Drug Class: Antiviral combinations
Highlights of Prescribing Information
COMPLERA™ (emtricitabine, rilpivirine, tenofovir disoproxil fumarate) tablets, for oral use
GILEAD ACCESS PROGRAM
Initial U.S. Approval: 2011
WARNING: POSTTREATMENT ACUTE EXACERBATION OF HEPATITIS B
See full prescribing information for complete boxed warning.
Severe acute exacerbations of hepatitis B virus (HBV) have been reported in patients coinfected with HIV-1 and HBV who have discontinued products containing emtricitabine (FTC) and/or tenofovir disoproxil fumarate (TDF), two of the components of COMPLERA. Closely monitor hepatic function with both clinical and laboratory follow-up for at least several months in patients who are coinfected with HIV-1 and HBV and discontinue COMPLERA. If appropriate, initiation of anti-hepatitis B therapy may be warranted. (5.1)
Recent Major Changes
| Warnings and Precautions | |
| Immune Reconstituition Syndrome (5.9) | 11/2019 |
Indications and Usage for Complera
COMPLERA, a combination of two nucleoside analog HIV-1 reverse transcriptase inhibitors (emtricitabine and tenofovir disoproxil fumarate) and one non-nucleoside reverse transcriptase inhibitor (rilpivirine), is indicated for use as a complete regimen for the treatment of HIV-1 infection in patients weighing at least 35 kg (1) as initial therapy in those with no antiretroviral treatment history and with HIV-1 RNA less than or equal to 100,000 copies/mL at the start of therapy, or (2) or to replace a stable antiretroviral regiment in those who are virologically suppressed (HIV-1 RNA <50 copies/mL) on a stable antiretroviral regimen for at least 6 months with no treatment failure and no known substitutions associated with resistance to the individual components of COMPLERA. (1, 14)
Limitations of Use:
- More rilpivirine-treated subjects with HIV-1 RNA greater than 100,000 copies/mL at the start of therapy experienced virologic failure (HIV-1 RNA ≥50 copies/mL) compared to rilpivirine-treated subjects with HIV-1 RNA less than or equal to 100,000 copies/mL. (1, 14)
Complera Dosage and Administration
- Testing: Prior to or when initiating COMPLERA, test for hepatitis B virus infection. Prior to initiation and during treatment with COMPLERA, on a clinically appropriate schedule, assess serum creatinine, estimated creatinine clearance, urine glucose, and urine protein in all patients. In patients with chronic kidney disease, also assess serum phosphorus. (2.1)
- Recommended dosage in adults and pediatric patients weighing at least 35 kg: One tablet taken orally once daily with food. (2.2)
- For pregnant patients who are already on COMPLERA prior to pregnancy and who are virologically suppressed (HIV-1 RNA less than 50 copies per mL), one tablet taken once daily may be continued. Lower exposures of rilpivirine were observed during pregnancy; therefore, viral load should be monitored closely. (2.3)
- Renal impairment: Not recommended in patients with estimated creatinine clearance below 50 mL per minute. (2.4)
- Recommended dosage with rifabutin coadministration: an additional 25 mg tablet of rilpivirine (Edurant) once per day taken concomitantly with COMPLERA and with a meal for the duration of the rifabutin coadministration. (2.5, 7.6, 12.3)
Dosage Forms and Strengths
Tablets: 200 mg of emtricitabine, 25 mg of rilpivirine, and 300 mg of tenofovir disoproxil fumarate. (3)
Contraindications
COMPLERA is contraindicated when coadministered with drugs which may result in loss of virologic response and possible resistance to COMPLERA. (4)
Warnings and Precautions
- Skin and Hypersensitivity Reactions: Severe skin and hypersensitivity reactions have been reported during postmarketing experience, including cases of Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS). Immediately discontinue treatment if hypersensitivity or rash with systemic symptoms or elevations in hepatic serum biochemistries develops and closely monitor clinical status, including hepatic serum biochemistries. (5.2)
- Hepatotoxicity: Hepatic adverse events have been reported in patients receiving a rilpivirine-containing regimen. Monitor liver-associated tests before and during treatment with COMPLERA in patients with underlying hepatic disease or marked elevations in liver-associated tests. Also consider monitoring liver-associated tests in patients without risk factors. (5.3)
- Depressive disorders: Severe depressive disorders have been reported. Immediate medical evaluation is recommended for severe depressive disorders. (5.4)
- New onset or worsening renal impairment: Can include acute renal failure and Fanconi syndrome. Avoid administering COMPLERA with concurrent or recent use of nephrotoxic drugs. (5.5)
- Decreases in bone mineral density (BMD): Consider monitoring BMD in patients with a history of pathologic fracture or other risk factors of osteoporosis or bone loss. (5.6)
