Drug Detail:Betadine ophthalmic solution (Povidone iodine ophthalmic [ po-vi-done-eye-oh-dine ])
Drug Class: Ophthalmic anti-infectives
DESCRIPTION: Povidone-Iodine is a broad-spectrum microbicide with the chemical formulas: 2-pyrrolidinone, 1- ethenyl-, homopolymer, compound with iodine; 1-vinyl-2-pyrrolidinone polymer, compound with iodine.
The structural formula is as follows:
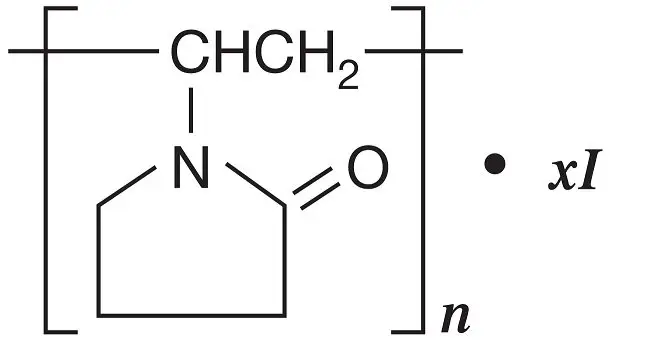
BETADINE* 5% Sterile Ophthalmic Prep Solution contains 5% povidone-iodine (0.5% available iodine) as a sterile dark brown solution stabilized by glycerin. Inactive Ingredients: purified water, citric acid, glycerin, nonoxynol-9, sodium chloride, sodium hydroxide, and dibasic sodium phosphate.
CLINICAL PHARMACOLOGY: A placebo-controlled study in 38 normal volunteers yielded data for 36 subjects who showed a mean log10 reduction of 3.05 log10 units in total aerobes at 10 minutes following prepping the skin with BETADINE* 5% Sterile Ophthalmic Prep Solution compared with reduction of 1.58 log10 units after prepping with vehicle free of the iodine complex. This placebo-controlled study indicates a mean log10 reduction by the iodine complex compared with the control solution of 1.47 log10 units at 10 minutes and 1.79 log10 units at 45 minutes. The base-line mean aerobic bacterial count was 7,586 organisms/cm2.
INDICATIONS AND USAGE: BETADINE* 5% Sterile Ophthalmic Prep Solution for the eye is indicated for prepping of the periocular region (lids, brow, and cheek) and irrigation of the ocular surface (cornea, conjunctiva, and palpebral fornices).
CONTRAINDICATIONS: Do not use on individuals known to be sensitive to iodine, or other components of this product.
Precautions
General: No studies are available in patients with thyroid disorders; therefore, caution is advised in using BETADINE* 5% Sterile Ophthalmic Prep Solution in these patients due to the possibility of iodine absorption.
Carcinogenesis, Mutagenesis, Impairment of Fertility: No long term studies in animals have been performed to evaluate the carcinogenic or mutagenic potential of povidone-iodine. One report of the mutagenic potential of povidone-iodine indicated that it was positive in a modification of the Ames S. typhimurium model, but these results could not be reproduced by another researcher. Another test using mouse lymphoma and Balb/3T3 cells showed that povidone-iodine has no significant mutagenic or transformation capabilities. Other data indicated that it does not produce mutagenic effects in mice or hamsters according to the dominant lethal test, micronucleus test, and chromosome analysis.
Pregnancy: Animal reproduction studies have not been conducted with BETADINE* 5% Sterile Ophthalmic Prep Solution. It is also not known whether BETADINE* 5% Sterile Ophthalmic Prep Solution can cause fetal harm when administered to a pregnant woman or can affect reproductive capacity. BETADINE* 5% Sterile Ophthalmic Prep Solution should only be used on a pregnant woman if clearly needed.
Geriatric Use: No overall differences in safety or effectiveness have been observed between elderly and younger patients.
ADVERSE REACTIONS: Local sensitivity has been exhibited by some individuals to povidone-iodine ophthalmic solution.
DOSAGE AND ADMINISTRATION: While the inner surface and contents of the immediate container (i.e. bottle) are sterile, the outer surface of the bottle is not sterile. The use of the bottle in a sterile field should be avoided. BETADINE* 5% Sterile Ophthalmic Prep Solution is used as follows:
BETADINE* 5% Sterile Ophthalmic Prep Solution is used as follows:
- Make sure container is intact before use. To open, COMPLETELY TWIST OFF TAB, do not pull off.
- Gently pour entire contents of bottle into a sterile prep cup. Saturate sterile cotton-tipped applicator to prep lashes and lid margins using one or more applicators per lid; repeat once.
- Saturate sterile prep sponge or other suitable material to prep lids, brow and cheek in a circular ever-expanding fashion until the entire field is covered; repeat prep three (3) times.
- While separating the lids, irrigate the cornea, conjunctiva and palpebral fornices with BETADINE* 5% Sterile Ophthalmic Prep Solution using a sterile bulb syringe.
