Drug Detail:Breyanzi (Lisocabtagene maraleucel)
Drug Class: Miscellaneous antineoplastics
Highlights of Prescribing Information
BREYANZI® (lisocabtagene maraleucel) suspension for intravenous infusion
Initial U.S. Approval: 2021
WARNING: CYTOKINE RELEASE SYNDROME and NEUROLOGIC TOXICITIES
See full prescribing information for complete boxed warning.
- •
- Cytokine Release Syndrome (CRS), including fatal or life-threatening reactions, occurred in patients receiving BREYANZI. Do not administer BREYANZI to patients with active infection or inflammatory disorders. Treat severe or life-threatening CRS with tocilizumab with or without corticosteroids (2.2, 2.3, 5.1).
- •
- Neurologic toxicities, including fatal or life-threatening reactions, occurred in patients receiving BREYANZI, including concurrently with CRS, after CRS resolution, or in the absence of CRS. Monitor for neurologic events after treatment with BREYANZI. Provide supportive care and/or corticosteroids as needed (2.2, 2.3, 5.2).
- •
- BREYANZI is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS) called the BREYANZI REMS (5.3).
Recent Major Changes
Indications and Usage (1), LBCL after one line of therapy 6/2022
Dosage and Administration (2.1) 6/2022
Warnings and Precautions (5.1, 5.2, 5.5, 5.6, 5.9) 6/2022
Indications and Usage for Breyanzi
BREYANZI is a CD19-directed genetically modified autologous T cell immunotherapy indicated for the treatment of adult patients with large B-cell lymphoma (LBCL), including diffuse large B-cell lymphoma (DLBCL) not otherwise specified (including DLBCL arising from indolent lymphoma), high-grade B-cell lymphoma, primary mediastinal large B-cell lymphoma, and follicular lymphoma grade 3B, who have:
- •
- refractory disease to first-line chemoimmunotherapy or relapse within 12 months of first-line chemoimmunotherapy (1); or
- •
- refractory disease to first-line chemoimmunotherapy or relapse after first-line chemoimmunotherapy and are not eligible for hematopoietic stem cell transplantation (HSCT) due to comorbidities or age (1); or
- •
- relapsed or refractory disease after two or more lines of systemic therapy (1).
Limitations of Use: BREYANZI is not indicated for the treatment of patients with primary central nervous system lymphoma (1, 14).
Breyanzi Dosage and Administration
For autologous use only. For intravenous use only.
- •
- Do NOT use a leukodepleting filter (2.2).
- •
- Administer a lymphodepleting regimen of fludarabine and cyclophosphamide before infusion of BREYANZI (2.2).
- •
- Verify the patient’s identity prior to infusion (2.2).
- •
- Premedicate with acetaminophen and an H1 antihistamine (2.2).
- •
- Confirm availability of tocilizumab prior to infusion (2.2, 5.1).
- •
- Dosing of BREYANZI is based on the number of chimeric antigen receptor (CAR)-positive viable T cells (2.1).
- •
- For LBCL after one line of therapy, the dose is 90 to 110 × 106 CAR-positive viable T cells (2.1).
- •
- For LBCL after two or more lines of therapy, the dose is 50 to 110 × 106 CAR-positive viable T cells (2.1).
- •
- Administer BREYANZI in a REMS-certified healthcare facility (2.2, 5.1, 5.2, 5.3).
Dosage Forms and Strengths
- •
- BREYANZI is a cell suspension for infusion (3).
- •
- A single dose of BREYANZI consists of 1:1 CAR-positive viable T cells of the CD8 and CD4 components, with each component supplied separately in one to four single-dose 5 mL vials (3). Each mL contains ≥ 1.5 × 106 to 70 × 106 CAR-positive viable T cells (3).
Contraindications
None (4).
Warnings and Precautions
- •
- Hypersensitivity Reactions: Monitor for hypersensitivity reactions during infusion (5.4).
- •
- Serious Infections: Monitor patients for signs and symptoms of infection; treat appropriately (5.5).
- •
- Prolonged Cytopenias: Patients may exhibit Grade 3 or higher cytopenias for several weeks following BREYANZI infusion. Monitor complete blood counts (5.6).
- •
- Hypogammaglobulinemia: Monitor and consider immunoglobulin replacement therapy (5.7).
- •
- Secondary Malignancies: In the event that a secondary malignancy occurs after treatment with BREYANZI, contact Bristol-Myers Squibb at 1-888-805-4555 (5.8).
- •
- Effects on Ability to Drive and Use Machines: Advise patients to refrain from driving and engaging in hazardous occupations or activities, such as operating heavy or potentially dangerous machinery for at least 8 weeks after BREYANZI administration (5.9).
Adverse Reactions/Side Effects
The most common nonlaboratory adverse reactions (incidence ≥ 30%) are fever, cytokine release syndrome, fatigue, musculoskeletal pain, and nausea. The most common Grade 3-4 laboratory abnormalities (≥ 30%) include lymphocyte count decrease, neutrophil count decrease, platelet count decrease, and hemoglobin decrease (6.1).
To report SUSPECTED ADVERSE REACTIONS, contact Bristol-Myers Squibb at 1-800-721-5072 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 6/2023
Full Prescribing Information
WARNING: CYTOKINE RELEASE SYNDROME and NEUROLOGIC TOXICITIES
- •
- Cytokine Release Syndrome (CRS), including fatal or life-threatening reactions, occurred in patients receiving BREYANZI. Do not administer BREYANZI to patients with active infection or inflammatory disorders. Treat severe or life-threatening CRS with tocilizumab with or without corticosteroids [see Dosage and Administration (2.2, 2.3) and Warnings and Precautions (5.1)].
- •
- Neurologic toxicities, including fatal or life-threatening reactions, occurred in patients receiving BREYANZI, including concurrently with CRS, after CRS resolution, or in the absence of CRS. Monitor for neurologic events after treatment with BREYANZI. Provide supportive care and/or corticosteroids as needed [see Dosage and Administration (2.2, 2.3) and Warnings and Precautions (5.2)].
- •
- BREYANZI is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS) called the BREYANZI REMS [see Warnings and Precautions (5.3)].
1. Indications and Usage for Breyanzi
BREYANZI is a CD19-directed genetically modified autologous T cell immunotherapy indicated for the treatment of adult patients with large B-cell lymphoma (LBCL), including diffuse large B-cell lymphoma (DLBCL) not otherwise specified (including DLBCL arising from indolent lymphoma), high-grade B-cell lymphoma, primary mediastinal large B-cell lymphoma, and follicular lymphoma grade 3B who have:
- •
- refractory disease to first-line chemoimmunotherapy or relapse within 12 months of first-line chemoimmunotherapy; or
- •
- refractory disease to first-line chemoimmunotherapy or relapse after first-line chemoimmunotherapy and are not eligible for hematopoietic stem cell transplantation (HSCT) due to comorbidities or age; or
- •
- relapsed or refractory disease after two or more lines of systemic therapy.
2. Breyanzi Dosage and Administration
For autologous use only. For intravenous use only.
2.1 Dose
See the respective Certificate of Release for Infusion (RFI Certificate) for each component, for the actual cell counts and volumes to be infused [see Dosage and Administration (2.2) and Dosage Forms and Strengths (3)].
Relapsed or Refractory LBCL After One Line of Therapy
A single dose of BREYANZI contains 90 to 110 × 106 CAR-positive viable T cells (consisting of 1:1 CAR-positive viable T cells of the CD8 and CD4 components), with each component supplied separately in one to four single-dose vials.
Relapsed or Refractory LBCL After Two or More Lines of Therapy
A single dose of BREYANZI contains 50 to 110 × 106 CAR-positive viable T cells (consisting of 1:1 CAR-positive viable T cells of the CD8 and CD4 components), with each component supplied separately in one to four single-dose vials.
2.2 Administration
BREYANZI is for autologous use only. The patient’s identity must match the patient identifiers on the BREYANZI cartons, vials and syringe labels. Do not infuse BREYANZI if the information on the patient-specific labels does not match the intended patient.
Preparing the Patient for BREYANZI
Confirm the availability of BREYANZI before starting lymphodepleting chemotherapy.
Pretreatment
Administer the lymphodepleting chemotherapy regimen before infusion of BREYANZI: fludarabine 30 mg/m2/day intravenously (IV), and cyclophosphamide 300 mg/m2/day IV for 3 days. See the prescribing information for fludarabine and cyclophosphamide for information on dose adjustment in renal impairment.
Infuse BREYANZI 2 to 7 days after completion of lymphodepleting chemotherapy.
Delay the infusion of BREYANZI if the patient has unresolved serious adverse events from preceding chemotherapies, active uncontrolled infection, or active graft-versus-host disease (GVHD).
Premedication
To minimize the risk of infusion reactions, premedicate the patient with acetaminophen (650 mg orally) and diphenhydramine (25-50 mg, IV or orally), or another H1-antihistamine, 30 to 60 minutes prior to treatment with BREYANZI.
Avoid prophylactic use of systemic corticosteroids, as they may interfere with the activity of BREYANZI.
Receipt of BREYANZI
- •
- BREYANZI is shipped directly to the cell-associated lab or clinical pharmacy associated with the infusion center in the vapor phase of a liquid nitrogen shipper.
- •
- Confirm the patient’s identity with the patient identifiers on the shipper.
- •
- If the patient is not expected to be ready for administration before the shipper expires and the infusion site is qualified for onsite storage, transfer BREYANZI to onsite vapor phase of liquid nitrogen storage prior to preparation.
- •
- If the patient is not expected to be ready for administration before the shipper expires and the infusion site is not qualified for onsite storage, contact Bristol-Myers Squibb at 1-888-805-4555 to arrange for return shipment.
Preparing BREYANZI
Before thawing the vials
- •
- Confirm the patient’s identity with the patient identifiers on the RFI Certificate.
- •
- Read the RFI Certificate (affixed inside the shipper) for information on the number of syringes you will need to administer the CD8 and CD4 components (syringe labels are provided with the RFI Certificate). There is a separate RFI Certificate for each cell component.
- •
- Confirm tocilizumab and emergency equipment are available prior to infusion and during the recovery period.
- •
- Confirm the infusion time in advance and adjust the start time of BREYANZI thaw such that it will be available for infusion when the patient is ready.
Thawing the vials
- 1.
- Confirm the patient’s identity with the patient identifiers on the outer carton and on the syringe labels.