- Concomitant use of COMPLERA with drugs with a known risk to prolong the QTc interval of the electrocardiogram may increase the risk of Torsade de Pointes. (5.7)
- Lactic acidosis/severe hepatomegaly with steatosis: Discontinue treatment in patients who develop symptoms or laboratory findings suggestive of lactic acidosis or pronounced hepatotoxicity. (5.8)
- Immune reconstitution syndrome: May necessitate further evaluation and treatment. (5.9)
Adverse Reactions/Side Effects
- Most common adverse reactions to rilpivirine (incidence greater than or equal to 2%, Grades 2–4) are depressive disorders, insomnia, and headache. (6.1)
- Most common adverse reactions to emtricitabine and tenofovir disoproxil fumarate (incidence greater than or equal to 10%) are diarrhea, nausea, fatigue, headache, dizziness, depression, insomnia, abnormal dreams, and rash. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Gilead Sciences, Inc. at [email protected] or the US FDA at 1-800-FDA-1088 or at www.fda.gov/medwatch
Drug Interactions
- COMPLERA is a complete regimen for the treatment of HIV-1 infection; therefore, coadministration with other antiretroviral medications for treatment of HIV-1 infection is not recommended. (7.1)
- Consult the Full Prescribing Information prior to and during treatment for important drug interactions. (4, 5.7, 7)
Use In Specific Populations
- Pregnancy: Monitor viral load closely during pregnancy as rilpivirine exposures were generally lower during pregnancy. (2.3, 8.1, 12.3)
- Lactation: Breastfeeding not recommended due to the potential for HIV-1 transmission. (8.2)
- Pediatrics: Not recommended for patients weighing less than 35 kg. (8.4)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 11/2019
Related/similar drugs
Biktarvy, Truvada, tenofovir, emtricitabine, Atripla, Stribild, EpzicomFull Prescribing Information
WARNING: POSTTREATMENT ACUTE EXACERBATION OF HEPATITIS B
Severe acute exacerbations of hepatitis B virus (HBV) have been reported in patients who are coinfected with HBV and HIV-1 and have discontinued products containing emtricitabine (FTC) and/or tenofovir disoproxil fumarate (TDF), two of the components of COMPLERA.
Closely monitor hepatic function with both clinical and laboratory follow-up for at least several months in patients who are coinfected with HIV-1 and HBV and discontinue COMPLERA. If appropriate, initiation of anti-hepatitis B therapy may be warranted [see Warnings and Precautions (5.1)].
1. Indications and Usage for Complera
COMPLERA™ is indicated as a complete regimen for the treatment of HIV-1 infection in adults and pediatric patients weighing at least 35 kg:
- as initial therapy in those with no antiretroviral treatment history with HIV-1 RNA less than or equal to 100,000 copies/mL at the start of therapy or
- to replace a stable antiretroviral regimen in those who are virologically suppressed (HIV-1 RNA less than 50 copies/mL) on a stable antiretroviral regimen for at least 6 months with no treatment failure and no known substitutions associated with resistance to the individual components of COMPLERA [see Microbiology (12.4) and Clinical Studies (14)].
2. Complera Dosage and Administration
2.1 Testing Prior to Initiation and During Treatment with COMPLERA
Prior to or when initiating COMPLERA, test patients for hepatitis B virus infection [see Warnings and Precautions (5.1)].
Prior to initiation of COMPLERA, and during treatment with COMPLERA, on a clinically appropriate schedule, assess serum creatinine, estimated creatinine clearance, urine glucose and urine protein in all patients. In patients with chronic kidney disease, also assess serum phosphorus [see Warnings and Precautions (5.5)].
2.2 Recommended Dosage
COMPLERA is a three-drug fixed dose combination product containing 200 mg of emtricitabine (FTC), 25 mg of rilpivirine (RPV), and 300 mg of tenofovir disoproxil fumarate (TDF). The recommended dosage of COMPLERA in adult and pediatric patients weighing at least 35 kg is one tablet taken orally once daily with food [see Use in Specific Populations (8.4) and Clinical Pharmacology (12.3)].
2.3 Recommended Dosage During Pregnancy
For pregnant patients who are already on COMPLERA prior to pregnancy and are virologically suppressed (HIV-1 RNA less than 50 copies per mL), one tablet of COMPLERA taken once daily may be continued. Lower exposures of rilpivirine, a component of COMPLERA, were observed during pregnancy, therefore viral load should be monitored closely [see Use in Specific Populations (8.1) and Clinical Pharmacology (12.3)].
2.4 Not Recommended in Patients with Moderate or Severe Renal Impairment
COMPLERA is not recommended in patients with moderate or severe renal impairment (estimated creatinine clearance below 50 mL per minute) [see Warnings and Precautions (5.5) and Use in Specific Populations (8.6)].
