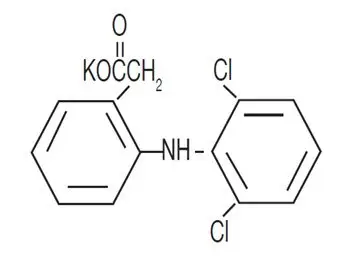
Drug Detail:Cataflam (Diclofenac [ dye-kloe-fen-ak ])
Drug Class: Nonsteroidal anti-inflammatory drugs
Cataflam - Clinical Pharmacology
Pharmacokinetics
Absorption
Diclofenac is 100% absorbed after oral administration compared to IV administration as measured by urine recovery. However, due to first pass metabolism, only about 50% of the absorbed dose is systemically available (see Table 1). In some fasting volunteers, measurable plasma levels are observed within 10 minutes of dosing with CATAFLAM. Peak plasma levels are achieved approximately 1 hour in fasting normal volunteers, with a range of .33 to 2 hours. Food has no significant effect on the extent of diclofenac absorption. However, there is usually a delay in the onset of absorption and a reduction in peak plasma levels of approximately 30%.
| PK Parameter | Normal Healthy Adults (20-52 years) | |
|---|---|---|
| Mean | Coefficient of Variation (%) | |
| Absolute Bioavailability (%) [N = 7] | 55 | 40 |
| Tmax (hr)[N = 65] | 1.0 | 76 |
| Oral Clearance (CL/F; mL/min) [N = 61] | 622 | 21 |
| Renal Clearance (% unchanged drug in urine) [N = 7] | < 1 | - |
| Apparent Volume of Distribution (V/F; L/kg) [N = 61] | 1.3 | 33 |
| Terminal Half-life (hr) [N = 48] | 1.9 | 29 |
Contraindications
CATAFLAM™ is contraindicated in the following patients:
- Known hypersensitivity (e.g., anaphylactic reactions and serious skin reactions) to diclofenac or any components of the drug product (see WARNINGS: Anaphylactic Reactions, Serious Skin Reactions).
- History of asthma, urticaria, or allergic type reactions after taking aspirin or other NSAIDs. Severe, sometimes fatal, anaphylactic reactions to NSAIDs have been reported in such patients (see WARNINGS: Anaphylactic Reactions, Exacerbation of Asthma Related to Aspirin Sensitivity).
- In the setting of coronary artery bypass graft (CABG) surgery (see WARNINGS: Cardiovascular Thrombotic Events).
Warnings
Cardiovascular Thrombotic Events
Clinical trials of several COX-2 selective and nonselective NSAIDs of up to three years duration have shown an increased risk of serious cardiovascular (CV) thrombotic events, including myocardial infarction (MI) and stroke, which can be fatal. Based on available data, it is unclear that the risk for CV thrombotic events is similar for all NSAIDs. The relative increase in serious CV thrombotic events over baseline conferred by NSAID use appears to be similar in those with and without known CV disease or risk factors for CV disease. However, patients with known CV disease or risk factors had a higher absolute incidence of excess serious CV thrombotic events, due to their increased baseline rate. Some observational studies found that this increased risk of serious CV thrombotic events began as early as the first weeks of treatment. The increase in CV thrombotic risk has been observed most consistently at higher doses.
To minimize the potential risk for an adverse CV event in NSAID treated patients, use the lowest effective dose for the shortest duration possible. Physicians and patients should remain alert for the development of such events, throughout the entire treatment course, even in the absence of previous CV symptoms. Patients should be informed about the symptoms of serious CV events and the steps to take if they occur.
There is no consistent evidence that concurrent use of aspirin mitigates the increased risk of serious CV thrombotic events associated with NSAID use. The concurrent use of aspirin and an NSAID, such as diclofenac, increases the risk of serious gastrointestinal (GI) events (see WARNINGS: Gastrointestinal Bleeding, Ulceration, and Perforation).
