Drug Detail:Beser (Fluticasone topical [ floo-tik-a-sone-top-i-kal ])
Drug Class: Topical steroids
Highlights of Prescribing Information
See full prescribing information for BESER (FLUTICASONE PROPIONATE) LOTION.
BESER™ (FLUTICASONE PROPIONATE) lotion, for topical use
Initial U.S. Approval: 1990
Recent Major Changes
| Indications and Usage (1) | 01/2015 |
Indications and Usage for Beser Lotion
Beser Lotion is a corticosteroid indicated for the relief of the inflammatory and pruritic manifestations of atopic dermatitis in patients 3 months of age and older. (1)
Beser Lotion Dosage and Administration
- Apply a thin film to the affected skin areas once daily. Rub in gently. (2)
- Discontinue use when control is achieved. (2)
- Reassess diagnosis if no improvement in 2 weeks. (2)
- The safety and efficacy of Beser Lotion have not been established beyond four weeks of use. (2)
- Avoid use under occlusion or application to diaper area. (2)
- Not for ophthalmic, oral, or intravaginal use. (2)
Dosage Forms and Strengths
- Lotion, 0.05%, supplied in 60 mL bottles. (3)
Contraindications
- None. (4)
Warnings and Precautions
- Hypothalamic-Pituitary-Adrenal (HPA) Axis Suppression: Reversible HPA axis suppression and resulting glucocorticoid insufficiency can occur during or after withdrawal of treatment. Risk factors include the use of high-potency topical corticosteroids, use over large surface area, prolonged use, use under occlusion, concomitant use with other corticosteroid-containing products, altered skin barrier, liver failure, and use in pediatric patients. Minimize risk by mitigating the risk factors and use product as recommended. Modify use if HPA axis suppression is suspected. (5.1, 8.4)
- Skin Irritation and Sensitization: Beser Lotion contains the excipient imidurea which releases formaldehyde as a breakdown product. Formaldehyde may cause allergic sensitization or irritation upon contact with the skin. Discontinue use if irritation or sensitization develops. (5.2)
Adverse Reactions/Side Effects
The most common adverse reactions (2%) were burning/stinging at the application site. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Medimetriks Pharmaceuticals, Inc., at 1-973-882-7512 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 10/2018
Related/similar drugs
prednisone, fluticasone topical, methylprednisolone, Medrol, Dupixent, Medrol Dosepak, Solu-MedrolFull Prescribing Information
1. Indications and Usage for Beser Lotion
Beser Lotion is a corticosteroid indicated for the relief of the inflammatory and pruritic manifestations of atopic dermatitis in patients 3 months of age or older.
2. Beser Lotion Dosage and Administration
Apply a thin film of Beser Lotion to the affected skin areas once daily. Rub in gently.
Discontinue use when control is achieved. If no improvement is seen within 2 weeks, reassessment of the diagnosis may be necessary.
The safety and efficacy of Beser Lotion have not been established beyond 4 weeks of use.
Avoid use with occlusive dressings or application to the diaper area [see Warnings and Precautions (5.1) and (5.2)].
Beser Lotion is for topical use only, and not for ophthalmic, oral, or intravaginal use.
3. Dosage Forms and Strengths
Lotion, 0.05%. Each gram of Beser (fluticasone propionate) Lotion, 0.05% contains 0.5 mg fluticasone propionate in a white to off-white lotion base. Beser (fluticasone propionate) Lotion, 0.05% is supplied in 60 mL bottles.
5. Warnings and Precautions
5.1 Hypothalamic-Pituitary-Adrenal (HPA) Axis Suppression and Other Adverse Endocrine Effects
Topical corticosteroids, including Beser Lotion, can produce reversible HPA axis suppression with the potential for glucocorticoid insufficiency. Risk factors that predispose to HPA axis suppression include the use of high-potency topical corticosteroids, large treatment surface areas, prolonged use, use under occlusion, concomitant use of more than one corticosteroid-containing product, altered skin barrier, and liver failure. Pediatric patients may be at greater risk of HPA axis suppression due to their higher skin surface area to body mass ratios [see Use in Specific Populations (8.4)].
HPA axis suppression may occur during or after withdrawal of treatment. If HPA axis suppression is suspected, gradually withdraw the drug, reduce the frequency of application, or substitute a less potent topical corticosteroid. Evaluation of HPA axis suppression may be done by using the cosyntropin stimulation test.
The effects of fluticasone propionate lotion, 0.05% on HPA axis function in pediatric patients were investigated in two trials. Among a total of 89 evaluable subjects from the two trials who were treated with fluticasone propionate lotion, 0.05% twice daily for 3 to 4 weeks, a single subject with >90% body surface area treated showed laboratory evidence of transient suppression immediately post-treatment. The post cosyntropin stimulation test serum cortisol returned to a normal level (22.1 μg/dL) within one week of the final treatment visit [see Use In Specific Populations (8.4) and Clinical Pharmacology (12.2)].