2.5 Recommended Dosage with Rifabutin Coadministration
If COMPLERA is coadministered with rifabutin, take an additional 25 mg tablet of rilpivirine (Edurant) with COMPLERA once daily with a meal for the duration of the rifabutin coadministration [see Drug Interactions (7.6) and Clinical Pharmacology (12.3)].
3. Dosage Forms and Strengths
Each COMPLERA tablet contains 200 mg of emtricitabine (FTC), 27.5 mg of rilpivirine hydrochloride (equivalent to 25 mg of rilpivirine [RPV]), and 300 mg of tenofovir disoproxil fumarate (TDF, equivalent to 245 mg of tenofovir disoproxil).
The tablets are white, capsule shaped, film coated and debossed with "GSI" on both sides.
4. Contraindications
COMPLERA is contraindicated when coadministered with the following drugs; coadministration may result in loss of virologic response and possible resistance to COMPLERA or to the class of NNRTIs [see Warnings and Precautions (5.7), Drug Interactions (7), and Clinical Pharmacology (12.3)]:
- Anticonvulsants: carbamazepine, oxcarbazepine, phenobarbital, phenytoin
- Antimycobacterials: rifampin, rifapentine
- Glucocorticoid (systemic): dexamethasone (more than a single-dose)
- Herbal Products: St John's wort (Hypericum perforatum)
- Proton Pump Inhibitors: e.g., dexlansoprazole, esomeprazole, lansoprazole, omeprazole, pantoprazole, rabeprazole
5. Warnings and Precautions
5.1 Severe Acute Exacerbation of Hepatitis B in Patients Coinfected with HIV-1 and HBV
Test all patients with HIV-1 for the presence of chronic hepatitis B virus (HBV) before or when initiating antiretroviral therapy [see Dosage and Administration (2.1)].
Severe acute exacerbations of hepatitis B (e.g., liver decompensation and liver failure) have been reported in patients who are coinfected with HBV and HIV-1 and have discontinued products containing FTC and/or TDF, two of the components of COMPLERA. Patients coinfected with HIV-1 and HBV who discontinue COMPLERA should be closely monitored with both clinical and laboratory follow-up for at least several months after stopping treatment with COMPLERA. If appropriate, initiation of anti-hepatitis B therapy may be warranted, especially in patients with advanced liver disease or cirrhosis, since posttreatment exacerbation of hepatitis may lead to hepatic decompensation and liver failure.
5.2 Skin and Hypersensitivity Reactions
Severe skin and hypersensitivity reactions have been reported during the postmarketing experience, including cases of Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) with RPV-containing regimens. While some skin reactions were accompanied by constitutional symptoms such as fever, other skin reactions were associated with organ dysfunctions, including elevations in hepatic serum biochemistries. During the Phase 3 clinical trials, treatment-related rashes with at least Grade 2 severity were reported in 1% of subjects receiving RPV plus FTC/TDF. Overall, most rashes were Grade 1 or 2 and occurred in the first four to six weeks of therapy [see Adverse Reactions (6.1 and 6.2)].
Discontinue COMPLERA immediately if signs or symptoms of severe skin or hypersensitivity reactions develop, including but not limited to, severe rash or rash accompanied by fever, blisters, mucosal involvement, conjunctivitis, facial edema, angioedema, hepatitis, or eosinophilia. Clinical status including laboratory parameters should be monitored and appropriate therapy should be initiated.
5.3 Hepatotoxicity
Hepatic adverse events have been reported in patients receiving an RPV-containing regimen. Patients with underlying hepatitis B or C virus infection, or marked elevations in liver-associated tests prior to treatment, may be at increased risk for worsening or development of liver-associated test elevations with use of COMPLERA. A few cases of hepatic toxicity have been reported in adult patients receiving an RPV-containing regimen who had no pre-existing hepatic disease or other identifiable risk factors. Appropriate laboratory testing prior to initiating therapy and monitoring for hepatotoxicity during therapy with COMPLERA is recommended in patients with underlying hepatic disease such as hepatitis B or C, or in patients with marked elevations in liver-associated tests prior to treatment initiation. Liver-associated test monitoring should also be considered for patients without pre-existing hepatic dysfunction or other risk factors.
5.4 Depressive Disorders
The adverse reaction depressive disorders (depressed mood, depression, dysphoria, major depression, mood altered, negative thoughts, suicide attempt, suicidal ideation) has been reported with RPV. Patients with severe depressive symptoms should seek immediate medical evaluation to assess the possibility that the symptoms are related to COMPLERA, and if so, to determine whether the risks of continued therapy outweigh the benefits.