Gastrointestinal Bleeding, Ulceration, and Perforation
NSAIDs, including diclofenac, cause serious gastrointestinal (GI) adverse events including inflammation, bleeding, ulceration, and perforation of the esophagus, stomach, small intestine, or large intestine, which can be fatal. These serious adverse events can occur at any time, with or without warning symptoms, in patients treated with NSAIDs. Only one in five patients, who develop a serious upper GI adverse event on NSAID therapy, is symptomatic. Upper GI ulcers, gross bleeding or perforation caused by NSAIDs occurred in approximately 1% of patients treated for 3-6 months, and in about 2%-4% of patients treated for one year. However, even short-term therapy is not without risk.
Hepatotoxicity
In clinical trials of diclofenac-containing products, meaningful elevations (i.e., more than 3 times the ULN) of AST (SGOT) were observed in about 2% of approximately 5,700 patients at some time during diclofenac treatment (ALT was not measured in all studies).
In a large, open-label, controlled trial of 3,700 patients treated with oral diclofenac sodium for 2-6 months, patients were monitored first at 8 weeks and 1,200 patients were monitored again at 24 weeks. Meaningful elevations of ALT and/or AST occurred in about 4% of patients and included marked elevations (greater than 8 times the ULN) in about 1% of the 3,700 patients. In that open-label study, a higher incidence of borderline (less than 3 times the ULN), moderate (3-8 times the ULN), and marked (greater than 8 times the ULN) elevations of ALT or AST was observed in patients receiving diclofenac when compared to other NSAIDs. Elevations in transaminases were seen more frequently in patients with osteoarthritis than in those with rheumatoid arthritis.
Almost all meaningful elevations in transaminases were detected before patients became symptomatic. Abnormal tests occurred during the first 2 months of therapy with diclofenac in 42 of the 51 patients in all trials who developed marked transaminase elevations.
In postmarketing reports, cases of drug-induced hepatotoxicity have been reported in the first month, and in some cases, the first 2 months of therapy, but can occur at any time during treatment with diclofenac. Postmarketing surveillance has reported cases of severe hepatic reactions, including liver necrosis, jaundice, fulminant hepatitis with and without jaundice, and liver failure. Some of these reported cases resulted in fatalities or liver transplantation.
In a European retrospective population-based, case-controlled study, 10 cases of diclofenac associated drug-induced liver injury with current use compared with nonuse of diclofenac were associated with a statistically significant 4-fold adjusted odds ratio of liver injury. In this particular study, based on an overall number of 10 cases of liver injury associated with diclofenac, the adjusted odds ratio increased further with female gender, doses of 150 mg or more, and duration of use for more than 90 days.
Physicians should measure transaminases at baseline and periodically in patients receiving long-term therapy with diclofenac, because severe hepatotoxicity may develop without a prodrome of distinguishing symptoms. The optimum times for making the first and subsequent transaminase measurements are not known. Based on clinical trial data and postmarketing experiences, transaminases should be monitored within 4 to 8 weeks after initiating treatment with diclofenac. However, severe hepatic reactions can occur at any time during treatment with diclofenac.
If abnormal liver tests persist or worsen, if clinical signs and/or symptoms consistent with liver disease develop, or if systemic manifestations occur (e.g., eosinophilia, rash, abdominal pain, diarrhea, dark urine, etc.), CATAFLAM should be discontinued immediately.
Inform patients of the warning signs and symptoms of hepatotoxicity (e.g., nausea, fatigue, lethargy, diarrhea, pruritus, jaundice, right upper quadrant tenderness, and "flu-like" symptoms). If clinical signs and symptoms consistent with liver disease develop, or if systemic manifestations occur (e.g., eosinophilia, rash, etc.), discontinue CATAFLAM immediately, and perform a clinical evaluation of the patient.
To minimize the potential risk for an adverse liver related event in patients treated with CATAFLAM, use the lowest effective dose for the shortest duration possible. Exercise caution when prescribing CATAFLAM with concomitant drugs that are known to be potentially hepatotoxic (e.g., acetaminophen, antibiotics, anti-epileptics).