- After the BETADINE* 5% Sterile Ophthalmic Prep Solution has been left in contact for two minutes, sterile saline solution in a bulb syringe should be used to flush the residual prep solution from the cornea, conjunctiva, and the palpebral fornices.
HOW SUPPLIED: BETADINE* 5% Sterile Ophthalmic Prep Solution is packaged under sterile conditions, and supplied in 1 fl oz (30 mL) form sealed blue HDPE bottles. Twenty-four (24) bottles are packed in each shipper.
NDC 0065-0411-30
Store at 15°C to 25°C (59°F-77°F).
Rx Only
Single-use only
@2021 Alcon Inc.
Alcon
Manufactured for:
Alcon Laboratories, Inc.
6201 South Freeway
Fort Worth, TX 76134
Manufactured by:
Woodstock Sterile Solutions, Inc.
Woodstock, IL 60098
*BETADINE is a registered trademark of Purdue Products L.P.
STW-AC6024-642R01
Principal Display Panel
NDC 0065-0411-30
Betadine* 5%
Sterile Ophthalmic Prep Solution
(povidone-iodine
ophthalmic solution)
For Pre-Operative Prep and
Irrigation of the Ocular and
Periocular Surfaces
Flush eye thoroughly with
sterile saline solution after
each use.
Rx Only
1 Fl. Oz. (30 mL)
Alcon
Indications:
For prepping of the periocular
region (lids, brow, and cheek)
and irrigation of the ocular
surface (cornea, conjunctiva,
and palpebral fornices).
Contraindications:
Do not use on individuals
known to be sensitive to iodine
or other components of this
product.
Warning:
For external use only.
NOT for intraocular injection or
irrigation.
Read package insert for full use
and directions and precautions.
Storage:
15°- 25°C (59°- 77°F).
Active Ingredient: povidone-iodine 5%
(0.5% available iodine).
Inactive Ingredients:
purified water, citric acid, dibasic sodium
phosphate, glycerin, nonoxynol-9, sodium
chloride, sodium hydroxide.
Single use only.
Manufactured for:
Alcon Laboratories, Inc.
Fort Worth, Texas 76134
Manufactured by:
Woodstock Sterile Solutions
Woodstock, IL 60098
*BETADINE Reg. TM of Purdue Products L.P.
Alcon
Alcon Laboratories, Inc.
6201 South Freeway
Fort Worth, Texas 76134
9008803-0515
NDC 0065-0411-30
Betadine* 5%
Sterile Ophthalmic Prep Solution
(povidone-iodine ophthalmic solution)
For Pre-Operative Prep and Irrigation of the Ocular and Periocular Surfaces
Flush eye thoroughly with sterile saline solution after each use.
Rx Only
1 Fl. Oz. (30 mL)
Alcon®
a Novartis company
Indications:
For prepping of the periocular region (lids, brow, and cheek) and irrigation of the ocular surface (cornea, conjunctiva, and palpebral fornices).
Contraindications:
Do not use on individuals known to be sensitive to iodine or other components of this product.
Warning:
For external use only.
NOT for intraocular injection or irrigation.
Read package insert for full use and directions and precautions.
Storage:
15°- 25°C (59°- 77°F).
©2014 Novartis
Active Ingredient: povidone-iodine 5% (0.5% available iodine).
Inactive Ingredients: purified water, citric acid, dibasic sodium phosphate, glycerin, nonoxynol-9, sodium chloride, sodium hydroxide.
Single use only.
Manufactured for:
Alcon Laboratories, Inc.
Fort Worth, Texas 76134
Manufactured by:
Catalent Pharma Solutions, LLC
Woodstock, IL 60098
*BETADINE Reg. TM of Purdue Products L.P.
Alcon®
Alcon Laboratories, Inc.
6201 South Freeway
Fort Worth, Texas 76134
9008803-0514
Rx only NDC 0065-0411-30
Betadine*
5% Sterile
Ophthalmic Prep Solution
(povidone-iodine
ophthalmic solution)
(0.5% available iodine)
1 fl. oz. (30mL)
SINGLE USE ONLY
See package insert.
Alcon
Mfd. for
Alcon Laboratories, Inc.
Fort Worth, Texas 76134 USA
*BETADINE Reg. TM of Purdue Products L.P.
H12639-0515

Rx only NDC 0065-0411-30
Betadine®
5% Sterile
Ophthalmic Prep Solution
(povidone-iodine
ophthalmic solution)
(0.5% available iodine)
1 fl. oz. (30mL)
SINGLE USE ONLY
See package insert.
Alcon
Mfd. for
Alcon Laboratories, Inc.
Fort Worth, Texas 76134 USA
H12639-0514

| BETADINE
povidone-iodine solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Alcon Laboratories, Inc. (008018525) |
| Registrant - Alcon Laboratories, Inc. (008018525) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Woodstock Sterile Solutions, Inc. | 117895702 | manufacture(0065-0411) | |