During the Phase 3 trials in adults (N=1368) through 96 weeks, the incidence of depressive disorders (regardless of causality, severity) reported among RPV (n=686) or efavirenz (EFV, n=682) was 9% and 8%, respectively. Most events were mild or moderate in severity. The incidence of Grades 3 and 4 depressive disorders (regardless of causality) was 1% for both RPV and EFV. The incidence of discontinuation due to depressive disorders among RPV or EFV was 1% in each arm. Suicidal ideation was reported in 4 subjects in each arm while suicide attempt was reported in 2 subjects in the RPV arm.
During the Phase 2 trial in pediatric subjects 12 to less than 18 years of age (N=36) receiving RPV through 48 weeks, the incidence of depressive disorders (regardless of causality, severity) was 19.4% (7/36). Most events were mild or moderate in severity. The incidence of Grade 3 and 4 depressive disorders (regardless of causality) was 5.6% (2/36). None of the subjects discontinued due to depressive disorders. Suicidal ideation and suicide attempt were reported in 1 subject.
5.5 New Onset or Worsening Renal Impairment
Renal impairment, including cases of acute renal failure and Fanconi syndrome (renal tubular injury with severe hypophosphatemia), has been reported with the use of TDF [see Adverse Reactions (6.2)].
Prior to initiation of COMPLERA, and during treatment with COMPLERA, on a clinically appropriate schedule, assess serum creatinine, estimated creatinine clearance, urine glucose, and urine protein in all patients. In patients with chronic kidney disease, also assess serum phosphorus.
COMPLERA should be avoided with concurrent or recent use of a nephrotoxic agent (e.g., high-dose or multiple nonsteroidal anti-inflammatory drugs [NSAIDs]) [see Drug Interactions (7.4)]. Cases of acute renal failure after initiation of high-dose or multiple NSAIDs have been reported in HIV-infected patients with risk factors for renal dysfunction who appeared stable on TDF. Some patients required hospitalization and renal replacement therapy. Alternatives to NSAIDs should be considered, if needed, in patients at risk for renal dysfunction.
Persistent or worsening bone pain, pain in extremities, fractures, and/or muscular pain or weakness may be manifestations of proximal renal tubulopathy and should prompt an evaluation of renal function in at-risk patients.
Emtricitabine and TDF are principally eliminated by the kidney; however, RPV is not. Since COMPLERA is a combination product and the dose of the individual components cannot be altered, COMPLERA is not recommended in patients with estimated creatinine clearance below 50 mL per minute [see Use in Specific Populations (8.6)].
5.7 Risk of Adverse Reactions or Loss of Virologic Response Due to Drug Interactions
The concomitant use of COMPLERA and other drugs may result in potentially significant drug interactions, some of which may lead to [see Dosage and Administration (2.5), Contraindications (4), and Drug Interactions (7)]:
- Loss of therapeutic effect of COMPLERA and possible development of resistance due to reduced exposure to RPV.
- Possible clinically significant adverse reaction from greater exposures of components of COMPLERA.
In healthy subjects, 75 mg once daily and 300 mg once daily doses of RPV (3 times and 12 times the dose in COMPLERA) have been shown to prolong the QTc interval of the electrocardiogram. Consider alternatives to COMPLERA when coadministered with a drug that is known to have a risk of Torsade de Pointes [see Drug Interactions (7) and Clinical Pharmacology (12.2)].
See Table 4 for steps to prevent or manage these possible and known significant drug interactions, including dosing recommendations. Consider the potential for drug interactions prior to and during COMPLERA therapy and review concomitant medications during COMPLERA therapy.
5.8 Lactic Acidosis/Severe Hepatomegaly with Steatosis
Lactic acidosis and severe hepatomegaly with steatosis, including fatal cases, have been reported with the use of nucleoside analogs, including TDF and FTC, components of COMPLERA, alone or in combination with other antiretrovirals. Treatment with COMPLERA should be suspended in any patient who develops clinical or laboratory findings suggestive of lactic acidosis or pronounced hepatotoxicity (which may include hepatomegaly and steatosis even in the absence of marked transaminase elevations).
5.9 Immune Reconstitution Syndrome
Immune reconstitution syndrome has been reported in patients treated with combination antiretroviral therapy, including the components of COMPLERA. During the initial phase of combination antiretroviral treatment, patients whose immune system responds may develop an inflammatory response to indolent or residual opportunistic infections (such as Mycobacterium avium infection, cytomegalovirus, Pneumocystis jirovecii pneumonia [PCP], or tuberculosis), which may necessitate further evaluation and treatment.
Autoimmune disorders (such as Graves' disease, polymyositis, Guillain-Barré syndrome, and autoimmune hepatitis) have also been reported to occur in the setting of immune reconstitution; however, the time to onset is more variable and can occur many months after initiation of treatment.