Hypertension
NSAIDs, including CATAFLAM can lead to new onset of hypertension or worsening of preexisting hypertension, either of which may contribute to the increased incidence of CV events. Patients taking angiotensin converting enzyme (ACE) inhibitors, thiazides diuretics or loop diuretics may have impaired response to these therapies when taking NSAIDs (see PRECAUTIONS: Drug Interactions).
Monitor blood pressure (BP) during the initiation of NSAID treatment and throughout the course of therapy.
Heart Failure and Edema
The Coxib and traditional NSAID Trialists' Collaboration meta-analysis of randomized controlled trials demonstrated an approximately two-fold increase in hospitalization for heart failure in COX-2 selective-treated patients and nonselective NSAID treated patients compared to placebo- treated patients. In a Danish National Registry study of patients with heart failure, NSAID use increased the risk of MI, hospitalization for heart failure, and death.
Additionally, fluid retention and edema have been observed in some patients treated with NSAIDs. Use of diclofenac may blunt the CV effects of several therapeutic agents used to treat these medical conditions (e.g., diuretics, ACE-inhibitors, or angiotensin receptor blockers [ARBs]) (see PRECAUTIONS: Drug Interactions).
Avoid the use of CATAFLAM in patients with severe heart failure unless the benefits are expected to outweigh the risk of worsening heart failure. If CATAFLAM is used in patients with severe heart failure, monitor patients for signs of worsening heart failure.
Anaphylactic Reactions
Diclofenac has been associated with anaphylactic reactions in patients with and without known hypersensitivity to diclofenac and in patients with aspirin-sensitive asthma (see CONTRAINDICATIONS, WARNINGS: Exacerbation of Asthma Related to Aspirin Sensitivity).
Exacerbation of Asthma Related to Aspirin Sensitivity
A subpopulation of patients with asthma may have aspirin-sensitive asthma which may include chronic rhinosinusitis complicated by nasal polyps; severe, potentially fatal bronchospasm; and/or intolerance to aspirin and other NSAIDs. Because cross-reactivity between aspirin and other NSAIDs has been reported in such aspirin-sensitive patients, CATAFLAM is contraindicated in patients with this form of aspirin sensitivity (see CONTRAINDICATIONS). When CATAFLAM is used in patients with preexisting asthma (without known aspirin sensitivity), monitor patients for changes in the signs and symptoms of asthma.
Serious Skin Reactions
NSAIDs, including diclofenac, can cause serious skin adverse events such as exfoliative dermatitis, Stevens-Johnson Syndrome (SJS), and toxic epidermal necrolysis (TEN), which can be fatal. These serious events may occur without warning. Inform patients about the signs and symptoms of serious skin reactions and discontinue the use of CATAFLAM at the first appearance of skin rash or any other sign of hypersensitivity. CATAFLAM is contraindicated in patients with previous serious skin reactions to NSAIDs (see CONTRAINDICATIONS).
Hematological Toxicity
Anemia has occurred in NSAID-treated patients. This may be due to occult or gross blood loss, fluid retention, or an incompletely described effect upon erythropoiesis. If a patient treated with CATAFLAM have any signs or symptoms of anemia, monitor hemoglobin or hematocrit.
NSAIDs, including CATAFLAM, may increase the risk of bleeding events. Co-morbid conditions such as coagulation disorders, concomitant use of warfarin and other anticoagulants, antiplatelet agents (e.g., aspirin), serotonin reuptake inhibitors (SSRIs) and serotonin norepinephrine reuptake inhibitors (SNRIs) may increase this risk. Monitor these patients for signs of bleeding (see PRECAUTIONS: Drug Interactions).
Precautions
Laboratory Monitoring
Because serious GI bleeding, hepatotoxicity, and renal injury can occur without warning symptoms or signs, consider monitoring patients on long-term NSAID treatment with a CBC and a chemistry profile periodically (see WARNINGS: Gastrointestinal Bleeding, Ulceration and Perforation, and Hepatotoxicity).
