Drug Detail:Butorphanol (monograph) (Medically reviewed)
Drug Class:
WARNING: ADDICTION, ABUSE, AND MISUSE; LIFE-THREATENING RESPIRATORY DEPRESSION; NEONATAL OPIOID WITHDRAWAL SYNDROME; and RISKS FROM CONCOMITANT USE WITH BENZODIAZEPINES OR OTHER CNS DEPRESSANTS
Butorphanol - Clinical Pharmacology
Pharmacokinetics
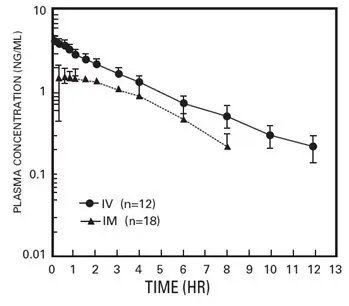
Butorphanol tartrate injection is rapidly absorbed after IM injection and peak plasma levels are reached in 20 to 40 minutes.
Following its initial absorption/distribution phase, the single dose pharmacokinetics of butorphanol by the intravenous and intramuscular routes of administration are similar (see Figure 1).
 |
Serum protein binding is independent of concentration over the range achieved in clinical practice (up to 7 ng/mL) with a bound fraction of approximately 80%.
The volume of distribution of butorphanol varies from 305 to 901 liters and total body clearance from 52 to 154 liters/hr (see Table 1).
| Parameters | Young | Elderly |
|---|---|---|
|
||
| AUC (inf)†
(hr ∙ ng/mL) | 7.24 (1.57)‡
(4.40–9.77)§ | 8.71 (2.02) (4.76–13.03) |
| Half-life (hr) | 4.56 (1.67) (2.06–8.70) | 5.61 (1.36) (3.25–8.79) |
| Volume of Distribution¶ (L) | 487 (155) (305–901) | 552 (124) (305–737) |
| Total body Clearance (L/hr) | 99 (23) (70–154) | 82 (21) (52–143) |
The drug is transported across the blood-brain and placental barriers and into human milk (see PRECAUTIONS: Labor or Delivery and Nursing Mothers).
Butorphanol is extensively metabolized in the liver. Metabolism is qualitatively and quantitatively similar following intravenous or intramuscular administration. Oral bioavailability is only 5 to 17% because of extensive first pass metabolism of butorphanol.
The major metabolite of butorphanol is hydroxybutorphanol, while norbutorphanol is produced in small amounts. Both have been detected in plasma following administration of butorphanol, with norbutorphanol present at trace levels at most time points. The elimination half-life of hydroxybutorphanol is about 18 hours and, as a consequence, considerable accumulation (~5-fold) occurs when butorphanol is dosed to steady state.
Elimination occurs by urine and fecal excretion. When 3H labeled butorphanol is administered to normal subjects, most (70 to 80%) of the dose is recovered in the urine, while approximately 15% is recovered in the feces.
About 5% of the dose is recovered in the urine as butorphanol. Forty-nine percent is eliminated in the urine as hydroxybutorphanol. Less than 5% is excreted in the urine as norbutorphanol.
Butorphanol pharmacokinetics in the elderly differ from younger patients (see Table 1).
In renally impaired patients with creatinine clearances <30 mL/min, the elimination half-life was approximately doubled and the total body clearance was approximately one half (10.5 hours [clearance 150 L/h] compared to 5.8 hours [clearance 260 L/h] in healthy subjects). No effect on Cmax or Tmax was observed after a single dose.
After intravenous administration to patients with hepatic impairment, the elimination half-life of butorphanol was approximately tripled and total body clearance was approximately one half (half-life 16.8 hours, clearance 92 L/h) compared to healthy subjects (half-life 4.8 hours, clearance 175 L/h). The exposure of hepatically impaired patients to butorphanol was significantly greater (about 2-fold) than that in healthy subjects.