6. Adverse Reactions/Side Effects
The following adverse reactions are discussed in other sections of the labeling:
- Severe Acute Exacerbations of Hepatitis B in Patients Coinfected with HIV-1 and HBV [see Warnings and Precautions (5.1)].
- Skin and Hypersensitivity Reactions [see Warnings and Precautions (5.2)].
- Hepatotoxicity [see Warnings and Precautions (5.3)].
- Depressive Disorders [see Warnings and Precautions (5.4)].
- New Onset or Worsening Renal Impairment [see Warnings and Precautions (5.5)].
- Bone Loss and Mineralization Defects [see Warnings and Precautions (5.6)].
- Lactic Acidosis/Severe Hepatomegaly with Steatosis [see Warnings and Precautions (5.8)].
- Immune Reconstitution Syndrome [see Warnings and Precautions (5.9)].
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Adverse Reactions from Clinical Trials Experience in Adult Subjects
In HIV-1-Infected Adult Subjects With No Antiretroviral Treatment History
6.2 Postmarketing Experience
The following adverse reactions have been identified during postmarketing experience in patients receiving RPV- or TDF-containing regimens. Because postmarketing reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
7. Drug Interactions
7.1 Not Recommended with Other Antiretroviral Medications
Because COMPLERA is a complete regimen, coadministration with other antiretroviral medications for the treatment of HIV-1 infection is not recommended. Comprehensive information regarding potential drug-drug interactions with other antiretroviral medications is not provided.
This section describes clinically relevant drug interactions with COMPLERA. Drug interaction studies were conducted with the components of COMPLERA (FTC, RPV, and TDF as single agents) or with COMPLERA as a combination product [see Dosage and Administration (2), Contraindications (4), and Clinical Pharmacology (12.3)].
7.2 Drugs Inducing or Inhibiting CYP3A Enzymes
Rilpivirine is primarily metabolized by cytochrome P450 (CYP) 3A, and drugs that induce or inhibit CYP3A may thus affect the clearance of RPV [see Contraindications (4), Warnings and Precautions (5.7), and Clinical Pharmacology (12.3)]. Coadministration of RPV and drugs that induce CYP3A may result in decreased plasma concentrations of RPV and loss of virologic response and possible resistance to RPV or to the class of NNRTIs. Coadministration of RPV and drugs that inhibit CYP3A may result in increased plasma concentrations of RPV.
7.3 Drugs Increasing Gastric pH
Coadministration of RPV with drugs that increase gastric pH may decrease plasma concentrations of RPV and loss of virologic response and possible resistance to RPV or to the class of NNRTIs. Use of RPV with proton pump inhibitors is contraindicated and use of RPV with H2-receptor antagonists requires staggered administration [see Contraindications (4) and Clinical Pharmacology (12.3)].
7.4 Drugs Affecting Renal Function
Because FTC and tenofovir are primarily eliminated by the kidneys through a combination of glomerular filtration and active tubular secretion, coadministration of COMPLERA with drugs that reduce renal function or compete for active tubular secretion may increase serum concentrations of FTC, tenofovir, and/or other renally eliminated drugs. Some examples of drugs that are eliminated by active tubular secretion include, but are not limited to, acyclovir, adefovir dipivoxil, cidofovir, ganciclovir, valacyclovir, valganciclovir, aminoglycosides (e.g., gentamicin), and high-dose or multiple NSAIDs [see Warnings and Precautions (5.5)].
7.5 QT Prolonging Drugs
There is limited information available on the potential for a pharmacodynamic interaction between RPV and drugs that prolong the QTc interval of the electrocardiogram. In a study of healthy subjects, 75 mg once daily and 300 mg once daily doses of RPV (3 times and 12 times the dose in COMPLERA) have been shown to prolong the QTc interval of the electrocardiogram [see Warnings and Precautions (5.7) and Clinical Pharmacology (12.2)]. Consider alternatives to COMPLERA when coadministered with a drug with a known risk of Torsade de Pointes.
7.6 Significant Drug Interactions
Important drug interaction information for COMPLERA is summarized in Table 4. The drug interactions described are based on studies conducted with FTC, RPV, or TDF as individual medications or with COMPLERA as a combination product, or are potential drug interactions [see Clinical Pharmacology (12.3), Tables 9–14]. For list of contraindicated drugs, [see Contraindications (4)].
