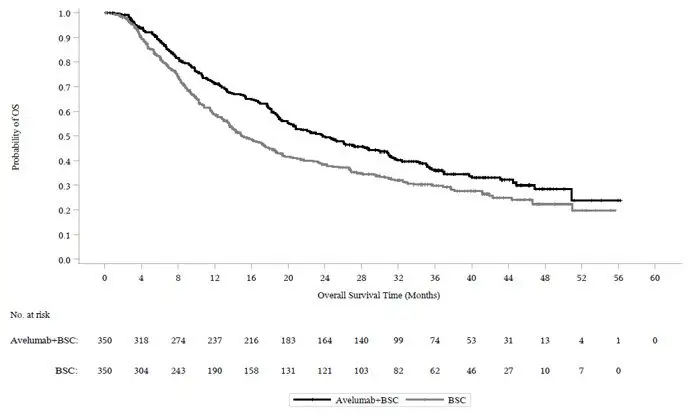
In the pre-specified endpoint of OS among patients with PD-L1-positive tumors (n=358, 51%), the hazard ratio was 0.69 (95% CI: 0.52, 0.90) in the updated OS analysis for patients randomized to BAVENCIO plus BSC versus BSC alone. In an exploratory analysis of patients with PD-L1-negative tumors (n=270, 39%), the updated OS hazard ratio was 0.82 (95% CI: 0.62, 1.09).
Previously-Treated Urothelial Carcinoma
The efficacy and safety of BAVENCIO was demonstrated in the UC cohorts of the JAVELIN Solid Tumor trial, an open-label, single-arm, multi-center study that included 242 patients with locally advanced or metastatic urothelial carcinoma (UC) with disease progression on or after platinum-containing chemotherapy or who had disease progression within 12 months of treatment with a platinum-containing neoadjuvant or adjuvant chemotherapy regimen. Patients with active or history of central nervous system metastasis; other malignancies within the last 5 years; organ transplant; conditions requiring therapeutic immune suppression; or active infection with HIV, hepatitis B, or hepatitis C were excluded. Patients with autoimmune disease, other than type I diabetes, vitiligo, psoriasis, or thyroid disease that did not require immunosuppressive treatment, were excluded. Patients were included regardless of their PD-L1 status.
Patients received BAVENCIO at a dose of 10 mg/kg intravenously every 2 weeks until radiographic or clinical progression or unacceptable toxicity. Tumor response assessments were performed every 6 weeks. Efficacy outcome measures included confirmed overall response rate (ORR), as assessed by an Independent Endpoint Review Committee (IERC) using Response Evaluation Criteria in Solid Tumors (RECIST) v1.1, and duration of response (DOR). Efficacy was evaluated in patients who were followed for a minimum of both 13 weeks and 6 months at the time of data cut-off.
Baseline demographic and disease characteristics for the 226 patients with a minimum of 13 weeks of follow-up were median age 68 years (range: 30 to 89), 72% male, 80% White, and 34% and 66% of patients had an ECOG performance status 0 and 1, respectively. Forty-four percent of patients had non-bladder urothelial carcinoma including 23% of patients with upper tract disease, and 83% of patients had visceral metastases (baseline target and/or non-target lesions present outside of the lymph nodes). Nine (4%) patients had disease progression following prior platinum-containing neoadjuvant or adjuvant therapy only. Forty-seven percent of patients only received prior cisplatin-based regimens, 32% received only prior carboplatin-based regimens, and 20% received both cisplatin and carboplatin-based regimens. At baseline, 17% of patients had a hemoglobin < 10 g/dL and 34% of patients had liver metastases.
Efficacy results are presented in Table 11. The median time to response was 2.0 months (range: 1.3 to 11.0) among patients followed for either ≥ 13 weeks or ≥ 6 months. Using a clinical trial assay to assess PD-L1 staining, with 16% of patients not evaluable, there were no clear differences in response rates based on PD-L1 tumor expression. Among the total 30 responding patients followed for ≥ 13 weeks, 22 patients (73%) had an ongoing response of 6 months or longer and 4 patients (13%) had ongoing responses of 12 months or longer. Among the total 26 responding patients followed for ≥ 6 months, 22 patients (85%) had ongoing responses of 6 months or longer and 4 patients (15%) had ongoing responses of 12 months or longer.
| Efficacy Endpoints | ≥ 13 Weeks Follow-Up (N=226) | ≥ 6 Months Follow-Up (N=161) |
|---|---|---|
| CI: Confidence interval; NE: Not estimable; + denotes a censored value. | ||
| Confirmed Overall Response Rate (ORR) | ||
| Overall Response Rate n (%) | 30 (13.3%) | 26 (16.1%) |
| (95% CI) | (9.1, 18.4) | (10.8, 22.8) |
| Complete Response (CR) n (%) | 9 (4.0%) | 9 (5.6%) |
| Partial Response (PR) n (%) | 21 (9.3%) | 17 (10.6%) |
| Duration of Response (DOR) | ||
| Median, months (range) | NE (1.4+ to 17.4+) | NE (1.4+ to 17.4+) |
14.3 Advanced Renal Cell Carcinoma
The efficacy and safety of BAVENCIO in combination with axitinib was demonstrated in the JAVELIN Renal 101 trial (NCT02684006), a randomized, multicenter, open-label, study of BAVENCIO in combination with axitinib in 886 patients with untreated advanced RCC regardless of tumor PD-L1 expression [intent-to-treat (ITT) population]. Patients with autoimmune disease or conditions requiring systemic immunosuppression were excluded.