(see PRECAUTIONS: Hepatic and Renal Disease, Drug Interactions and Geriatric Use and CLINICAL PHARMACOLOGY: Individualization of Dosage).
Clinical Trials
The effectiveness of opioid analgesics varies in different pain syndromes. Studies with butorphanol tartrate injection have been performed in postoperative (primarily abdominal and orthopedic) pain and pain during labor and delivery, as preoperative and preanesthetic medication, and as a supplement to balanced anesthesia (see below).
Individualization of Dosage
Use of butorphanol in geriatric patients, patients with renal impairment, patients with hepatic impairment and during labor requires extra caution (see below and the appropriate sections in PRECAUTIONS).
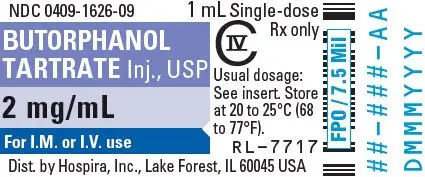
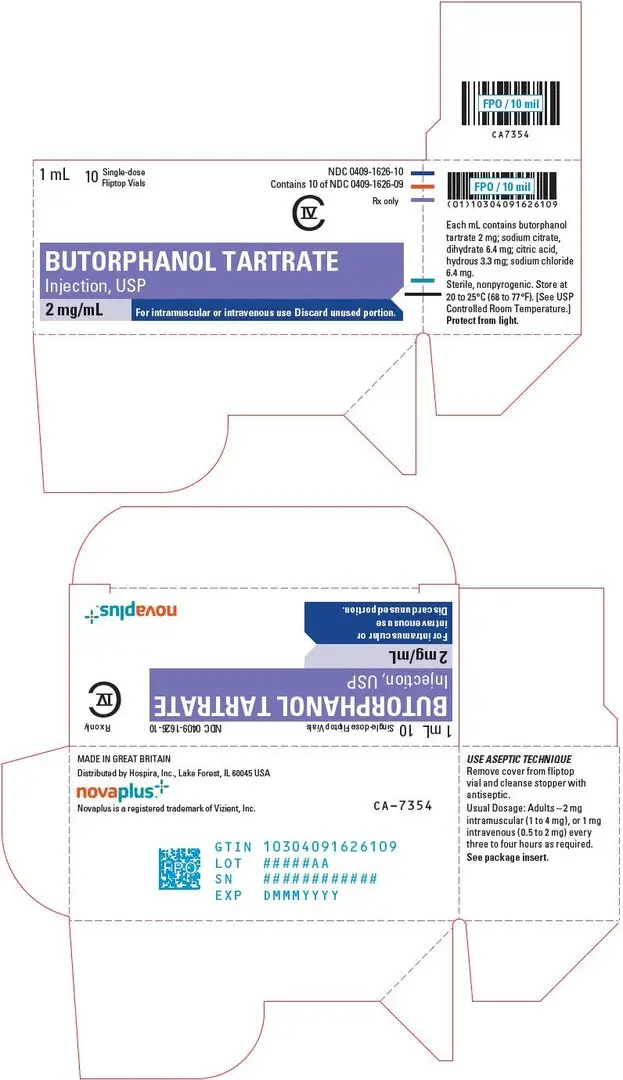
For pain relief the recommended initial dosage regimen of butorphanol tartrate injection is 1 mg IV or 2 mg IM with repeated doses every three to four hours as necessary. This dosage regimen is likely to be effective for the majority of patients. Dosage adjustments of butorphanol injection should be based on observations of its beneficial and adverse effects. The initial dose in the elderly and in patients with renal or hepatic impairment should generally be half the recommended adult dose (0.5 mg IV and 1 mg IM). Repeat doses in these patients should be determined by the patient's response rather than at fixed intervals but will generally be no less than 6 hours (see PRECAUTIONS).