Cushing's syndrome, hyperglycemia, and unmasking of latent diabetes mellitus can also result from systemic absorption of topical corticosteroids.
Use of more than one corticosteroid-containing product at the same time may increase the total systemic absorption of topical corticosteroids.
5.2 Local Adverse Reactions
Beser Lotion may cause local adverse reactions, including skin atrophy [see Adverse Reactions (6.1, 6.2)]. The risk is greater with use under occlusion and with higher potency products.
Beser Lotion contains the excipient imidurea which releases formaldehyde as a breakdown product. Formaldehyde may cause allergic sensitization or irritation upon contact with the skin. Avoid using Beser Lotion in individuals with hypersensitivity to formaldehyde as it may prevent healing or worsen dermatitis.
If irritation develops, discontinue Beser Lotion and institute appropriate therapy.
6. Adverse Reactions/Side Effects
The following adverse reactions are discussed in greater detail in other sections of the labeling:
- HPA Axis Suppression and Other Adverse Endocrine Effects [see Warnings and Precautions (5.1)]
- Local Adverse Reactions [see Warnings and Precautions (5.2)]
- Concomitant Skin Infections [see Warnings and Precautions (5.3)]
6.1 Clinical Trials Experience: Controlled Clinical Trials
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In 2 multicenter vehicle-controlled clinical trials of once-daily application of fluticasone propionate lotion, 0.05% by 196 adult and 242 pediatric patients, the total incidence of adverse reactions considered drug related by investigators was approximately 4%. These were local cutaneous reactions, usually mild and self-limiting, and consisted primarily of burning/stinging (2%). All other drug-related events occurred with an incidence of less than 1%, and included were contact dermatitis, exacerbation of atopic dermatitis, folliculitis of legs, pruritus, pustules on arm, rash, and skin infection. See Table 1.
The incidence of adverse reactions between the 242 pediatric subjects (age 3 months to < 17 years) and 196 adult subjects (17 years or older) was similar (4% and 5%, respectively).
| Adverse Reactions | Fluticasone Propionate Lotion, 0.05% n=221 | Vehicle n=217 |
|---|---|---|
| Burning/Stinging skin | 4 (2%) | 3 (1%) |
| Contact Dermatitis | 0 | 1 (<1%) |
| Exacerbation of Atopic dermatitis | 0 | 1 (<1%) |
| Folliculitis of legs | 2 (<1%) | 0 |
| Irritant Contact Dermatitis | 0 | 1 (<1%) |
| Pruritus | 1 (<1%) | 1 (<1%) |
| Pustules on Arms | 1 (<1%) | 0 |
| Rash | 1 (<1%) | 2 (<1%) |
| Skin Infection | 0 | 3 (1%) |
During the clinical trials, eczema herpeticum occurred in a 33-year old male patient treated with fluticasone propionate lotion, 0.05%.
Table 2 summarizes all adverse events by body system that occurred in at least 1% of patients in either the drug or vehicle group in the phase 3 controlled clinical trials.
| Body System | Fluticasone Propionate Lotion, 0.05% (N=221) | Vehicle Lotion (N=217) |
|---|---|---|
| Any Adverse Event | 77 (35%) | 82 (38%) |
| Skin | ||
| Burning and Stinging | 4 (2%) | 3 (1%) |
| Pruritus | 3 (1%) | 5 (2%) |
| Rash | 2 (<1%) | 3 (1%) |
| Skin Infection | 0 | 3 (1%) |
| Ear, Nose, Throat | ||
| Common Cold | 9 (4%) | 5 (2%) |
| Ear Infection | 3 (1%) | 3 (1%) |
| Nasal Sinus Infection | 2 (<1%) | 4 (2%) |
| Rhinitis | 1 (<1%) | 3 (1%) |
| Upper Respiratory Tract Infection | 6 (3%) | 7 (3%) |
| Gastrointestinal | ||
| Normal Tooth Eruption | 2 (<1%) | 3 (1%) |
| Diarrhea | 3 (1%) | 0 |
| Vomiting | 3 (1%) | 2 (<1%) |
| Lower Respiratory | ||
| Cough | 7 (3%) | 6 (3%) |
| Influenza | 5 (2%) | 0 |
| Wheeze | 0 | 3 (1%) |
| Neurology | ||
| Headache | 4 (2%) | 5 (2%) |
| Non-Site Specific | ||
| Fever | 8 (4%) | 8 (4%) |
| Seasonal Allergy | 2 (<1%) | 3 (1%) |
6.2 Clinical Trials Experience: Pediatric Open Label Trials
In an open label HPA axis suppression trial of 44 pediatric subjects (ages ≥3 months to ≤6 years) fluticasone propionate lotion, 0.05% was applied twice daily (rather than the indicated dosing regimen of once daily) to at least 35% of body surface area for 3 or 4 weeks. Subjects whose lesions cleared after 2 or 3 weeks of treatment continued to apply fluticasone propionate lotion, 0.05% for an additional week. The overall incidence of adverse reactions was 14%. These were local, cutaneous reactions and included dry skin (7%), stinging at application site (5%), and excoriation (2%). Additionally, a 4-month-old patient treated with fluticasone propionate lotion, 0.05% had marked elevations of the hepatic enzymes AST and ALT. [see Use in Specific Populations (8.4)]
In another open label HPA axis suppression trial in which fluticasone propionate lotion, 0.05% was also applied twice daily (rather than the indicated dosing regimen of once daily), 56 pediatric subjects (ages ≥3 months to 12 months), were enrolled [see Use in Specific Populations (8.4)].