Randomization was stratified according to Eastern Cooperative Oncology Group (ECOG) Performance Status (PS) (0 vs. 1) and region (United States vs. Canada/Western Europe vs. the rest of the world). Patients were randomized (1:1) to one of the following treatment arms:
- BAVENCIO 10 mg/kg intravenous infusion every 2 weeks in combination with axitinib 5 mg twice daily orally (N=442). Patients who tolerated axitinib 5 mg twice daily without Grade 2 or greater axitinib-related adverse events for 2 consecutive weeks could increase to 7 mg and then subsequently to 10 mg twice daily. Axitinib could be interrupted or reduced to 3 mg twice daily and subsequently to 2 mg twice daily to manage toxicity.
- Sunitinib 50 mg once daily orally for 4 weeks followed by 2 weeks off (N=444) until radiographic or clinical progression or unacceptable toxicity.
Treatment with BAVENCIO and axitinib continued until RECIST v1.1-defined progression of disease by Blinded Independent Central Review (BICR) assessment or unacceptable toxicity. Administration BAVENCIO and axitinib was permitted beyond RECIST-defined disease progression if the patient was clinically stable and considered to be deriving clinical benefit by the investigator. Assessment of tumor status was performed at baseline, after randomization at 6 weeks, then every 6 weeks thereafter up to 18 months after randomization, and every 12 weeks thereafter until documented confirmed disease progression by BICR.
Baseline characteristics were a median age of 61 years (range: 27 to 88), 38% of patients were 65 years or older, 75% were male, 75% were White, and the ECOG PS was 0 (63%) or 1 (37%), respectively. Patient distribution by International Metastatic Renal Cell Carcinoma Database (IMDC) risk groups was 21% favorable, 62% intermediate, and 16% poor.
The major efficacy outcome measures were progression-free survival (PFS), as assessed by an BICR using RECIST v1.1 and overall survival (OS) in patients with PD-L1-positive tumors using a clinical trial assay (PD-L1 expression level ≥ 1%). Since PFS was statistically significant in patients with PD-L1-positive tumors [HR 0.61 (95% CI: 0.48, 0.79)], it was then tested in the ITT population and a statistically significant improvement in PFS in the ITT population was also demonstrated.
With a median overall survival follow-up of 19 months, overall survival data were immature with 27% deaths in the ITT population.
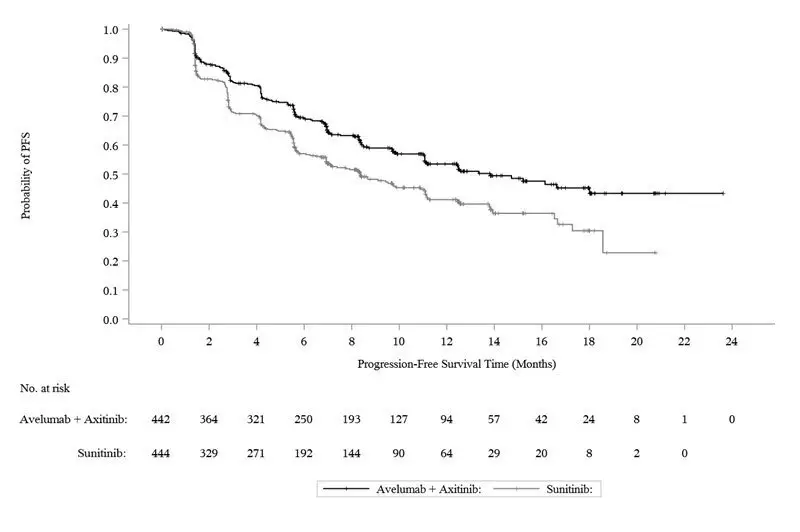
Efficacy results are presented in Table 12 and Figure 2.