The usual preoperative dose is 2 mg IM given 60 to 90 minutes before surgery or 2 mg IV shortly before induction. This is approximately equivalent in sedative effect to 10 mg morphine or 80 mg of meperidine. This single preoperative dose should be individualized based on age, body weight, physical status, underlying pathological condition, use of other drugs, type of anesthesia to be used and the surgical procedure involved.
During maintenance in balanced anesthesia the usual incremental dose of butorphanol tartrate is 0.5 to 1 mg IV. The incremental dose may be higher, up to 0.06 mg/kg (4 mg/70 kg), depending on previous sedative, analgesic, and hypnotic drugs administered. The total dose of butorphanol injection will vary; however, patients seldom require less than 4 mg or more than 12.5 mg (approximately 0.06 to 0.18 mg/kg).
As with other opioids of this class, butorphanol injection may not provide adequate intraoperative analgesia in every patient or under all conditions. A failure to achieve successful analgesia during balanced anesthesia is commonly reflected by increases in general sympathetic tone. Consequently, if blood pressure or heart rate continue to rise, consideration should be given to adding a potent volatile liquid inhalation anesthetic or another intravenous medication.
In labor, the recommended initial dose of butorphanol tartrate is 1 or 2 mg IM or IV in mothers with fetuses of 37 weeks gestation or beyond and without signs of fetal distress. Dosage adjustments of butorphanol in labor should be based on initial response with consideration given to concomitant analgesic or sedative drugs and the expected time of delivery. A dose should not be repeated in less than four hours nor administered less than four hours prior to the anticipated delivery (see PRECAUTIONS).
Related/similar drugs
aspirin, acetaminophen, tramadol, naproxen, oxycodone, Tylenol, fentanylIndications and Usage for Butorphanol
Butorphanol Tartrate Injection is indicated
- -
- as a preoperative or pre-anesthetic medication
- -
- as a supplement to balanced anesthesia
- -
- for the relief of pain during labor, and
- -
- for the management of pain severe enough to require an opioid analgesic and for which alternative treatments are inadequate.
Contraindications
Butorphanol Tartrate Injection is contraindicated in:
- Patients with significant respiratory depression (see WARNINGS)
- Patients with acute of severe bronchial asthma in an unmonitored setting or in the absence of resuscitative equipment (see WARNINGS)
- Patients with known or suspected gastrointestinal obstruction, including paralytic ileus (see WARNINGS)
- Patients with hypersensitivity to butorphanol tartrate or any of the formulation excipients (e.g., anaphylaxis) (see WARNINGS)
Warnings
Precautions
Hepatic and Renal Disease
In patients with hepatic or renal impairment, the initial dose of Butorphanol Tartrate Injection should generally be half the recommended adult dose (0.5 mg IV and 1 mg IM). Repeat doses in these patients should be determined by the patient's response rather than at fixed intervals but will generally be no less than 6 hours apart (see CLINICAL PHARMACOLOGY: Pharmacokinetics and Individualization of Dosage sections).
Pregnancy
Labor or Delivery
Opioids cross the placenta and may produce respiratory depression and psycho-physiologic effects in neonates. An opioid antagonist, such as naloxone, must be available for reversal of opioid-induced respiratory depression in the neonate. Butorphanol tartrate is not recommended for use in pregnant women during or immediately prior to labor, when other analgesic techniques are more appropriate. Opioid analgesics, including butorphanol tartrate, can prolong labor through actions which temporarily reduce the strength, duration, and frequency of uterine contractions. However, this effect is not consistent and may be offset by an increased rate of cervical dilation, which tends to shorten labor. Monitor neonates exposed to opioid analgesics during labor for signs of excess sedation and respiratory depression.
Butorphanol has been detected in milk following administration of Butorphanol Tartrate Injection to nursing mothers. The amount an infant would receive is probably clinically insignificant (estimated 4 µg/L of milk in a mother receiving 2 mg IM four times a day).