Once the vials of CAR-positive viable T cells (CD8 component and CD4 component) are removed from frozen storage, the thaw must be carried to completion and the cells administered within 2 hours. - 2.
- Remove the CD8 component carton and CD4 component carton from the outer carton.
- 3.
- Confirm the patient’s identity with the patient identifiers on the inner carton.
- 4.
- Open each inner carton and visually inspect the vial(s) for damage. If the vials are damaged, contact Bristol-Myers Squibb at 1-888-805-4555.
- 5.
- Confirm the patient’s identity with the patient identifiers on the vials.
- 6.
- Carefully remove the vials from the cartons, place vials on a protective barrier pad, and thaw at room temperature until there is no visible ice in the vials. Thaw all of the vials at the same time.
Keep the CD8 and CD4 components separate.
Dose preparation
- •
- Prepare BREYANZI using sterile technique.
- •
- Based on the concentration of CAR-positive viable T cells for each component, more than one vial of each of the CD8 and CD4 components may be required to complete a dose. A separate syringe should be prepared for each CD8 or CD4 component vial received.
Note: The volume to be drawn up and infused may differ for each component as indicated on the RFI Certificate. Do NOT draw up excess volume into the syringe. - •
- Each vial contains 5 mL with a total extractable volume of 4.6 mL of CD8 or CD4 component T cells. The RFI Certificate for each component indicates the volume (mL) of cells to be drawn up into each syringe. Use the smallest Luer-lock tip syringe necessary (1, 3, or 5 mL) to draw up the specified volume from each vial. A 5 mL syringe should not be used for volumes less than 3 mL.
- 7.
-
Prepare the syringe(s) of the CD8 component first. Affix the CD8 syringe labels to the syringe(s) prior to pulling the required volume into the syringe(s).
Note: It is important to confirm that the volume drawn up for each component matches the volume specified in the respective RFI Certificate. Do NOT draw up excess volume into the syringe.
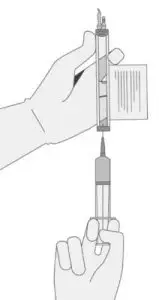
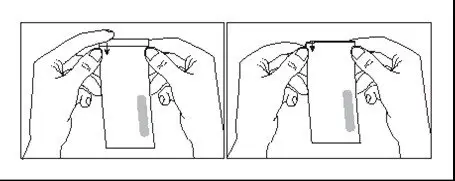
Withdrawal of the required volume of cells from each vial into a separate syringe should be carried out using the following instructions:
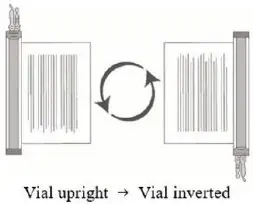
- 8.
- Hold the thawed vial(s) upright and gently invert the vial(s) 5 times to mix the cell product. If any clumping is apparent, continue to invert the vial(s) until clumps have dispersed and cells appear to be evenly resuspended.
- 9.
- Visually inspect the thawed vial(s) for damage or leaks. Do not use if the vial is damaged or if the clumps do not disperse; contact Bristol-Myers Squibb at 1-888-805-4555. The liquid in the vials should be slightly opaque to opaque, colorless to yellow or brownish-yellow.
- 10.
- Remove the polyaluminum cover (if present) from the bottom of the vial and swab the septum with an alcohol wipe. Allow to air dry before proceeding.
NOTE: The absence of the polyaluminum cover does not impact the sterility of the vial.
- 11.
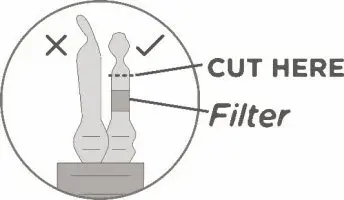
- Keeping the vial(s) upright, cut the seal on the tubing line on the top of the vial immediately above the filter to open the air vent on the vial.
NOTE: Be careful to select the correct tubing line with the filter. Cut ONLY the tubing with a filter.
- 12.
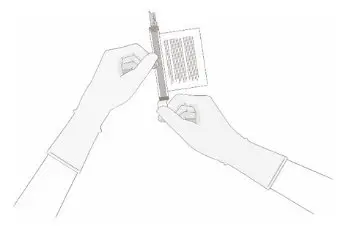
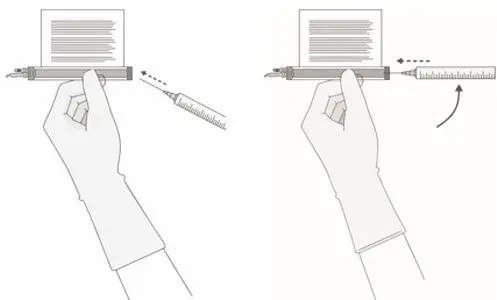
- Hold a 20-gauge, 1-1 ½ inch needle, with the opening of the needle tip away from the retrieval port septum.
- a.
- Insert the needle into the septum at a 45°- 60° angle to puncture the retrieval port septum.
- b.
- Increase the angle of the needle gradually as the needle enters the vial.
- 13.
- WITHOUT drawing air into the syringe, slowly withdraw the target volume (as specified in the RFI Certificate). Carefully inspect the syringe for signs of debris prior to proceeding. If there is debris, contact Bristol-Myers Squibb at 1-888-805-4555.
- 14.
- Verify that the volume of CD8/CD4 component matches the volume specified for the relevant component in the RFI Certificate.
Once the volume is verified, remove the syringe/needle from the vial, carefully detach the needle from the syringe and cap the syringe.
- 15.
- Continue to keep the vial horizontal and return it to the carton to avoid leaking from the vial.
- 16.
- Dispose of any unused portion of BREYANZI (according to local biosafety guidelines).
- 17.
- Repeat the process steps 7-16 for the CD4 Component.
- 18.
- Transport the labeled CD8 and CD4 syringes to the bedside by placing with protective barrier pad inside an insulated room temperature container.
BREYANZI Administration
- •
- Do NOT use a leukodepleting filter.
- •
- Ensure tocilizumab and emergency equipment are available prior to infusion and during the recovery period.
- •
- Confirm the patient’s identity matches the patient identifiers on the syringe label.
- •
- Once BREYANZI has been drawn into syringes, proceed with administration as soon as possible. The total time from removal from frozen storage to patient administration should not exceed 2 hours as indicated by the time entered on the syringe label.
- 1.
- Use intravenous normal saline to flush all the infusion tubing prior to and after each CD8 or CD4 component administration.
- 2.
- Administer the entire volume of the CD8 component intravenously at an infusion rate of approximately 0.5 mL/minute, using the closest port or Y-arm.
NOTE: The time for infusion will vary but will usually be less than 15 minutes for each component. - 3.
- If more than one syringe is required for a full cell dose of the CD8 component, administer the volume in each syringe consecutively without any time between administering the contents of the syringes (unless there is a clinical reason (e.g., infusion reaction) to hold the dose).
- 4.
- After the CD8 component has been administered, flush the tubing with normal saline, using enough volume to clear the tubing and the length of the IV catheter.
- 5.
- Administer the CD4 component second, immediately after administration of the CD8 component is complete, using steps 1-4, as described for the CD8 component. Following administration of the CD4 component, flush the tubing with normal saline, using enough volume to clear the tubing and the length of the IV catheter.
BREYANZI contains human blood cells that are genetically modified with replication-incompetent, self-inactivating lentiviral vector. Follow universal precautions and local biosafety guidelines applicable for the handling and disposal, to avoid potential transmission of infectious diseases.
Monitoring
- •
- Administer BREYANZI at a REMS-certified healthcare facility.
- •
- Monitor patients daily for at least 7 days following BREYANZI infusion at a REMS-certified healthcare facility for signs and symptoms of CRS and neurologic toxicities.
- •
- Instruct patients to remain within proximity of the certified healthcare facility for at least 4 weeks following infusion.
- •
- Instruct patients to refrain from driving or hazardous activities for at least 8 weeks following infusion.
2.3 Management of Severe Adverse Reactions
Cytokine Release Syndrome
Identify cytokine release syndrome (CRS) based on clinical presentation [see Warnings and Precautions (5.1)]. Evaluate for and treat other causes of fever, hypoxia, and hypotension. If CRS is suspected, manage according to the recommendations in Table 1. Patients who experience Grade 2 or higher CRS (e.g., hypotension not responsive to fluids, or hypoxia requiring supplemental oxygenation) should be monitored with continuous cardiac telemetry and pulse oximetry. For patients experiencing severe CRS, consider performing an echocardiogram to assess cardiac function. For severe or life-threatening CRS, consider intensive-care supportive therapy.
If concurrent neurologic toxicity is suspected during CRS, administer:
- •
- Corticosteroids according to the more aggressive intervention based on the CRS and neurologic toxicity grades in Tables 1 and 2
- •
- Tocilizumab according to the CRS grade in Table 1
- •
- Antiseizure medication according to the neurologic toxicity in Table 2
| CRS Gradea | Tocilizumab | Corticosteroidsb |
|---|---|---|
| a Lee criteria for grading CRS (Lee et al, 2014). | ||
| b If corticosteroids are initiated, continue corticosteroids for at least 3 doses or until complete resolution of symptoms, and consider corticosteroid taper. | ||
|
Grade 1
|
If less than 72 hours after infusion, consider tocilizumab 8 mg/kg IV over 1 hour (not to exceed 800 mg). |
If less than 72 hours after infusion, consider dexamethasone 10 mg IV every 24 hours. |
|
Grade 2
|
Administer tocilizumab 8 mg/kg IV over 1 hour (not to exceed 800 mg). |
If less than 72 hours after infusion, administer dexamethasone 10 mg IV every 12-24 hours. |
|
If no improvement within 24 hours or rapid progression, repeat tocilizumab and escalate dose and frequency of dexamethasone (10-20 mg IV every 6 to 12 hours). |
||
|
Grade 3 |
Per Grade 2. |
Administer dexamethasone 10 mg IV every 12 hours. |
|
Symptoms require and respond to aggressive intervention. |
If no improvement within 24 hours or rapid progression of CRS, repeat tocilizumab and escalate dose and frequency of dexamethasone (10-20 mg IV every 6 to 12 hours). |
|
|
Grade 4
|
Per Grade 2. |
Administer dexamethasone 20 mg IV every 6 hours. |
|
If no improvement within 24 hours or rapid progression of CRS, escalate tocilizumab and corticosteroid use. If no improvement or continued rapid progression, maximize dexamethasone, switch to high-dose methylprednisolone 2 mg/kg if needed. After 2 doses of tocilizumab, consider alternative immunosuppressants. Do not exceed 3 doses tocilizumab in 24 hours, or 4 doses in total. |
||
Neurologic Toxicity
Monitor patients for signs and symptoms of neurologic toxicities (Table 2). Rule out other causes of neurologic symptoms. Provide intensive care supportive therapy for severe or life-threatening neurologic toxicities. If neurologic toxicity is suspected, manage according to the recommendations in Table 2.