The adverse reactions included 2 cases of Herpes simplex at the application site (3.6%) and 3 cases of bacterial skin infections (5.4%).
6.3 Postmarketing Experience
The following local adverse reactions have been identified during post-approval use of fluticasone propionate lotion, 0.05%: erythema, edema/swelling, and bleeding.
The following systemic adverse reactions have been identified during post-approval use of fluticasone propionate cream and fluticasone propionate ointment: immunosuppression/Pneumocystis jirovecii pneumonia/leukopenia/thrombocytopenia; hyperglycemia/ glycosuria; Cushing syndrome; generalized body edema/blurred vision; and acute urticarial reaction (edema, urticaria, pruritus, and throat swelling).
The following local adverse reactions have also been reported with the use of topical corticosteroids, and they may occur more frequently with the use of occlusive dressings or higher potency corticosteroids. These reactions include: acneiform eruptions, hypopigmentation, perioral dermatitis, skin atrophy, striae, hypertrichosis and miliaria.
Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
8. Use In Specific Populations
8.3 Nursing Mothers
Systemically administered corticosteroids appear in human milk and can suppress growth, interfere with endogenous corticosteroid production, or cause other untoward effects. It is not known whether topical administration of corticosteroids could result in sufficient systemic absorption to produce detectable quantities in human milk. Because many drugs are excreted in human milk, caution should be exercised when Beser Lotion is administered to a nursing woman.
8.4 Pediatric Use
Beser Lotion may be used in pediatric patients as young as 3 months of age. The safety and effectiveness of Beser Lotion in pediatric patients below 3 months of age have not been established.
Because of a higher ratio of skin surface area to body mass, pediatric patients are at a greater risk than adults of systemic effects when treated with topical drugs. They are, therefore, also at greater risk of HPA axis suppression and adrenal insufficiency upon the use of topical corticosteroids [see Warnings and Precautions (5.1)].
In an HPA axis suppression trial, none of the 40 evaluable pediatric subjects, 4 months old to < 6 years old, with moderate to severe atopic dermatitis covering ≥ 35% Body Surface Area (BSA) who were treated with an exaggerated dosing regimen (twice daily) of fluticasone propionate lotion, 0.05% experienced adrenal suppression (defined as a 30-minute post-stimulation cortisol level ≤18 micrograms/dL) [see Warnings and Precautions (5.1) and Clinical Pharmacology (12.2)].
In another HPA axis suppression trial, one of 49 (2%) evaluable pediatric subjects, 3 months to 11 months old, with moderate to severe atopic dermatitis covering ≥ 35% Body Surface Area (BSA) who applied an exaggerated dosing regimen (twice daily) of fluticasone propionate lotion, 0.05% experienced reversible adrenal suppression (defined as a 30-minute post-stimulation cortisol level ≤18 micrograms/dL) following 4 weeks of therapy [see Warnings and Precautions (5.1) and Clinical Pharmacology (12.2)].
Systemic effects such as Cushing's syndrome, linear growth retardation, delayed weight gain, and intracranial hypertension have been reported in pediatric patients, especially those with prolonged exposure to large doses of high-potency topical corticosteroids, or concomitant use of more than one corticosteroid product.
Local adverse reactions including skin atrophy have also been reported with use of topical corticosteroids in pediatric patients.
Parents of pediatric patients should be advised not to use this medication in the treatment of diaper dermatitis unless directed by a physician. Beser Lotion should not be applied in the diaper areas as diapers or plastic pants may constitute occlusive dressing.