Drug Interactions
See Table 2 for clinically significant drug interactions with diclofenac.
| Drugs That Interfere with Hemostasis | |
| Clinical Impact: |
|
| Intervention: | Monitor patients with concomitant use of CATAFLAM with anticoagulants (e.g., warfarin), antiplatelet agents (e.g., aspirin), selective serotonin reuptake inhibitors (SSRIs), and serotonin norepinephrine reuptake inhibitors (SNRIs) for signs of bleeding (see WARNINGS: Hematological Toxicity). |
| Aspirin | |
| Clinical Impact: | Controlled clinical studies showed that the concomitant use of NSAIDs and analgesic doses of aspirin does not produce any greater therapeutic effect than the use of NSAIDs alone. In a clinical study, the concomitant use of an NSAID and aspirin was associated with a significantly increased incidence of GI adverse reactions as compared to use of the NSAID alone (see WARNINGS: Gastrointestinal Bleeding, Ulceration, and Perforation). |
| Intervention: | Concomitant use of CATAFLAM and analgesic doses of aspirin is not generally recommended because of the increased risk of bleeding (see WARNINGS: Hematological Toxicity). CATAFLAM is not a substitute for low-dose aspirin for cardiovascular protection. |
| ACE Inhibitors, Angiotensin Receptor Blockers, and Beta-Blockers | |
| Clinical Impact: |
|
| Intervention: |
|
| Diuretics | |
| Clinical Impact: | Clinical studies, as well as post-marketing observations, showed that NSAIDs reduced the natriuretic effect of loop diuretics (e.g., furosemide) and thiazide diuretics in some patients. This effect has been attributed to the NSAID inhibition of renal prostaglandin synthesis. |
| Intervention: | During concomitant use of CATAFLAM with diuretics, observe patients for signs of worsening renal function, in addition to assuring diuretic efficacy including antihypertensive effects (see WARNINGS: Renal Toxicity and Hyperkalemia). |
| Digoxin | |
| Clinical Impact: | The concomitant use of diclofenac with digoxin has been reported to increase the serum concentration and prolong the half-life of digoxin. |
| Intervention: | During concomitant use of CATAFLAM and digoxin, monitor serum digoxin levels. |
| Lithium | |
| Clinical Impact: | NSAIDs have produced elevations in plasma lithium levels and reductions in renal lithium clearance. The mean minimum lithium concentration increased 15%, and the renal clearance decreased by approximately 20%. This effect has been attributed to NSAID inhibition of renal prostaglandin synthesis. |
| Intervention: | During concomitant use of CATAFLAM and lithium, monitor patients for signs of lithium toxicity. |
| Methotrexate | |
| Clinical Impact: | Concomitant use of NSAIDs and methotrexate may increase the risk for methotrexate toxicity (e.g., neutropenia, thrombocytopenia, renal dysfunction). |
| Intervention: | During concomitant use of CATAFLAM and methotrexate, monitor patients for methotrexate toxicity. |
| Cyclosporine | |
| Clinical Impact: | Concomitant use of CATAFLAM and cyclosporine may increase cyclosporine's nephrotoxicity. |
| Intervention: | During concomitant use of CATAFLAM and cyclosporine, monitor patients for signs of worsening renal function. |
| NSAIDs and Salicylates | |
| Clinical Impact: | Concomitant use of diclofenac with other NSAIDs or salicylates (e.g., diflunisal, salsalate) increases the risk of GI toxicity, with little or no increase in efficacy (see WARNINGS: Gastrointestinal Bleeding, Ulceration, and Perforation). |
| Intervention: | The concomitant use of diclofenac with other NSAIDs or salicylates is not recommended. |
| Pemetrexed | |
| Clinical Impact: | Concomitant use of CATAFLAM and pemetrexed may increase the risk of pemetrexed associated myelosuppression, renal, and GI toxicity (see the pemetrexed prescribing information). |