If concurrent CRS is suspected during neurologic toxicity, administer:
- •
- Corticosteroids according to the more aggressive intervention based on the CRS and neurologic toxicity grades in Tables 1 and 2
- •
- Tocilizumab according to the CRS grade in Table 1
- •
- Antiseizure medication according to the neurologic toxicity in Table 2
| NT Gradea | Corticosteroids and Antiseizure Medication |
|---|---|
| a NCI CTCAE criteria for grading neurologic toxicities, version 4.03. | |
|
Grade 1 |
Start non-sedating, antiseizure medicines (e.g., levetiracetam) for seizure prophylaxis. |
|
Grade 2 |
Start non-sedating, antiseizure medicines (e.g., levetiracetam) for seizure prophylaxis. |
|
Grade 3 |
Start non-sedating, antiseizure medicines (e.g., levetiracetam) for seizure prophylaxis. |
|
Grade 4 |
Start non-sedating, antiseizure medicines (e.g., levetiracetam) for seizure prophylaxis. |
3. Dosage Forms and Strengths
BREYANZI is a cell suspension for infusion.
A single dose of BREYANZI contains CAR-positive viable T cells that consist of CD8 and CD4 components, with each component supplied separately in single-dose vials [see Dosage and Administration (2.1)].
More than one vial of each of the CD8 component and/or CD4 component may be needed to achieve the dose of BREYANZI.
Each vial contains between 6.9 × 106 and 322 x 106 CAR-positive viable T cells in 4.6 mL cell suspension (between 1.5 × 106 and 70 x 106 CAR-positive viable T cells/mL).
The infusion volume is calculated based on the concentration of cryopreserved drug product CAR-positive viable T cells. The volume may differ for each component infused. See the RFI Certificate for details [see How Supplied/Storage and Handling (16)].
5. Warnings and Precautions
5.1 Cytokine Release Syndrome
Cytokine release syndrome (CRS), including fatal or life-threatening reactions, occurred following treatment with BREYANZI. Among patients receiving BREYANZI for LBCL (N=418), CRS occur in 46% (190/418), including ≥ Grade 3 CRS (Lee grading system1) in 3.1% of patients.
In patients receiving BREYANZI after two or more lines of therapy for LBCL, CRS occurred in 46% (122/268), including ≥ Grade 3 CRS in 4.1% of patients. One patient had fatal CRS and 2 had ongoing CRS at time of death. The median time to onset was 5 days (range: 1 to 15 days). CRS resolved in 98% with a median duration of 5 days (range: 1 to 17 days).
In patients receiving BREYANZI after one line of therapy for LBCL, CRS occurred in 45% (68/150), including Grade 3 CRS in 1.3% of patients. The median time to onset was 4 days (range: 1 to 63 days). CRS resolved in all patients with a median duration of 4 days (range: 1 to 16 days).
The most common manifestations of CRS (≥ 10%) included fever (94%), hypotension (42%), tachycardia (28%), chills (23%), hypoxia (16%), and headache (12%) [see Adverse Reactions (6.1)].
Serious events that may be associated with CRS include cardiac arrhythmias (including atrial fibrillation and ventricular tachycardia), cardiac arrest, cardiac failure, diffuse alveolar damage, renal insufficiency, capillary leak syndrome, hypotension, hypoxia, and hemophagocytic lymphohistiocytosis/macrophage activation syndrome (HLH/MAS) [see Adverse Reactions (6.1)].
Ensure that 2 doses of tocilizumab are available prior to infusion of BREYANZI.
Of the 418 patients who received BREYANZI for LBCL, 23% received tocilizumab and/or a corticosteroid for CRS, including 10% who received tocilizumab only and 2.2% who received corticosteroids only.
Monitor patients daily for at least 7 days following BREYANZI infusion at a REMS-certified healthcare facility for signs and symptoms of CRS. Monitor patients for signs or symptoms of CRS for at least 4 weeks after infusion. At the first sign of CRS, institute treatment with supportive care, tocilizumab, or tocilizumab and corticosteroids as indicated [see Dosage and Administration (2.3)].
Counsel patients to seek immediate medical attention should signs or symptoms of CRS occur at any time [see Patient Counseling Information (17)].
5.2 Neurologic Toxicities
Neurologic toxicities that were fatal or life-threatening, including immune effector cell-associated neurotoxicity syndrome (ICANS), occurred following treatment with BREYANZI. Serious events including cerebral edema and seizures occurred with BREYANZI. Fatal and serious cases of leukoencephalopathy, some attributable to fludarabine, also occurred.
In patients receiving BREYANZI after two or more lines of therapy for LBCL, CAR T cell-associated neurologic toxicities occurred in 35% (95/268), including ≥ Grade 3 cases in 12% of patients. Three patients had fatal neurologic toxicity and 7 had ongoing neurologic toxicity at time of death. The median time to onset of neurotoxicity was 8 days (range: 1 to 46 days). Neurologic toxicities resolved in 85% with a median duration of 12 days (range: 1 to 87 days).
In patients receiving BREYANZI after one line of therapy for LBCL, CAR T cell-associated neurologic toxicities occurred in 27% (41/150) of patients, including Grade 3 cases in 7% of patients. The median time to onset of neurologic toxicity was 8 days (range: 1 to 63 days). The median duration of neurologic toxicity was 6 days (range: 1 to 119 days).
In all patients combined receiving BREYANZI for LBCL, neurologic toxicities occurred in 33% (136/418), including ≥ Grade 3 cases in 10% of patients. The median time to onset was 8 days (range: 1 to 63), with 87% of cases developing by 16 days. Neurologic toxicities resolved in 85% of patients with a median duration of 11 days (range: 1 to 119 days). Of patients developing neurotoxicity, 77% (105/136) also developed CRS.
The most common neurologic toxicities (≥ 5%) included encephalopathy (20%), tremor (13%), aphasia (8%), headache (6%), dizziness (6%), and delirium (5%).
Monitor patients daily at least for at least 7 days following BREYANZI infusion at a REMS-certified healthcare facility for signs and symptoms of neurologic toxicities and assess for other causes of neurological symptoms. Monitor patients for signs or symptoms of neurologic toxicities for at least 4 weeks after infusion and treat promptly. Manage neurologic toxicity with supportive care and/or corticosteroid as needed [see Dosage and Administration (2.3)].
Counsel patients to seek immediate medical attention should signs or symptoms of neurologic toxicity occur at any time [see Patient Counseling Information (17)].
5.3 BREYANZI REMS
Because of the risk of CRS and neurologic toxicities, BREYANZI is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS) called the BREYANZI REMS [see Boxed Warning and Warnings and Precautions (5.1, 5.2)]. The required components of the BREYANZI REMS are:
- •
- Healthcare facilities that dispense and administer BREYANZI must be enrolled and comply with the REMS requirements.
- •
- Certified healthcare facilities must have on-site, immediate access to tocilizumab.
- •
- Ensure that a minimum of 2 doses of tocilizumab are available for each patient for infusion within 2 hours after BREYANZI infusion, if needed for treatment of CRS.
- •
- Certified healthcare facilities must ensure that healthcare providers who prescribe, dispense, or administer BREYANZI are trained on the management of CRS and neurologic toxicities.
Further information is available at www.BreyanziREMS.com, or contact Bristol-Myers Squibb at 1-888-423-5436.
5.4 Hypersensitivity Reactions
Allergic reactions may occur with the infusion of BREYANZI. Serious hypersensitivity reactions, including anaphylaxis, may be due to dimethyl sulfoxide (DMSO).
5.5 Serious Infections
Severe infections, including life-threatening or fatal infections, have occurred in patients after BREYANZI infusion.
In patients receiving BREYANZI for LBCL, infections of any grade occurred in 36%, with Grade 3 or higher infections occurred in 12% of all patients. Grade 3 or higher infections with an unspecified pathogen occurred in 7%, bacterial infections in 4.3%, viral infections in 1.9%, and fungal infections in 0.5%.
Febrile neutropenia developed after BREYANZI infusion in 8% of patients with LBCL. Febrile neutropenia may be concurrent with CRS. In the event of febrile neutropenia, evaluate for infection and manage with broad-spectrum antibiotics, fluids, and other supportive care as medically indicated.
Monitor patients for signs and symptoms of infection before and after BREYANZI administration and treat appropriately. Administer prophylactic antimicrobials according to standard institutional guidelines.
Avoid administration of BREYANZI in patients with clinically significant, active systemic infections.
Viral Reactivation
Hepatitis B virus (HBV) reactivation, in some cases resulting in fulminant hepatitis, hepatic failure, and death, can occur in patients treated with drugs directed against B cells.
In patients who received BREYANZI for LBCL, 15 of the 16 patients with a prior history of HBV were treated with concurrent antiviral suppressive therapy. Perform screening for HBV, HCV, and HIV in accordance with clinical guidelines before collection of cells for manufacturing. In patients with prior history of HBV, consider concurrent antiviral suppressive therapy to prevent HBV reactivation per standard guidelines.
5.6 Prolonged Cytopenias
Patients may exhibit cytopenias not resolved for several weeks following lymphodepleting chemotherapy and BREYANZI infusion.
Grade 3 or higher cytopenias persisted at Day 29 following BREYANZI infusion in 36% of patients with LBCL and included thrombocytopenia in 28%, neutropenia in 21%, and anemia in 6%.
Monitor complete blood counts prior to and after BREYANZI administration.
5.7 Hypogammaglobulinemia
B-cell aplasia and hypogammaglobulinemia can occur in patients receiving BREYANZI.
In patients receiving BREYANZI for LBCL, hypogammaglobulinemia was reported as an adverse reaction in 11% of patients. Hypogammaglobulinemia, either as an adverse reaction or laboratory IgG level below 500 mg/dL after infusion, was reported in 28% of patients.