| Concomitant Drug Class: Drug Name | Effect on Concentration† | Clinical Comment |
|---|---|---|
|
||
| Antacids:
antacids (e.g., aluminum, magnesium hydroxide, or calcium carbonate) | ↔ RPV (antacids taken at least 2 hours before or at least 4 hours after RPV) ↓ RPV (concomitant intake) | Administer antacids at least 2 hours before or at least 4 hours after COMPLERA. |
| Anticonvulsants:
carbamazepine oxcarbazepine phenobarbital phenytoin | ↓ RPV | Coadministration is contraindicated due to potential for loss of virologic response and development of resistance. |
| Antimycobacterials:
rifampin rifapentine | ↓ RPV | Coadministration is contraindicated due to potential for loss of virologic response and development of resistance. |
| rifabutin | ↓ RPV‡ | If COMPLERA is coadministered with rifabutin, an additional 25 mg tablet of RPV (Edurant) once per day is recommended to be taken concomitantly with COMPLERA and with a meal for the duration of rifabutin coadministration. |
| Azole Antifungal Agents: fluconazole itraconazole ketoconazole posaconazole voriconazole | ↑ RPV‡,§
↓ ketoconazole‡,§ | No dose adjustment is required when COMPLERA is coadministered with azole antifungal agents. Clinically monitor for breakthrough fungal infections when azole antifungals are coadministered with COMPLERA. |
| Glucocorticoid (systemic):
dexamethasone (more than a single-dose treatment) | ↓ RPV | Coadministration is contraindicated due to potential for loss of virologic response and development of resistance. |
| Hepatitis C Antiviral Agents: ledipasvir/sofosbuvir sofosbuvir/velpatasvir sofosbuvir/velpatasvir/voxilaprevir | ↑ tenofovir‡ | Patients receiving COMPLERA concomitantly with HARVONI™ (ledipasvir/sofosbuvir), EPCLUSA™ (sofosbuvir/velpatasvir), or VOSEVI™ (sofosbuvir/velpatasvir/voxilaprevir) should be monitored for adverse reactions associated with TDF. |
| H2-Receptor Antagonists:
cimetidine famotidine nizatidine ranitidine | ↔ RPV‡,§
(famotidine taken 12 hours before RPV or 4 hours after RPV) ↓ RPV‡,§ (famotidine taken 2 hours before RPV) | Administer H2-receptor antagonists at least 12 hours before or at least 4 hours after COMPLERA. |
| Herbal Products:
St John's wort (Hypericum perforatum) | ↓ RPV | Coadministration is contraindicated due to potential for loss of virologic response and development of resistance. |
| Macrolide or Ketolide Antibiotics:
clarithromycin erythromycin telithromycin | ↑ RPV ↔ clarithromycin ↔ erythromycin ↔ telithromycin | Where possible, alternatives such as azithromycin should be considered. |
| Narcotic Analgesics: methadone | ↓ R(−) methadone‡
↓ S(+) methadone‡ ↔ RPV‡ ↔ methadone‡ (when used with tenofovir) | No dose adjustments are required when initiating coadministration of methadone with COMPLERA. However, clinical monitoring is recommended as methadone maintenance therapy may need to be adjusted in some patients. |
| Proton Pump Inhibitors:
e.g., dexlansoprazole esomeprazole lansoprazole omeprazole pantoprazole rabeprazole | ↓ RPV | Coadministration is contraindicated due to potential for loss of virologic response and development of resistance. |
7.7 Drugs with No Observed Interactions with COMPLERA
No clinically significant drug interactions have been observed between FTC and the following medications: famciclovir, ledipasvir/sofosbuvir, sofosbuvir/velpatasvir, sofosbuvir/velpatasvir/voxilaprevir, or TDF.
No clinically significant drug interactions have been observed between TDF and the following medications: entecavir, methadone, oral contraceptives, ribavirin, sofosbuvir, or tacrolimus in studies conducted in healthy subjects.
No clinically significant drug interactions have been observed between RPV and the following medications: acetaminophen, atorvastatin, chlorzoxazone, ethinyl estradiol, ledipasvir/sofosbuvir, norethindrone, sildenafil, simeprevir, sofosbuvir, sofosbuvir/velpatasvir, sofosbuvir/velpatasvir/voxilaprevir, or TDF. RPV did not have a clinically significant effect on the pharmacokinetics of digoxin or metformin.
8. Use In Specific Populations
8.1 Pregnancy
Data
8.4 Pediatric Use
The safety and effectiveness of COMPLERA as a complete regimen for the treatment of HIV-1 infection was established in pediatric subjects 12 years of age and older with body weight greater than or equal to 35 kg [see Dosage and Administration (2.2)]. Use of COMPLERA in this age group weighing at least 35 kg is supported by adequate and well-controlled studies of RPV+FTC+TDF in adults with HIV-1 infection as well as data from pediatric studies of the individual components of COMPLERA (RPV, FTC, and TDF) [see Clinical Pharmacology (12.3), and Clinical Studies (14.2)].
COMPLERA should only be administered to pediatric patients with a body weight greater than or equal to 35 kg. Because COMPLERA is a fixed-dose combination tablet, the dose of COMPLERA cannot be adjusted for patients of lower weight. Safety and effectiveness for COMPLERA have not been established in pediatric patients weighing less than 35 kg [see Adverse Reactions (6.1) and Clinical Pharmacology (12.3)].