| Efficacy Endpoints (Based on BICR Assessment) | BAVENCIO plus Axitinib (N=442) | Sunitinib (N=444) |
|---|---|---|
| BICR: Blinded Independent Central Review; CI: Confidence interval; NE: Not estimable. | ||
|
||
| Progression-Free Survival (PFS) | ||
| Events (%) | 180 (41) | 216 (49) |
| Median in months (95% CI) | 13.8 (11.1, NE) | 8.4 (6.9, 11.1) |
| Hazard ratio (95% CI) | 0.69 (0.56, 0.84) | |
| p-value* | 0.0002 | |
| Confirmed Objective Response Rate (ORR) | ||
| Objective Response Rate n (%) | 227 (51.4) | 114 (25.7) |
| (95% CI) | (46.6, 56.1) | (21.7, 30.0) |
| Complete Response (CR) n (%) | 15 (3.4) | 8 (1.8) |
| Partial Response (PR) n (%) | 212 (48) | 106 (24) |
Figure 2: K-M Estimates for PFS based on BICR Assessment – ITT
16. How is Bavencio supplied
BAVENCIO (avelumab) Injection is a sterile, preservative-free, and clear, colorless to slightly yellow solution for intravenous infusion supplied as a single-dose vial of 200 mg/10 mL (20 mg/mL), individually packed into a carton (NDC 44087-3535-1).
17. Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
| This Medication Guide has been approved by the U.S. Food and Drug Administration. | Revised: November 2020 | |||
| MEDICATION GUIDE
BAVENCIO® (buh-VEN-see-oh) (avelumab) injection |
||||
| What is the most important information I should know about BAVENCIO?
BAVENCIO is a medicine that may treat certain cancers by working with your immune system. BAVENCIO can cause your immune system to attack normal organs and tissues in any area of your body and can affect the way they work. These problems can sometimes become severe or life-threatening and can lead to death. You can have more than one of these problem at the same time. These problems may happen anytime during treatment or even after your treatment has ended. Call or see your healthcare provider right away if you get any new or worsening signs or symptoms, including: Lung problems. |
||||
|
|
|
||
Intestinal problems.
|
||||
|
|
|||
| Hormone gland problems. | ||||
|
|
|||
| Kidney problems. | ||||
|
|
|||
| Skin problems. | ||||
|
|
|||
Problems can also happen in other organs and tissues. These are not all of the signs or symptoms of immune system problems that can happen with BAVENCIO. Call or see your healthcare provider right away for any new or worsening signs or symptoms, which may include:
|
||||
| Infusion-related reactions can sometimes be severe or life-threatening. Signs and symptoms of infusion-related reactions may include: | ||||
|
|
|||
| Complications, including graft-versus-host-disease (GVHD), in people who have received a bone marrow (stem cell) transplant that uses donor stem cells (allogeneic). These complications can be serious and can lead to death. These complications may happen if you underwent transplantation either before or after being treated with BAVENCIO. Your healthcare provider will monitor you for these complications. Heart problems. When BAVENCIO is used with the medicine axitinib, severe heart problems can happen and can lead to death. Signs and symptoms of heart problems may include: |
||||
|
|
|||
| Getting medical treatment right away may help keep these problems from becoming more serious. Your healthcare provider will check you for these problems during your treatment with BAVENCIO. Your healthcare provider may treat you with corticosteroid or hormone replacement medicines. Your healthcare provider may also need to delay or completely stop treatment with BAVENCIO if you have severe side effects. | ||||
| What is BAVENCIO?
BAVENCIO is a prescription medicine used to treat:
|
||||
Before you receive BAVENCIO, tell your healthcare provider about all of your medical conditions, including if you:
|
||||
How will I receive BAVENCIO?
|
||||
| What are the possible side effects of BAVENCIO? BAVENCIO can cause serious side effects, including:
|
||||
|
|
|||
| The most common side effects of BAVENCIO as maintenance treatment in people with UC whose cancer responded or stabilized after platinum-containing chemotherapy as first treatment include: | ||||
|
|
|||
| The most common side effects of BAVENCIO in people with UC after platinum-containing chemotherapy that did not work, or is no longer working, include: | ||||
|
|
|||
| The most common side effects of BAVENCIO when given with axitinib in people with RCC include: | ||||
|
|
|||
| These are not all the possible side effects of BAVENCIO. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
||||
| General information about the safe and effective use of BAVENCIO.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. If you would like more information about BAVENCIO, talk with your healthcare provider. You can ask your pharmacist or healthcare provider for information about BAVENCIO that is written for health professionals. |
||||
| What are the ingredients in BAVENCIO?
Active ingredient: avelumab Inactive ingredients: D-mannitol, glacial acetic acid, polysorbate 20, sodium hydroxide, and Water for Injection Manufactured by: EMD Serono, Inc. One Technology Place, Rockland, MA 02370 USA, U.S. License No. 1773. Marketed by: EMD Serono, Inc. and Pfizer Inc., NY, NY 10017 USA. BAVENCIO is a trademark of Merck KGaA, Darmstadt, Germany. For more information, call toll-free 1-844-826-8371 or go to www.bavencio.com. |
||||
| BAVENCIO
avelumab injection, solution, concentrate |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - EMD Serono, Inc. (088514898) |