Monitor immunoglobulin levels after treatment with BREYANZI and manage using infection precautions, antibiotic prophylaxis, and immunoglobulin replacement as clinically indicated.
Live Vaccines
The safety of immunization with live viral vaccines during or following BREYANZI treatment has not been studied. Vaccination with live virus vaccines is not recommended for at least 6 weeks prior to the start of lymphodepleting chemotherapy, during BREYANZI treatment, and until immune recovery following treatment with BREYANZI.
5.8 Secondary Malignancies
Patients treated with BREYANZI may develop secondary malignancies. Monitor lifelong for secondary malignancies. In the event that a secondary malignancy occurs, contact Bristol-Myers Squibb at 1-888-805-4555 for reporting and to obtain instructions on collection of patient samples for testing.
5.9 Effects on Ability to Drive and Use Machines
Due to the potential for neurologic events, including altered mental status or seizures, patients receiving BREYANZI are at risk for developing altered or decreased consciousness or impaired coordination in the 8 weeks following BREYANZI administration. Advise patients to refrain from driving and engaging in hazardous occupations or activities, such as operating heavy or potentially dangerous machinery, for at least 8 weeks.
6. Adverse Reactions/Side Effects
The following adverse reactions are described elsewhere in the labeling:
- •
- Cytokine Release Syndrome [see Warnings and Precautions (5.1, 5.3)]
- •
- Neurologic Toxicities [see Warnings and Precautions (5.2, 5.3)]
- •
- Hypersensitivity Reactions [see Warnings and Precautions (5.4)]
- •
- Serious Infections [see Warnings and Precautions (5.5)]
- •
- Prolonged Cytopenias [see Warnings and Precautions (5.6)]
- •
- Hypogammaglobulinemia [see Warnings and Precautions (5.7)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety data described in the WARNINGS and PRECAUTIONS reflect exposure to a single dose of BREYANZI in 418 patients with relapsed or refractory (R/R) LBCL: one randomized, open-label study with 89 patients (TRANSFORM) and two open-label, single-arm studies with 61 patients (PILOT) and 268 patients (TRANSCEND).
Relapsed or Refractory LBCL After One Line of Therapy
TRANSFORM
The safety of BREYANZI was evaluated in the TRANSFORM, a randomized, open-label, multicenter study, in which patients with primary refractory LBCL or relapse within 1 year of first-line chemoimmunotherapy received BREYANZI (N=89) or standard therapy (N=91) [see Clinical Studies (14)]. Patients had not yet received treatment for relapsed or refractory lymphoma and were potential candidates for autologous HSCT. The trial excluded patients who were ineligible for transplant or who had age > 75 years, ECOG performance status >1, history of central nervous system (CNS) disorders (such as seizures or cerebrovascular ischemia), uncontrolled infection, CrCl < 45 mL/min, ALT > 5 times the upper limit of normal (ULN), LVEF < 40%, or absolute neutrophil count (ANC) < 1.0 × 109 cells/L or platelets < 50 × 109 cells/L in the absence of bone marrow involvement.
The planned dose of BREYANZI was 100 × 106 CAR-positive viable T cells. The median age of the BREYANZI-treated population was 59 years (range: 20 to 74 years); 47% were male; 58% were white, 11% were Asian, and 5% were black.
Serious adverse reactions occurred in 38% of patients. The most common nonlaboratory, serious adverse reactions (> 2%) were CRS, sepsis, fever, febrile neutropenia, headache, aphasia, COVID-19 infection, and pulmonary embolism.
Table 3 presents selected nonlaboratory adverse reactions in patients treated with BREYANZI, and Table 4 describes selected new or worsening Grade 3 or 4 laboratory abnormalities.
The most common nonlaboratory adverse reactions (≥ 20%) were fever, CRS, musculoskeletal pain, headache, fatigue, nausea, constipation, and dizziness.
| a Tachycardia includes atrial flutter, sinus tachycardia, supraventricular tachycardia, tachycardia. | ||
| b Abdominal pain includes abdominal pain, abdominal pain lower, abdominal tenderness. | ||
| c Fatigue includes asthenia, fatigue, malaise. | ||
| d Edema includes edema peripheral, localized edema, edema peripheral, pleural effusion swelling. | ||
| e Infections and infestations are grouped per high-level grouped term. | ||
| f Sepsis includes bacteremia, bacterial sepsis, enterococcal bacteremia, Escherichia bacteremia, Klebsiella bacteremia, Klebsiella sepsis, sepsis, septic shock, staphylococcal bacteremia. | ||
| g Musculoskeletal pain includes arthralgia, back pain, bone pain, flank pain, musculoskeletal chest pain, musculoskeletal pain, musculoskeletal stiffness, myalgia, neck pain, osteoarthritis, pain in extremity. | ||
| h Motor dysfunction includes fine motor skill dysfunction, muscle spasms, muscular weakness. | ||
| i Headache includes headache, migraine, migraine with aura. | ||
| j Dizziness includes dizziness, dizziness postural, syncope, vertigo. | ||
| k Tremor includes resting tremor, tremor, essential tremor. | ||
| l Insomnia includes insomnia, sleep disorder. | ||
| m Cough includes cough, productive cough. | ||
| n Rash includes catheter site rash, dermatitis acneiform, dermatitis exfoliative generalized, erythema multiforme, rash, rash maculo-papular, rash pruritic. | ||
| o Hypotension includes hypotension, orthostatic hypotension. | ||
| p Hemorrhage includes conjunctival hemorrhage, cystitis hemorrhagic, epistaxis, gastrointestinal hemorrhage, hematoma, hematuria, retinal hemorrhage, vaginal hemorrhage. | ||
|
Adverse Reaction |
Any Grade (%) |
Grade 3 or Higher (%) |
|
Blood and lymphatic system disorders |
||
|
Febrile neutropenia |
10 |
10 |
|
Cardiac disorders |
||
|
Tachycardiaa |
15 |
1.1 |
|
Gastrointestinal disorders |
||
|
Nausea |
24 |
0 |
|
Constipation |
20 |
2.2 |
|
Diarrhea |
18 |
0 |
|
Abdominal painb |
13 |
2.2 |
|
Vomiting |
11 |
0 |
|
General disorders and administration site conditions |
||
|
Fever |
55 |
3.4 |
|
Fatiguec |
28 |
1.1 |
|
Edemad |
13 |
0 |
|
Immune system disorders |
||
|
Cytokine release syndrome |
49 |
1.1 |
|
Infections and infestationse |
||
|
Bacterial infectious disorders |
12 |
6 |
|
Infections with pathogen unspecified |
12 |
6 |
|
Sepsisf |
10 |
7 |
|
Metabolism and nutrition disorders |
||
|
Decreased appetite |
15 |
0 |
|
Musculoskeletal and connective tissue disorders |
||
|
Musculoskeletal paing |
36 |
3.4 |
|
Motor dysfunctionh |
12 |
3.4 |
|
Nervous system disorders |
||
|
Headachei |
34 |
6 |
|
Dizzinessj |
20 |
1.1 |
|
Tremork |
11 |
1.1 |
|
Psychiatric disorders |
||
|
Insomnial |
15 |
0 |
|
Respiratory, thoracic and mediastinal disorders |
||
|
Coughm |
11 |
0 |
|
Skin and subcutaneous tissue disorders |
||
|
Rashn |
12 |
1.1 |
|
Vascular disorders |
||
|
Hypotensiono |
15 |
2.2 |
|
Hemorrhagep |
12 |
0 |
Other clinically important adverse reactions in < 10% of patients treated with BREYANZI included the following:
- •
- Immune system disorders: Hemophagocytic lymphohistiocytosis (1.1%)
- •
- Infections and infestations: Viral infection (9%), fungal infection (4.5%), pneumonia (2.2%)
- •
- Nervous system disorders: Encephalopathy (8%), aphasia (4.5%), peripheral neuropathy (4.5%), ataxia (3.4%), paresis (1.1%)
- •
- Psychiatric disorders: Delirium (2.2%)
- •
- Renal and urinary disorders: Renal failure (3.4%)
- •
- Respiratory, thoracic, and mediastinal disorders: Dyspnea (8%)
- •
- Vascular disorders: Thrombosis (8%), hypertension (7%)
| a Baseline lab values were assessed prior to lymphodepleting chemotherapy. | |
| b Based on 88 evaluable patients, defined as those with both a baseline grade and at least one post-baseline grade for the particular lab. | |
|
Laboratory Abnormalitya |
Grade 3 or 4 (%)b |
|
Lymphocyte count decreased |
98 |
|
Neutrophil count decreased |
89 |
|
Platelet count decreased |
48 |
|
Hemoglobin decreased |
32 |
Grade 4 laboratory abnormalities in ≥ 10% of patients were lymphocyte decrease (64%), neutrophil decrease (66%), and platelet decrease (34%).
PILOT
The safety of BREYANZI was evaluated in the PILOT study, a single-arm open-label study in transplant-ineligible patients with R/R LBCL after one line of chemoimmunotherapy [see Clinical Studies (14)]. The study enrolled patients who were ineligible for high-dose therapy and autologous HSCT due to organ function or age, but who had adequate organ function for CAR-T cell therapy. Patients with a history of relevant CNS disorders (such as seizures or cerebrovascular ischemia), ECOG performance status > 2, or uncontrolled infection were ineligible. The trial required left ventricular ejection fraction ≥ 40%, adequate oxygen saturation on room air with ≤ Grade 1 dyspnea, AST and ALT ≤ 5 x ULN, total bilirubin < 2.0 mg/dL, creatinine clearance > 30 mL/min, and adequate bone marrow function to receive lymphodepleting chemotherapy. The planned dose of BREYANZI was 100 × 106 CAR-positive viable T cells.
The median age was 74 years (range: 53 to 84 years), 90% were age ≥ 65 years, 61% were male. The ECOG performance status was 0 or 1 in 74% of patients and 2 in 26% of patients; 25% had CrCl < 60 ml/min; 20% had a baseline ANC < 1000/μL.
Serious adverse reactions occurred in 33% of patients. The most common nonlaboratory, serious adverse reactions (> 2%) were CRS, confusional state, gastrointestinal hemorrhage, muscular weakness, musculoskeletal pain, pulmonary embolism, and sepsis.
Table 5 presents selected nonlaboratory adverse reactions, and Table 6 describes selected new or worsening Grade 3 or 4 laboratory abnormalities.