8.5 Geriatric Use
Clinical studies of FTC, RPV, or TDF did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. In general, dose selection for elderly patients should be cautious, keeping in mind the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy [see Clinical Pharmacology (12.3)].
8.6 Renal Impairment
Because COMPLERA is a fixed-dose combination, and cannot be dose adjusted, it is not recommended in patients with moderate, severe, or end-stage renal impairment (estimated creatinine clearance below 50 mL per minute) or that require dialysis [see Warnings and Precautions (5.5) and Clinical Pharmacology (12.3)].
10. Overdosage
If overdose occurs the patient must be monitored for evidence of toxicity. Treatment of overdose with COMPLERA consists of general supportive measures, including monitoring of vital signs and ECG (QT interval) as well as observation of the clinical status of the patient.
11. Complera Description
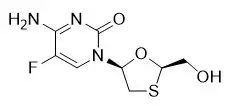
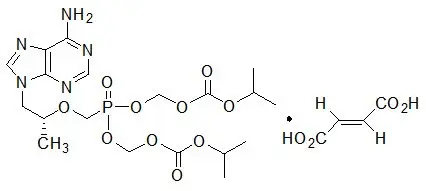
COMPLERA is a fixed-dose combination tablet containing FTC, rilpivirine hydrochloride, and TDF. Emtricitabine (FTC) is a synthetic nucleoside analog of cytidine. Rilpivirine (RPV) is a non-nucleoside reverse transcriptase inhibitor. Tenofovir disoproxil fumarate (TDF) is converted in vivo to tenofovir, an acyclic nucleoside phosphonate (nucleotide) analog of adenosine 5′-monophosphate.
COMPLERA tablets are for oral administration. Each tablet contains 200 mg of FTC, 27.5 mg of rilpivirine hydrochloride (equivalent to 25 mg of RPV), and 300 mg of TDF (equivalent to 245 mg of tenofovir disoproxil) as active ingredients. The tablets include the following inactive ingredients: croscarmellose sodium, lactose monohydrate, magnesium stearate, microcrystalline cellulose, polysorbate 20, povidone, pregelatinized starch. The tablets are film coated with a coating material containing hypromellose, lactose monohydrate, polyethylene glycol, titanium dioxide, triacetin.
12. Complera - Clinical Pharmacology
12.1 Mechanism of Action
COMPLERA is a fixed-dose combination of the antiretroviral drugs FTC, RPV, and TDF [see Microbiology (12.4)].
12.3 Pharmacokinetics
Specific Populations
Pediatric Patients
Pediatric trials have not been conducted using COMPLERA tablets. Pediatric information is based on trials conducted with the individual components of COMPLERA [see Use in Specific Populations (8.4)].
Rilpivirine: The pharmacokinetics of RPV in antiretroviral treatment-naïve HIV-1- infected pediatric subjects 12 to less than 18 years of age receiving RPV 25 mg once daily were comparable to those in treatment-naïve HIV-1-infected adults receiving RPV 25 mg once daily (See Table 7). There was no clinically significant impact of body weight on RPV pharmacokinetics in pediatric subjects in trial C213 (33 to 93 kg).
| Parameter | RPV 25 mg once daily N=34 |
|---|---|
| AUC24h (ng∙h/mL) | |
| Mean ± Standard Deviation | 2424 ± 1024 |
| Median (Range) | 2269 (417–5166) |
| C0h (ng/mL) | |
| Mean ± Standard Deviation | 85 ± 40 |
| Median (Range) | 79 (7–202) |
Pregnancy and Postpartum
The exposure (C0h and AUC24h) to total RPV after intake of RPV 25 mg once daily as part of an antiretroviral regimen was 30 to 40% lower during pregnancy (similar for the second and third trimester), compared with postpartum (see Table 8). However, the exposure during pregnancy was not significantly different from exposures obtained in Phase 3 trials of RPV-containing regimens. Based on the exposure-response relationship for RPV, this decrease is not considered clinically relevant in patients who are virologically suppressed. The protein binding of RPV was similar (>99%) during the second trimester, third trimester, and postpartum.