| Intervention: | During concomitant use of CATAFLAM and pemetrexed, in patients with renal impairment whose creatinine clearance ranges from 45 mL/min to 79 mL/min, monitor for myelosuppression, renal and GI toxicity. NSAIDs with short elimination half-lives (e.g., diclofenac, indomethacin) should be avoided for a period of two days before, the day of, and two days following administration of pemetrexed. In the absence of data regarding potential interaction between pemetrexed and NSAIDs with longer half-lives (e.g., meloxicam, nabumetone), patients taking these NSAIDs should interrupt dosing for at least five days before, the day of, and two days following pemetrexed administration. |
| CYP2C9 Inhibitors or Inducers | |
| Clinical Impact: | Diclofenac is metabolized by cytochrome P450 enzymes, predominantly by CYP2C9. Co-administration of diclofenac with CYP2C9 inhibitors (e.g., voriconazole) may enhance the exposure and toxicity of diclofenac whereas co-administration with CYP2C9 inducers (e.g., rifampin) may lead to compromised efficacy of diclofenac. |
| Intervention: | A dosage adjustment may be warranted when diclofenac is administered with CYP2C9 inhibitors or inducers (see CLINICAL PHARMACOLOGY: Pharmacokinetics). |
Adverse Reactions/Side Effects
The following adverse reactions are discussed in greater detail in other sections of the labeling:
- Cardiovascular Thrombotic Events (see WARNINGS)
- GI Bleeding, Ulceration and Perforation (see WARNINGS)
- Hepatotoxicity (see WARNINGS)
- Hypertension (see WARNINGS)
- Heart Failure and Edema (see WARNINGS)
- Renal Toxicity and Hyperkalemia (see WARNINGS)
- Anaphylactic Reactions (see WARNINGS)
- Serious Skin Reactions (see WARNINGS)
- Hematologic Toxicity (see WARNINGS)
| Medication Guide for Nonsteroidal Anti-Inflammatory Drugs (NSAIDs) | ||
| What is the most important information I should know about medicines called Nonsteroidal Anti- Inflammatory Drugs (NSAIDs)? | ||
NSAIDs can cause serious side effects, including:
Avoid taking NSAIDs after a recent heart attack, unless your healthcare provider tells you to. You may have an increased risk of another heart attack if you take NSAIDs after a recent heart attack.
|
||
|
||
|
|
|
NSAIDs should only be used:
|
||
| What are NSAIDs?
NSAIDs are used to treat pain and redness, swelling, and heat (inflammation) from medical conditions such as different types of arthritis, menstrual cramps, and other types of short-term pain. |
||
| Who should not take NSAIDs? Do not take NSAIDs:
|
||
Before taking NSAIDs, tell your healthcare provider about all of your medical conditions, including if you:
What are the possible side effects of NSAIDs? NSAIDs can cause serious side effects, including: See "What is the most important information I should know about medicines called Nonsteroidal Anti-Inflammatory Drugs (NSAIDs)?"
|
||
|
|
|
| Stop taking your NSAID and call your healthcare provider right away if you get any of the following symptoms: | ||
|
|
|
| If you take too much of your NSAID, call your healthcare provider or get medical help right away. These are not all the possible side effects of NSAIDs. For more information, ask your healthcare provider or pharmacist about NSAIDs. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1- 800-FDA-1088. Other information about NSAIDs
|
||
| General information about the safe and effective use of NSAIDs
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use NSAIDs for a condition for which they were not prescribed. Do not give NSAIDs to other people, even if they have the same symptoms that you have. They may harm them. If you would like more information about NSAIDs, talk with your healthcare provider. You can ask your pharmacist or healthcare provider for information about NSAIDs that is written for health professionals. For more information, please visit: www.blucrestpharma.com or call BluCrest at 1-877-938-4525 This Medication Guide has been approved by the U.S. Food and Drug Administration. The brands listed are trademarks of their respective owners. Manufactured for: Blucrest Pharmaceuticals, LLC. Hazlet, NJ 07730 Revised: 03/2021 |
||
| CATAFLAM
diclofenac potassium tablet |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Labeler - BLUCREST PHARMACEUTICALS, LLC (117424533) |