The most common nonlaboratory adverse reactions (≥ 20%) were fatigue, CRS, fever, nausea, encephalopathy, hypotension, musculoskeletal pain, and edema.
| a Tachycardia includes atrial fibrillation, sinus tachycardia, tachycardia. | ||
| b Fatigue includes asthenia, fatigue, malaise. | ||
| c Edema includes edema peripheral, pleural effusion, swelling. | ||
| d Grouped per high-level grouped term. | ||
| e Upper respiratory tract infection includes nasal congestion, paranasal sinus hypersecretion, rhinitis, rhinorrhea, upper respiratory tract infection. | ||
| f Musculoskeletal pain includes arthralgia, back pain, bone pain, flank pain, musculoskeletal chest pain, musculoskeletal pain, musculoskeletal stiffness, myalgia, neck pain, osteoarthritis, pain in extremity, spinal pain. | ||
| g Encephalopathy includes amnesia, apraxia, cognitive disorder, confusional state, depressed level of consciousness, disturbance in attention, dyscalculia, encephalopathy, lethargy, memory impairment, mental status changes, somnolence. | ||
| h Dizziness includes dizziness, dizziness postural, syncope, vertigo. | ||
| i Tremor includes resting tremor, tremor. | ||
| j Cough includes cough, productive cough. | ||
| k Dyspnea includes acute respiratory distress syndrome, dyspnea, tachypnea, wheezing. | ||
| l Hypotension includes hypotension, orthostatic hypotension. | ||
|
Adverse Reaction |
Any Grade (%) |
Grade 3 or Higher (%) |
|
Cardiac disorders |
||
|
Tachycardiaa |
10 |
0 |
|
Gastrointestinal disorders |
||
|
Nausea |
25 |
1.6 |
|
Diarrhea |
15 |
0 |
|
Constipation |
11 |
0 |
|
General disorders and administration site conditions |
||
|
Fatigueb |
44 |
1.6 |
|
Fever |
38 |
1.6 |
|
Edemac |
20 |
0 |
|
Immune system disorders |
||
|
Cytokine release syndrome |
39 |
1.6 |
|
Infections and infestations |
||
|
Infections with pathogen unspecifiedd |
13 |
4.9 |
|
Upper respiratory tract infectione |
13 |
0 |
|
Bacterial infectious disorders |
10 |
3.3 |
|
Metabolism and nutrition disorders |
||
|
Decreased appetite |
13 |
1.6 |
|
Musculoskeletal and connective tissue disorders |
||
|
Musculoskeletal painf |
23 |
4.9 |
|
Nervous system disorders |
||
|
Encephalopathyg |
23 |
4.9 |
|
Dizzinessh |
16 |
1.6 |
|
Tremori |
16 |
0 |
|
Headache |
11 |
1.6 |
|
Psychiatric disorders |
||
|
Insomnia |
11 |
0 |
|
Respiratory, thoracic and mediastinal disorders |
||
|
Coughj |
18 |
0 |
|
Dyspneak |
16 |
4.9 |
|
Vascular disorders |
||
|
Hypotensionl |
23 |
1.6 |
|
Hypertension |
10 |
4.9 |
Other clinically important adverse reactions in < 10% of patients included the following:
- •
- Blood and lymphatic system disorders: Febrile neutropenia (1.6%)
- •
- Eye disorders: Vision blurred (3.3%)
- •
- Gastrointestinal disorders: Vomiting (8%), abdominal pain (7%), gastrointestinal hemorrhage (4.9%)
- •
- Infections and infestations: Fungal infection (4.9%), sepsis (3.3%), viral infection (3.3%)
- •
- Nervous system disorders: Motor dysfunction (7%), aphasia (4.9%), ataxia (4.9%), peripheral neuropathy (4.9%)
- •
- Psychiatric disorders: Delirium (3.3%)
- •
- Renal and urinary disorders: Renal failure (7%)
- •
- Respiratory, thoracic, and mediastinal disorders: Hypoxia (4.9%)
- •
- Skin and subcutaneous tissue disorders: Rash (7%)
- •
- Vascular disorders: Thrombosis (7%)
| a Baseline lab values were assessed prior to lymphodepleting chemotherapy. | |
|
Laboratory Abnormalitya |
Grade 3 or 4 (%) |
|
Lymphocyte count decreased |
97 |
|
Neutrophil count decreased |
80 |
|
Hemoglobin decreased |
30 |
|
Platelet count decreased |
26 |
Grade 4 laboratory abnormalities in ≥ 10% of patients were lymphocyte decrease (95%), neutrophil decrease (57%), and platelet decrease (20%).
Relapsed or Refractory LBCL After Two or More Lines of Therapy
TRANSCEND
The safety of BREYANZI was evaluated in the TRANSCEND study, in which 268 adult patients with R/R LBCL after 2 or more prior lines of therapy received a single dose of CAR-positive viable T cells [see Clinical Studies (14)]. Patients with a history of CNS disorders (such as seizures or cerebrovascular ischemia) or autoimmune disease requiring systemic immunosuppression were ineligible. The median age of the study population was 63 years (range: 18 to 86 years); 65% were male. The Eastern Cooperative Oncology Group (ECOG) performance status at screening was 0 in 41% of patients, 1 in 58% of patients, and 2 in 1.5% of patients.
Serious adverse reactions occurred in 46% of patients. The most common nonlaboratory, serious adverse reactions (> 2%) were CRS, encephalopathy, sepsis, febrile neutropenia, aphasia, pneumonia, fever, hypotension, dizziness, and delirium. Fatal adverse reactions occurred in 4% of patients.
Table 7 presents selected nonlaboratory adverse reactions reported in patients treated with BREYANZI, and Table 8 describes selected new or worsening Grade 3 or 4 laboratory abnormalities.
The most common nonlaboratory adverse reactions (≥ 20%) were fatigue, CRS, musculoskeletal pain, nausea, headache, encephalopathy, infections (pathogen unspecified), decreased appetite, diarrhea, hypotension, tachycardia, dizziness, cough, constipation, abdominal pain, vomiting, and edema.
| Adverse Reaction | Any Grade (%) | Grade 3 or Higher (%) |
|---|---|---|
| a Tachycardia includes heart rate increased, sinus tachycardia, tachycardia. | ||
| b Abdominal pain includes abdominal discomfort, abdominal pain, abdominal pain lower, abdominal pain upper, abdominal tenderness. | ||
| c Fatigue includes asthenia, fatigue, malaise. | ||
| d Edema includes edema, edema peripheral, fluid overload, fluid retention, generalized edema, hypervolemia, peripheral swelling, pulmonary congestion, pulmonary edema, swelling. | ||
| e Infections and infestations are grouped by pathogen type and selected clinical syndromes. | ||
| f Infections with pathogen unspecified contains febrile neutropenia (9%). | ||
| g Bacterial infection includes infections by pathogen type plus appendicitis, diverticulitis, peritonitis, skin infection, tooth infection. | ||
| h Upper respiratory tract infections include nasopharyngitis, pharyngitis, rhinitis, rhinovirus infection, sinusitis, upper respiratory tract congestion, upper respiratory tract infection. | ||
| i Musculoskeletal pain includes arthralgia, back pain, bone pain, musculoskeletal chest pain, musculoskeletal discomfort, musculoskeletal pain, musculoskeletal stiffness, myalgia, neck pain, pain in extremity, spinal pain. | ||
| j Motor dysfunction includes eyelid ptosis, motor dysfunction, muscle rigidity, muscle spasms, muscle spasticity, muscle tightness, muscle twitching, muscular weakness, myoclonus, myopathy. | ||
| k Headache includes headache, head discomfort, migraine, sinus headache. | ||
| l Encephalopathy includes amnesia, bradyphrenia, cognitive disorder, confusional state, depersonalization/derealization disorder, depressed level of consciousness, disturbance in attention, encephalopathy, flat affect, hypersomnia, incoherent, lethargy, leukoencephalopathy, loss of consciousness, memory impairment, mental impairment, mental status changes, somnolence. | ||
| m Dizziness includes dizziness, presyncope, syncope, vertigo. | ||
| n Tremor includes essential tremor, resting tremor, tremor. | ||
| o Peripheral neuropathy includes hyperesthesia, hypoesthesia, meralgia paresthetica, neuralgia, neuropathy peripheral, paresthesia, peripheral sensory neuropathy, sciatica, sensory loss. | ||
| p Aphasia includes aphasia, disorganized speech, dysarthria, dysphemia, dysphonia, slow speech, speech disorder. | ||
| q Insomnia includes insomnia, somnambulism. | ||
| r Anxiety includes anxiety, panic attack. | ||
| s Delirium includes agitation, delirium, delusion, disorientation, hallucination, ‘hallucination, visual’, irritability, restlessness. | ||
| t Renal failure includes acute kidney injury, blood creatinine increased, chronic kidney disease, renal failure, renal injury. | ||
| u Cough includes cough, productive cough, upper-airway cough syndrome. | ||
| v Dyspnea includes acute respiratory failure, dyspnea, dyspnea exertional, respiratory failure. | ||
| w Rash includes erythema, dermatitis acneiform, perineal rash, rash, rash erythematous, rash macular, rash maculo-papular, rash morbilliform, rash papular, rash pruritic, rash pustular. | ||
| x Hypotension includes hypotension, orthostatic hypotension. | ||
| y Hemorrhage includes catheter site hemorrhage, conjunctival hemorrhage, epistaxis, hematoma, hematuria, hemorrhage, hemorrhage intracranial, pulmonary hemorrhage, retinal hemorrhage, vaginal hemorrhage. | ||
|
Cardiac disorders |
||
|
Tachycardiaa |
25 |
0 |
|
Gastrointestinal disorders |
||
|
Nausea |
33 |
1.5 |
|
Diarrhea |
26 |
0.4 |
|
Constipation |
23 |
0 |
|
Abdominal painb |
21 |
3.0 |
|
Vomiting |
21 |
0.4 |
|
General disorders and administration site conditions |
||
|
Fatiguec |
48 |
3.4 |
|
Edemad |
21 |
1.1 |
|
Fever |
16 |
0 |
|
Chills |
12 |
0 |
|
Immune system disorders |
||
|
Cytokine release syndrome |
46 |
4.1 |
|
Infections and infestationse |
||
|
Infection with pathogen unspecifiedf |
29 |
16 |
|
Bacterial infectiong |
13 |
5 |
|
Upper respiratory tract infectionh |
13 |
0.7 |
|
Viral infection |
10 |
1.5 |
|
Metabolism and nutrition disorders |
||
|
Decreased appetite |
28 |
2.6 |
|
Musculoskeletal and connective tissue disorders |
||
|
Musculoskeletal paini |
37 |
2.2 |
|
Motor dysfunctionj |
10 |
1.1 |
|
Nervous system disorders |
||
|
Headachek |
30 |
1.1 |
|
Encephalopathyl |
29 |
9 |
|
Dizzinessm |
24 |
2.6 |
|
Tremorn |
16 |
0 |
|
Peripheral neuropathyo |