8.5 Geriatric Use
A limited number of patients above 65 years of age have been treated with fluticasone propionate lotion, 0.05% in US and non-US clinical trials. Specifically only 8 patients above 65 years of age were treated with fluticasone propionate lotion, 0.05% in controlled clinical trials. The number of patients is too small to permit separate analyses of efficacy and safety.
11. Beser Lotion Description
Beser (fluticasone propionate) Lotion, 0.05% contains fluticasone propionate [S-Fluoromethyl 6α, 9α-difluoro-11β-hydroxy-16α-methyl-3-oxo-17α-propionyloxyandrosta-1,4-diene-17β-carbothioate], a synthetic fluorinated corticosteroid, for topical use. The topical corticosteroids constitute a class of primarily synthetic steroids used as anti-inflammatory and antipruritic agents.
Chemically, fluticasone propionate is C25H31F3O5S. It has the following structural formula:
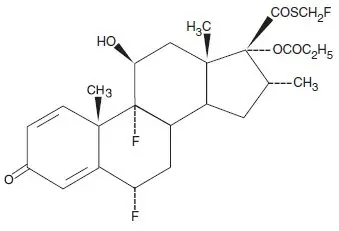
Fluticasone propionate has a molecular weight of 500.6. It is a white to off-white powder and is practically insoluble in water, freely soluble in dimethyl sulfoxide and dimethylformamide, and slightly soluble in methanol and 95% ethanol.
Each gram of Beser Lotion contains 0.5 mg fluticasone propionate in a white to off white lotion base of anhydrous citric acid, cetomacrogol 1000, cetostearyl alcohol, dimethicone 360, imidurea, isopropyl myristate, methylparaben, mineral oil, propylene glycol, propylparaben, purified water, and sodium citrate.
12. Beser Lotion - Clinical Pharmacology
12.1 Mechanism of Action
Corticosteroids play a role in cellular signaling, immune function, inflammation, and protein regulation; however, the precise mechanism of action of Beser Lotion in atopic dermatitis is unknown.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
In an oral (gavage) mouse carcinogenicity study, doses of 0.1, 0.3 and 1 mg/kg/day fluticasone propionate were administered to mice for 18 months. Fluticasone propionate demonstrated no tumorigenic potential at oral doses up to 1 mg/kg/day (less than the MRHD in adults based on body surface area comparisons) in this study.
In a dermal mouse carcinogenicity study, 0.05% fluticasone propionate ointment (40 μl) was topically administered for 1, 3 or 7 days/week for 80 weeks. Fluticasone propionate demonstrated no tumorigenic potential at dermal doses up to 6.7 μg/kg/day (less than the MRHD in adults based on body surface area comparisons) in this study.
Fluticasone propionate revealed no evidence of mutagenic or clastogenic potential based on the results of five in vitro genotoxicity tests (Ames assay, E. coli fluctuation test, S. cerevisiae gene conversion test, Chinese hamster ovary cell chromosome aberration assay and human lymphocyte chromosome aberration assay) and one in vivo genotoxicity test (mouse micronucleus assay).
No evidence of impairment of fertility or effect on mating performance was observed in a fertility and general reproductive performance study conducted in male and female rats at subcutaneous doses up to 50 μg/kg/day (less than the MRHD in adults based on body surface area comparisons).
14. Clinical Studies
Fluticasone propionate lotion, 0.05% applied once daily was superior to vehicle in the treatment of atopic dermatitis in two clinical trials. The two trials enrolled 438 subjects with atopic dermatitis aged 3 months and older, of which 169 subjects were selected as having clinically significant signs of erythema, infiltration/ papulation, and erosion/oozing/crusting at baseline. Clinically significant was defined as having moderate or severe involvement for at least two of the three signs (erythema, infiltrations/papulation, or erosion/oozing/crusting), in at least 2 body regions. Subjects who had moderate to severe disease in a single body region were excluded from the analysis.
Table 3 presents the percentage of subjects who completely cleared of erythema, infiltration/papulation and erosion/oozing/crusting at Week 4 out of those subjects with clinically significant baseline signs.
| Fluticasone Propionate Lotion, 0.05% | Vehicle | |
|---|---|---|
| Study 1 | 9/45 (20%) | 0/37 (0%) |
| Study 2 | 7/44 (16%) | 1/43 (2%) |
16. How is Beser Lotion supplied
Beser (fluticasone propionate) Lotion, 0.05% is white to off-white in color, and supplied as follows:
60 mL bottle - NDC 43538-970-60
| BESER
fluticasone propionate lotion |
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| Labeler - Medimetriks Pharmaceuticals, Inc. (019903816) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Perrigo, Inc. | 600093611 | MANUFACTURE(43538-970) , PACK(43538-970) , LABEL(43538-970) , ANALYSIS(43538-970) | |