There have been rare reports of infant respiratory distress/apnea following the administration of Butorphanol Tartrate Injection during labor. The reports of respiratory distress/apnea have been associated with administration of a dose within 2 hours of delivery, use of multiple doses, use with additional analgesic or sedative drugs, or use in preterm pregnancies (see OVERDOSAGE: Treatment). In a study of 119 patients, the administration of 1 mg of IV Butorphanol Tartrate Injection during labor was associated with transient (10–90 minutes) sinusoidal fetal heart rate patterns, but 16 was not associated with adverse neonatal outcomes. In the presence of an abnormal fetal heart rate pattern, Butorphanol Tartrate Injection should be used with caution.
Nursing Mothers
Butorphanol has been detected in milk following administration of Butorphanol Tartrate Injection to nursing mothers. The amount an infant would receive is probably clinically insignificant (estimated 4 µg/L of milk in a mother receiving 2 mg IM four times a day).
The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for butorphanol tartrate and any potential adverse effects on the breastfed infant from butorphanol tartrate or from the underlying maternal condition.
Infants exposed to butorphanol tartrate through breast milk should be monitored for excess sedation and respiratory depression. Withdrawal symptoms can occur in breastfed infants when maternal administration of an opioid analgesic is stopped, or when breast-feeding is stopped.
Pediatric Use
Butorphanol is not recommended for use in patients below 18 years of age because safety and efficacy have not been established in this population.
Geriatric Use
Elderly patients (aged 65 years or older) may have increased sensitivity to butorphanol tartrate. In general, use caution when selecting a dosage for an elderly patient, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function and of concomitant disease or other drug therapy.
Respiratory depression is the chief risk for elderly patients treated with opioids, and has occurred after large initial doses were administered to patients who were not opioid-tolerant or when opioids were co-administered with other agents that depress respiration. Titrate the dosage of butorphanol tartrate slowly in geriatric patients (see WARNINGS).
This drug is known to be substantially excreted by the kidney, and the risk of adverse reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
Drug Abuse and Dependence
Overdosage
Treatment of Overdose
In case of overdose, priorities are the reestablishment of a patent and protected airway and institution of assisted of controlled ventilation, If needed. Employ other supportive measures (including oxygen and vasopressors) in the management of circulatory shock and pulmonary edema as indicated. Cardiac arrest or arrhythmias will require advanced life-support techniques.
The opioid antagonist, naloxone or nalmefene, are specific antidotes to respiratory depression resulting from opioid overdose. For clinically significant respiratory or circulatory depression secondary to butorphanol tartrate overdose, administer an opioid antagonist. As butorphanol is a mixed opioid agonist/antagonist, larger doses of naloxone or nalmefene may be needed to reverse the effects of an overdose.
Opioid antagonists should not be administered in the absence of clinically significant respiratory or circulatory depression secondary to butorphanol tartrate overdose.
Because the duration of opioid reversal is expected to be less than the duration of action of butorphanol in Butorphanol Tartrate Injection, carefully monitor the patient until spontaneous respiration is reliably re-established. If the response to an opioid antagonist is suboptimal or only brief in nature, administer additional antagonist as directed by the product's prescribing information.
In an individual physically dependent on opioids, administration of the recommended usual dosage of the antagonist will precipitate an acute withdrawal syndrome. The severity of the withdrawal symptoms experienced will depend on the degree of physical dependence and the dose of the antagonist administered. If a decision is made to treat serious respiratory depression in the physically dependent patient, administration of the antagonist should be begun with care and by titration with smaller than usual doses of the antagonist.
| BUTORPHANOL TARTRATE
butorphanol tartrate injection, solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Hospira, Inc. (141588017) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Hospira, Inc. | 030606222 | ANALYSIS(0409-1626) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Hospira, Inc. | 093132819 | ANALYSIS(0409-1626) , LABEL(0409-1626) , MANUFACTURE(0409-1626) , PACK(0409-1626) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Hospira, Inc. | 827731089 | ANALYSIS(0409-1626) | |