11 |
0 |
|
Aphasiap |
10 |
2.2 |
|
Psychiatric disorders |
||
|
Insomniaq |
14 |
0.4 |
|
Anxietyr |
10 |
0 |
|
Deliriums |
10 |
2.2 |
|
Renal and urinary disorders |
||
|
Renal failuret |
11 |
3.0 |
|
Respiratory, thoracic, and mediastinal disorders |
||
|
Coughu |
23 |
0 |
|
Dyspneav |
16 |
2.6 |
|
Skin and subcutaneous tissue disorders |
||
|
Rashw |
13 |
0.4 |
|
Vascular disorders |
||
|
Hypotensionx |
26 |
3.4 |
|
Hypertension |
14 |
4.5 |
|
Hemorrhagey |
10 |
1.5 |
Other clinically important adverse reactions in < 10% of patients included the following:
- •
- Cardiac disorders: Arrhythmia (6%), cardiomyopathy (1.5%)
- •
- Gastrointestinal disorders: Gastrointestinal hemorrhage (4.1%)
- •
- Infections and infestations: Pneumonia (8%), fungal infections (8%), sepsis (4.5%), urinary tract infection (4.1%)
- •
- Metabolism and nutrition disorders: Tumor lysis syndrome (0.7%)
- •
- Nervous system disorders: Ataxia or gait disturbance (7%), visual disturbance (5%), paresis (2.6%), cerebrovascular events (1.9%), seizure (1.1%), brain edema (0.4%)
- •
- Procedural complications: Infusion-related reaction (1.9%)
- •
- Respiratory, thoracic, and mediastinal disorders: Pleural effusion (7%), hypoxia (6%)
- •
- Vascular disorder: Thrombosis (7%)
| Laboratory Abnormalitya | Grade 3 or 4 (%)b |
|---|---|
| a Baseline lab values were assessed prior to lymphodepleting chemotherapy. | |
| b The denominator varied from 239 to 268, based on the number of patients with a baseline value and at least one post-treatment value for the particular lab. | |
|
Lymphocyte count decreased |
95 |
|
Neutrophil count decreased |
88 |
|
Platelet count decreased |
41 |
|
Hemoglobin decreased |
32 |
|
Fibrinogen decreased |
14 |
|
Phosphate decreased |
16 |
6.2 Immunogenicity
BREYANZI has the potential to induce anti-product antibodies. The immunogenicity of BREYANZI has been evaluated using an electrochemiluminescence (ECL) immunoassay for the detection of binding antibodies against the extracellular CD19-binding domain of BREYANZI. Pre-existing anti-product antibodies were detected in 11% (28/261) of patients in TRANSCEND, 1% (1/89) of patients in TRANSFORM and 0% (0/51) of patients in PILOT. Treatment-induced or treatment-boosted anti-product antibodies were detected in 11% (27/257) in TRANSCEND, 1% (1/89) in TRANSFORM, and 2% (1/49) in PILOT, of patients, respectively. Due to the small number of patients who had anti-product antibodies, the relationship between anti-product antibody status and efficacy, safety, or pharmacokinetics was not conclusive.
8. Use In Specific Populations
8.1 Pregnancy
Risk Summary
There are no available data with BREYANZI use in pregnant women. No animal reproductive and developmental toxicity studies have been conducted with BREYANZI to assess whether it can cause fetal harm when administered to a pregnant woman.
It is not known if BREYANZI has the potential to be transferred to the fetus. Based on the mechanism of action, if the transduced cells cross the placenta, they may cause fetal toxicity, including B-cell lymphocytopenia and hypogammaglobulinemia. Therefore, BREYANZI is not recommended for women who are pregnant, and pregnancy after BREYANZI infusion should be discussed with the treating physician.
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
8.2 Lactation
Risk Summary
There is no information regarding the presence of BREYANZI in human milk, the effect on the breastfed infant, and the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for BREYANZI and any potential adverse effects on the breastfed infant from BREYANZI or from the underlying maternal condition.
8.3 Females and Males of Reproductive Potential
Pregnancy Testing
Pregnancy status of females with reproductive potential should be verified. Sexually active females of reproductive potential should have a pregnancy test prior to starting treatment with BREYANZI.
Contraception
See the prescribing information for fludarabine and cyclophosphamide for information on the need for effective contraception in patients who receive lymphodepleting chemotherapy.
There are insufficient exposure data to provide a recommendation concerning duration of contraception following treatment with BREYANZI.
8.4 Pediatric Use
The safety and efficacy of BREYANZI have not been established in pediatric patients.
8.5 Geriatric Use
In clinical trials of BREYANZI, 111 (41%) of 268 patients with two or more prior lines of therapy for LBCL, and 89 (59%) of 150 patients with one prior line of therapy for LBCL, were 65 years of age or older; 27 (10%) and 28 (19%) were 75 years of age or older, respectively. No clinically important differences in safety or effectiveness of BREYANZI were observed between patients aged ≥ 65 and younger patients.
11. Breyanzi Description
BREYANZI (lisocabtagene maraleucel) is a CD19-directed genetically modified autologous T cell immunotherapy administered as a defined composition of CAR-positive viable T cells (consisting of CD8 and CD4 components). The CAR is comprised of the FMC63 monoclonal antibody-derived single-chain variable fragment (scFv), IgG4 hinge region, CD28 transmembrane domain, 4-1BB (CD137) costimulatory domain, and CD3 zeta activation domain. In addition, BREYANZI includes a nonfunctional truncated epidermal growth factor receptor (EGFRt) that is co-expressed on the cell surface with the CD19-specific CAR.
BREYANZI is a T cell product. BREYANZI is prepared from the patient’s T cells, which are obtained via a standard leukapheresis procedure. The purified CD8-positive and CD4-positive T cells are separately activated and transduced with the replication-incompetent lentiviral vector containing the anti-CD19 CAR transgene. The transduced T cells are expanded in cell culture, washed, formulated into a suspension, and cryopreserved as separate CD8 and CD4 component vials that together constitute a single dose of BREYANZI. The product must pass a sterility test before release for shipping as a frozen suspension in patient-specific vials. The product is thawed prior to administration [see Dosage and Administration (2.2) and How Supplied/Storage and Handling (16)].
The BREYANZI formulation contains 75% (v/v) Cryostor® CS10 [containing 7.5% dimethylsulfoxide (v/v)], 24% (v/v) Multiple Electrolytes for Injection, Type 1, 1% (v/v) of 25% albumin (human).
12. Breyanzi - Clinical Pharmacology
12.1 Mechanism of Action
BREYANZI is a CD19-directed genetically modified autologous cell immunotherapy administered as a defined composition to reduce variability in CD8-positive and CD4-positive T cell dose. The CAR is comprised of an FMC63 monoclonal antibody-derived single chain variable fragment (scFv), IgG4 hinge region, CD28 transmembrane domain, 4-1BB (CD137) costimulatory domain, and CD3 zeta activation domain. CD3 zeta signaling is critical for initiating activation and antitumor activity, while 4-1BB (CD137) signaling enhances the expansion and persistence of BREYANZI.
CAR binding to CD19 expressed on the cell surface of tumor and normal B cells induces activation and proliferation of CAR T cells, release of pro-inflammatory cytokines, and cytotoxic killing of target cells.
12.2 Pharmacodynamics
Following BREYANZI infusion, pharmacodynamic responses were evaluated over a 4-week period by measuring transient elevation of soluble biomarkers such as cytokines, chemokines, and other molecules. Peak elevation of soluble biomarkers was observed within the first 14 days after BREYANZI infusion and returned to baseline levels within 28 days.
B-cell aplasia, defined as CD19+ B cells comprising less than 3% of peripheral blood lymphocytes, is an on-target effect of BREYANZI. B-cell aplasia was observed in the majority of patients for up to 1 year following BREYANZI infusion.
12.3 Pharmacokinetics
Following infusion, BREYANZI exhibited an initial expansion followed by a bi-exponential decline. The median time of maximal expansion in peripheral blood occurred 10-12 days after infusion. BREYANZI was present in peripheral blood for an estimated median of 12.1 months (range: 0.1+ to 24.2+ months).
Among patients who received two or more prior lines of therapy for LBCL (TRANSCEND), responders (N=135) had a 2.3-fold higher median Cmax than nonresponders (N=37) (35,335 vs. 15,527 copies/µg). Responders had a 1.8-fold higher median AUC0-28d than nonresponders (273,552 vs. 155,240 day*copies/µg).
In TRANSCEND, patients with higher CAR-T cell expansion tended to have higher rates of CRS and neurologic toxicities. Patients treated with tocilizumab (N=49) had a 3.6-fold and 3.7-fold higher median Cmax and AUC0-28d, respectively, compared to patients who did not receive tocilizumab (N=189). Similarly, patients who received corticosteroids (N=50) had a 3.8-fold and 3.7-fold higher median Cmax and AUC0-28d, respectively, compared to patients who did not receive corticosteroids (N=188).
Patients < 65 years old (N=142) had a 3.1-fold and 2.3-fold higher median Cmax and AUC0-28d, respectively, compared to patients ≥ 65 years old (N=96). Sex, race, ethnicity, and body weight did not show clear relationships to Cmax and AUC0-28d.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
No carcinogenicity or genotoxicity studies have been conducted with BREYANZI. No studies have been conducted to evaluate the effects of BREYANZI on fertility. In vitro studies with BREYANZI manufactured from healthy donors and patients showed no evidence for transformation and/or immortalization and no preferential integration near genes associated with oncogenic transformation.
14. Clinical Studies
Relapsed or Refractory LBCL After One Line of Therapy
TRANSFORM
A randomized, open-label, multicenter trial evaluated the efficacy of BREYANZI in adult patients with relapsed or refractory LBCL after first-line chemoimmunotherapy (TRANSFORM; NCT03575351). Patients had not yet received treatment for relapsed or refractory lymphoma, were potential candidates for autologous HSCT, and were required to have primary refractory disease or relapse within 12 months from complete response (CR) to initial chemoimmunotherapy. Eligibility criteria required adequate organ function and blood counts for HSCT.
In total, 184 patients were randomized in a 1:1 ratio to receive a single infusion of BREYANZI (planned dose, 100 × 106 CAR-positive viable T cells) or to receive standard therapy consisting of 3 cycles of chemoimmunotherapy followed by high-dose therapy and autologous HSCT in patients who attained CR or PR. All patients underwent leukapheresis prior to randomization.
Patients randomized to BREYANZI were to receive lymphodepleting chemotherapy consisting of fludarabine 30 mg/m2/day and cyclophosphamide 300 mg/m2/day concurrently for 3 days followed by BREYANZI infusion 2 to 7 days after completion of lymphodepleting chemotherapy. Bridging chemotherapy was permitted between leukapheresis and the start of lymphodepleting chemotherapy. BREYANZI was administered in the inpatient (79%) and outpatient (21%) setting.
In the overall study population, the median age was 59 years (range: 20 to 75 years), 57% were male, 59% were white, 10% were Asian, and 4% were black. Diagnoses included de novo DLBCL NOS (55%), high-grade B-cell lymphoma (23%), primary mediastinal large B-cell lymphoma (10%), and DLBCL arising from indolent lymphoma (8%). Of these patients, 73% had primary refractory disease to last therapy and 27% had relapsed disease within 12 months of achieving CR to first-line therapy.
Of 92 patients randomized to receive BREYANZI, 89 (97%) received BREYANZI. The median time from leukapheresis to product availability was 26 days (range: 19 to 84 days), and the median time from leukapheresis to product infusion was 36 days (range: 25 to 91 days). Fifty-eight (63%) patients received bridging therapy. One patient received a nonconforming product (manufacturing failure; 1.1%).
Of the 92 patients randomized to receive standard therapy, 91 started treatment and 43 (47%) received high-dose therapy and HSCT. The most common reason for not receiving HSCT was lack of efficacy of the salvage chemotherapy.
The primary efficacy measure was event-free survival (EFS) as determined by an independent review committee (IRC). Other efficacy measures included progression-free survival. Efficacy is summarized in Table 9 and Figure 1. The estimated 1-year EFS was 45% [95% CI: 29, 59] in the BREYANZI arm and 24% [95% CI: 14, 35] in the standard therapy arm.
Of the 92 patients in the BREYANZI arm, the estimated median DOR was not reached (95% CI: 7.9 months, NR) in patients who achieved CR (N=61) and 2.3 months (95% CI: 2.1, NR) in patients who achieved a best response of PR (N=18).
An interim analysis of overall survival was conducted at the time of the primary EFS analysis. The interim analysis of overall survival did not meet the criteria for statistical significance. Forty-six patients randomized to the standard therapy arm (50%) subsequently received BREYANZI on protocol.
| NR=not reached; CI=confidence interval. | ||
| a Per the Lugano criteria, as assessed by an IRC. | ||
| b EFS is defined as time from randomization to the earliest date of disease progression or relapse, death from any cause, failure to achieve CR or PR by 9 weeks post-randomization, or start of new lymphoma therapy due to efficacy concerns. | ||
| c Kaplan-Meier estimate. | ||
| d Based on a stratified Cox proportional hazards model. For all stratified analyses, stratification was based on response to first-line therapy (primary refractory vs relapsed) and second-line age-adjusted International Prognostic Index. | ||
| e Cochran-Mantel-Haenszel test. | ||
| f p-value is compared with 0.012 of the allocated alpha for this pre-specified interim analysis. | ||
|
Outcomea |
BREYANZI Arm (N=92) |
Standard Therapy Arm (N=92) |
|
Event-Free Survivalb |
||
|
Number of events, n (%) |
35 (38) |
63 (69) |
|
Median, months [95% CI]c |
10.1 [6.1, NR] |
2.3 [2.2, 4.3] |
|
Hazard ratio [95% CI]d |
0.34 [0.22, 0.52] |
|
|
One-sided p-value |
<0.0001f |
|
|
Complete Response Rate, % [95% CI] |
66 [56, 76] |
39 [29, 50] |
|
Difference in CR rate, % [95% CI] |
27 [12, 41] |
|
|
One-sided p-valuee |
<0.0001f |
|
|
Progression-Free Survival |
||
|
Number of events, n (%) |
28 (30) |
43 (47) |
|
Median, months [95% CI]c |
14.8 [6.6, NR] |
5.7 [3.9, 9.4] |
|
Hazard ratio [95% CI]d |
0.41 [0.25, 0.66] |
|
|
One-sided p-value |
0.0001f |
|
Figure 1: Kaplan-Meier Plot of IRC-Assessed Event-Free Survival (Intention-to- Treat Analysis)
PILOT
The efficacy of BREYANZI was evaluated in a single-arm, open-label, multicenter trial (PILOT; NCT03483103) in transplant-ineligible patients with relapsed or refractory LBCL after one line of chemoimmunotherapy. The study enrolled patients who were not eligible for high-dose therapy and autologous HSCT due to organ function or age, while also having adequate organ function for CAR-T cell therapy. The study required at least one of the following criteria: age ≥ 70 years, adjusted diffusing capacity of the lung for carbon monoxide (DLCO) ≤ 60%; LVEF < 50%; creatinine clearance < 60mL/min; AST or ALT greater than 2 × ULN, or ECOG performance status of 2. The planned dose of BREYANZI was 100 × 106 CAR-positive viable T cells. Bridging therapy for disease control was permitted between leukapheresis and the start of lymphodepleting chemotherapy. Of the 61 patients treated with BREYANZI, 32 (53%) received bridging therapy.
BREYANZI was administered two to seven days following completion of lymphodepleting chemotherapy. The lymphodepleting chemotherapy regimen consisted of fludarabine 30 mg/m2/day and cyclophosphamide 300 mg/m2/day concurrently for 3 days. BREYANZI was administered in the inpatient (67%) and outpatient (33%) setting.
Of 74 patients who underwent leukapheresis, 61 (82%) received BREYANZI and comprise the main efficacy population; 1 (1.4%) received CAR-positive T cells that did not meet the product specifications for BREYANZI (manufacturing failure); and 12 (16%) did not receive CAR-positive T cells for other reasons.
Of the 61 patients who received BREYANZI, the median age was 74 years (range: 53 to 84 years), 61% were male, 89% were white, 3% were Asian, and 2% were black. Diagnoses included de novo DLBCL NOS (51%), high-grade B-cell lymphoma (33%), and DLBCL arising from follicular lymphoma (15%). Of these patients, 53% had primary refractory disease, 23% had relapse within 12 months of completing first-line therapy, and 25% had relapse >12 months after first-line therapy.
Efficacy was based on complete response (CR) rate and duration of response (DOR), as determined by an independent review committee (IRC) using 2014 Lugano criteria (Tables 10 and 11). The median time to CR was 1 month (range 0.8 to 6.9 months).
| a Per the Lugano criteria, as assessed by an IRC. | ||
| b 2-sided 95% exact Clopper-Pearson confidence intervals. | ||
|
Outcomea |
BREYANZI-Treated (N=61) |
All Leukapheresed Patients (N=74) |
|
Overall Response, n (%) [95% CI]b |
49 (80%) [68%, 89%] |
50 (68%) [56%, 78%] |
|
Complete Response [95% CI] |
33 (54%) [41%, 67%] |
34 (46%) [34%, 58%] |
|
Partial Response [95% CI] |
16 (26%) [16%, 39%] |
16 (22%) [13%, 33%] |
|
CI=confidence interval; NR=Not reached |
||
| DOR=duration of response; CI=confidence interval; CR=complete response; PR=partial response; NR=not reached. | |
| a Per the Lugano criteria, as assessed by an IRC. | |
| b Kaplan-Meier method is used to obtain 2-sided 95% confidence intervals. | |
| c Kaplan-Meier estimate. | |
| † A + sign indicates a censored value | |
|
Outcomea |
BREYANZI-Treated (N=61) |
|
Number of Responders |
49 |
|
DOR |
|
|
Median [95% CI], monthsb Range, months† |
11.2 [5.1, NR] 0.0+ to 22.8+ |
|
DOR if Best Response is CR Median [95% CI], months Range, months Rate at 6 months [95% CI]c Rate at 12 months [95% CI]c |
NR [11.2, NR] 2.0+ to 22.8+ 83% [64, 93] 68% [45, 83] |
|
DOR if Best Response is PR |
|
|
Median [95% CI], months |
2.1 [1.4, 2.3] |
|
Range, months |
0.0+ to 7.9 |
|
Rate at 6 months [95% CI]c |
8.2% [0.5, 30.5] |
Relapsed or Refractory LBCL After Two or More Lines of Therapy
TRANSCEND
The efficacy of BREYANZI was evaluated in an open-label, multicenter, single-arm trial (TRANSCEND; NCT02631044) in adult patients with relapsed or refractory large B-cell non-Hodgkin lymphoma after at least 2 lines of therapy. The study included patients with ECOG performance status ≤ 2, prior autologous and/or allogeneic HSCT, and secondary CNS lymphoma involvement. The study excluded patients with a creatinine clearance of less than 30 mL/min, alanine aminotransferase > 5 times the upper limit of normal, or left ventricular ejection fraction (LVEF) < 40%. There was no prespecified threshold for blood counts; patients were eligible to enroll if they were assessed by the investigator to have adequate bone marrow function to receive lymphodepleting chemotherapy. Bridging therapy for disease control was permitted between apheresis and the start of lymphodepleting chemotherapy, including intrathecal chemotherapy or radiation therapy for treatment of CNS involvement with lymphoma.
BREYANZI was administered two to seven days following completion of lymphodepleting chemotherapy. The lymphodepleting chemotherapy regimen consisted of fludarabine 30 mg/m2/day and cyclophosphamide 300 mg/m2/day concurrently for 3 days. BREYANZI was administered in the inpatient and outpatient setting.
Of 299 patients who underwent leukapheresis for whom BREYANZI was manufactured in the dose range of 50 to 110 × 106 CAR-positive viable T cells:
- •
- 44 (15%) did not receive CAR-positive T cells either due to manufacturing failures (n=2), death (n=29), disease complications (n=6), or other reasons (n=7).
- •
- 204 (68%) received BREYANZI in the intended dose range, of whom 192 were evaluable for efficacy (main efficacy population); 12 were not evaluable due to absence of PET positive disease at study baseline or after bridging therapy.
- •
- 51 (17%) either received BREYANZI outside of the intended dose range (n=26) or received CAR-positive T cells that did not meet the product specifications for BREYANZI (manufacturing failures; n=25).
Of the 192 patients in the main efficacy population, the median age was 63 years (range: 18 to 86 years), 69% were male, 84% were white, 6% were black, and 4.7% were Asian. The median number of prior therapies was 3 (range: 1 to 8). Diagnoses were de novo DLBCL (53%), DLBCL transformed from indolent lymphoma (25%), high-grade B-cell lymphoma (14%), primary mediastinal large B-cell lymphoma (7%), follicular lymphoma, grade 3B (1.0%). Of these patients, 64% had disease refractory to last therapy, 53% had primary refractory disease, 37% had prior HSCT and 2.6% had CNS involvement.
Efficacy was based on complete response (CR) rate and duration of response (DOR), as determined by an independent review committee (IRC) using 2014 Lugano criteria (Tables 12 and 13). The median time to first response (CR or partial response [PR]) was 1.0 month (range: 0.7 to 8.9 months). The median time to first CR was 1.0 month (range 0.8 to 12.5 months). Of the 104 patients who achieved CR, 23 initially had stable disease (6 patients) or PR (17 patients), with a median time to improvement of 2.2 months (range: 0.7 to 11.6 months).
| BREYANZI-Treated
N=192 |
|
|---|---|
| a Per the Lugano criteria, as assessed by an IRC. | |
|
Overall Response Ratea, n
|
141 (73%) |
|
Complete Response, n
|
104 (54%) |
|
Partial Response, n
|
37 (19%) |
CI=confidence interval.
| BREYANZI-Treateda
N=192 |
|
|---|---|
| Number of Responders | 141 |
| a Evaluable for efficacy. | |
| b KM method was used to obtain 2-sided 95% confidence intervals. | |
| † A + sign indicates a censored value. | |
|
DOR (Months) | |
|
Median [95% CI]b Range† |
16.7 [5.3, NR] 0.0+ to 23.5+ |
|
DOR if Best Response is CR (Months) | |
|
Median [95% CI] Range |
NR [16.7, NR] 0.7+ to 23.5+ |
|
DOR if Best Response is PR (Months) | |
|
Median [95% CI] Range |
1.4 [1.1, 2.2] 0.0+ to 22.8+ |
DOR=duration of response; CI=confidence interval; CR=complete response; PR=partial response; NR=not reached
Response durations were longer in patients who achieved a CR, as compared to patients with a best response of PR (Table 13). Of the 104 patients who achieved CR, 68 (65%) had remission lasting at least 6 months and 64 (62%) had remission lasting at least 9 months.
Of the 287 patients who underwent leukapheresis and had radiographically evaluable disease, 27 additional patients achieved a response, apart from the responses noted in Table 12. The IRC-assessed overall response rate in the leukapheresed population (n=287) was 59% (95% CI: 53, 64), with a CR rate of 43% (95% CI: 37, 49) and PR rate of 15% (95% CI: 11, 20). These efficacy results include responses that may have been contributed solely by bridging therapy, responses after receipt of product outside of the intended dose range, and responses to product that did not meet release specifications.
15. References
- 1.
- Lee DW, Gardner R, Porter DL, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood 2014;124:188-195.
16. How is Breyanzi supplied
BREYANZI consists of genetically modified autologous T cells, supplied in vials as separate frozen suspensions of each CD8 component (NDC 73153-901-08) and CD4 component (NDC 73153-902-04). Each CD8 or CD4 component is packed in a carton containing up to 4 vials, depending upon the concentration of the cryopreserved drug product CAR-positive viable T cells. The cartons for each CD8 component and CD4 component are in an outer carton (NDC 73153-900-01). BREYANZI is shipped directly to the cell lab or clinical pharmacy associated with the infusion center in the vapor phase of a liquid nitrogen shipper. A Release for Infusion (RFI) Certificate for each component and patient-specific syringe labels are affixed inside the shipper.
- •
- Confirm patient identity upon receipt.
- •
- Store vials in the vapor phase of liquid nitrogen (less than or equal to minus 130°C) in a temperature-monitored system.
- •
- Thaw BREYANZI prior to infusion [see Dosage and Administration (2.2)].
17. Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Ensure that patients understand the risk (11%) of manufacturing failure. In case of a manufacturing failure, a second manufacturing of BREYANZI may be attempted. While the patient awaits the product, additional bridging therapy (not the lymphodepletion) may be necessary. This bridging therapy may be associated with adverse events during the pre-infusion period, which could delay or prevent the administration of BREYANZI.
Advise patients that they will be monitored daily for at least 7 days following the BREYANZI infusion at a REMS-certified healthcare facility and instruct patients to remain within 2 hours of a REMS-certified healthcare facility for at least 4 weeks following the infusion.
Prior to infusion, advise patients of the following risks:
- •
- Cytokine Release Syndrome (CRS) – Signs and symptoms of CRS (fever, chills, hypotension, tachycardia, hypoxia, and fatigue). Counsel patients to seek immediate medical attention should signs or symptoms of CRS occur at any time [see Warnings and Precautions (5.1) and Adverse Reactions (6.1)].
- •
- Neurologic Toxicities – Signs or symptoms associated with neurologic events including encephalopathy, confusion, decreased consciousness, speech disorders, tremor, and seizures. Counsel patients to seek immediate medical attention should signs or symptoms of neurologic toxicity occur at any time [see Warnings and Precautions (5.2) and Adverse Reactions (6.1)].
- •
- Serious Infections – Signs or symptoms associated with infection [see Warnings and Precautions (5.5) and Adverse Reactions (6.1)].
- •
- Prolonged Cytopenias – Signs or symptoms associated with bone marrow suppression including neutropenia, anemia, thrombocytopenia, or febrile neutropenia [see Warnings and Precautions (5.6) and Adverse Reactions (6.1)].
Advise patients of the need to:
- •
- Contact Bristol-Myers Squibb at 1-888-805-4555 if they are diagnosed with a secondary malignancy [see Warnings and Precautions (5.8)].
- •
- Refrain from driving or operating heavy or potentially dangerous machines until at least 8 weeks after BREYANZI administration [see Warnings and Precautions (5.9)].
Manufactured by:
Juno Therapeutics Inc., a Bristol-Myers Squibb Company, Bothell, WA 98021. US License No.: 2156.
Celgene Corporation, a Bristol-Myers Squibb Company, Summit, NJ 07901. US License No.: 2252.
Bristol-Myers Squibb Company, Devens, MA 01434. US License No.: 1713.
BREYANZI® is a trademark of Juno Therapeutics, Inc., a Bristol-Myers Squibb Company.
Pat. https://www.bms.com/patient-and-caregivers/our-medicines.html
BREPI.004/MG.004
| MEDICATION GUIDE
BREYANZI® (pronounced braye an' zee) (lisocabtagene maraleucel) |
|---|
| This Medication Guide has been approved by the U.S. Food and Drug Administration. Revised: June 2023 |
|
Read this Medication Guide before you start your BREYANZI treatment. The more you know about your treatment, the more active you can be in your care. Talk with your healthcare provider if you have questions about your health condition or treatment. Reading this Medication Guide does not take the place of talking with your healthcare provider about your treatment. |
|
What is the most important information I should know about BREYANZI?
It is important that you tell your healthcare providers that you have received BREYANZI and to show them your BREYANZI Patient Wallet Card. Your healthcare provider may give you other medicines to treat your side effects. |
|
What is BREYANZI?
BREYANZI is different than other cancer medicines because it is made from your own white blood cells, which have been genetically modified to recognize and attack your lymphoma cells. |
|
Before getting BREYANZI, tell your healthcare provider about all your medical problems, including if you have or have had:
Tell your healthcare provider about all the medications you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. |
|
How will I receive BREYANZI?
|
|
What should I avoid after receiving BREYANZI?
|
|
What are the possible or reasonably likely side effects of BREYANZI? The most common side effects of BREYANZI are:
BREYANZI can increase the risk of life-threatening infections that may lead to death. Tell your healthcare provider right away if you develop fever, chills, or any signs or symptoms of an infection. |
|
General information about the safe and effective use of BREYANZI
Juno Therapeutics Inc., a Bristol-Myers Squibb Company, Bothell, WA 98021. US License No.: 2156 Celgene Corporation, a Bristol-Myers Squibb Company, Summit, NJ 07901. US License No.: 2252. Bristol-Myers Squibb Company, Devens, MA 01434. US License No.: 1713. |
PRINCIPAL DISPLAY PANEL - Kit Carton
lisocabtagene maraleucel
Breyanzi™
lisocabtagene maraleucel
Breyanzi®
NDC 73153-900-01
Rx only
FOR AUTOLOGOUS & INTRAVENOUS USE ONLY
Dispense with Medication Guide
Dosage: See Release for Infusion Certificate (inside shipper).
Contains DMSO 7.5% v/v.
1 to 4 single-dose vials/component. Each vial
contains up to 70 x 106 CAR-positive viable T cells/mL.
4.6 mL per vial.
Store at ≤ -130°C; vapor phase of liquid nitrogen.
Do not filter or irradiate.
Not evaluated for infectious substances.
Discard unused portion.
Manufactured by:
Juno Therapeutics, Inc., a Bristol-Myers Squibb Company
Bothell, WA 98021
Phone: 1-888-805-4555
US License #: 2156
304029
VERIFY
PATIENT
ID
First: FIRST NAME
Last: LAST NAME
Date of birth: DD-MMM-YYYY
JOIN: XXXX-XXXXX
Aph ID/DIN: XXX XXX XXX XXX
CD8
component
CD4
component
Lot
XXXX-XXXXXY
XXXX-XXXXXY
Expiration
DD-MMM-YYYY
DD-MMM-YYYY
STOP
Confirm patient ID
prior to infusion
| BREYANZI
lisocabtagene maraleucel kit |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - Juno Therapeutics, Inc. (079290042) |