| Pharmacokinetics of total RPV (mean ±SD, tmax: median [range]) | Postpartum (6–12 Weeks) (n=11) | 2nd Trimester of pregnancy (n=15) | 3rd Trimester of pregnancy (n=13) |
|---|---|---|---|
| C0h, ng/mL | 111 ± 69.2 | 65.0 ± 23.9 | 63.5 ± 26.2 |
| Cmin, ng/mL | 84.0 ± 58.8 | 54.3 ± 25.8 | 52.9 ± 24.4 |
| Cmax, ng/mL | 167 ± 101 | 121 ± 45.9 | 123 ± 47.5 |
| tmax, h | 4.00 (2.03–25.08) | 4.00 (1.00–9.00) | 4.00 (2.00–24.93) |
| AUC24h, ng∙h/mL | 2,714 ± 1,535 | 1,792 ± 711 | 1,762 ± 662 |
12.4 Microbiology
Resistance
In HIV-1-Infected Adult Subjects With No Antiretroviral Treatment History
In the Week 96 pooled resistance analysis for adult subjects receiving RPV or EFV in combination with FTC/TDF in the Phase 3 clinical trials C209 and C215, the emergence of resistance was greater among subjects' viruses in the RPV + FTC/TDF arm compared to the EFV + FTC/TDF arm and was dependent on baseline viral load. In the pooled resistance analysis, 61% (47/77) of the subjects who qualified for resistance analysis (resistance analysis subjects) in the RPV + FTC/TDF arm had virus with genotypic and/or phenotypic resistance to RPV compared to 42% (18/43) of the resistance analysis subjects in the EFV + FTC/TDF arm who had genotypic and/or phenotypic resistance to EFV. Moreover, genotypic and/or phenotypic resistance to FTC or tenofovir emerged in viruses from 57% (44/77) of the resistance analysis subjects in the RPV arm compared to 26% (11/43) in the EFV arm.
Emerging NNRTI substitutions in the RPV resistance analysis of subjects' viruses included V90I, K101E/P/T, E138K/A/Q/G, V179I/L, Y181C/I, V189I, H221Y, F227C/L, and M230L, which were associated with an RPV phenotypic fold change range of 2.6–621. The E138K substitution emerged most frequently during RPV treatment, commonly in combination with the M184I substitution. The FTC and lamivudine resistance-associated substitutions M184I or V and NRTI resistance-associated substitutions (K65R/N, A62V, D67N/G, K70E, Y115F, K219E/R) emerged more frequently in the RPV resistance analysis subjects than in EFV resistance analysis subjects (See Table 15).
NNRTI- and NRTI-resistance substitutions emerged less frequently in the resistance analysis of viruses from subjects with baseline viral loads of ≤100,000 copies/mL compared to viruses from subjects with baseline viral loads of >100,000 copies/mL: 23% (10/44) compared to 77% (34/44) of NNRTI-resistance substitutions and 20% (9/44) compared to 80% (35/44) of NRTI-resistance substitutions. This difference was also observed for the individual FTC/lamivudine and tenofovir resistance substitutions: 22% (9/41) compared to 78% (32/41) for M184I/V and 0% (0/8) compared to 100% (8/8) for K65R/N. Additionally, NNRTI and/or NRTI-resistance substitutions emerged less frequently in the resistance analysis of the viruses from subjects with baseline CD4+ cell counts ≥200 cells/mm3 compared to the viruses from subjects with baseline CD4+ cell counts <200 cells/mm3: 32% (14/44) compared to 68% (30/44) of NNRTI-resistance substitutions and 27% (12/44) compared to 73% (32/44) of NRTI-resistance substitutions.
| C209 and C215 N=1096 |
||
|---|---|---|
| RPV + FTC/TDF | EFV + FTC/TDF | |
| N=550 | N=546 | |
|
||
| Subjects who Qualified for Resistance Analysis | 14% (77/550) | 8% (43/546) |
| Subjects with Evaluable Postbaseline Resistance Data | 70 | 31 |
| Emergent NNRTI Substitutions† | ||
| Any | 63% (44/70) | 55% (17/31) |
| V90I | 14% (10/70) | 0 |
| K101E/P/T/Q | 19% (13/70) | 10% (3/31) |
| K103N | 1% (1/70) | 39% (12/31) |
| E138K/A/Q/G | 40% (28/70) | 0 |
| E138K+M184I‡ | 30% (21/70) | 0 |
| V179I/D | 6% (4/70) | 10% (3/31) |
| Y181C/I/S | 13% (9/70) | 3% (1/31) |
| V189I | 9% (6/70) | 0 |
| H221Y | 10% (7/70) | 0 |
| Emergent NRTI Substitutions§ | ||
| Any | 63% (44/70) | 32% (10/31) |
| M184I/V | 59% (41/70) | 26% (8/31) |
| K65R/N | 11% (8/70) | 6% (2/31) |
| A62V, D67N/G, K70E, Y115F, or K219E/R¶ | 20% (14/70) | 3% (1/31) |
Cross Resistance
Rilpivirine:
13. Nonclinical Toxicology
14. Clinical Studies
14.2 Pediatric Subjects
The pharmacokinetics, safety, and efficacy of RPV in combination with other antiretroviral agents was evaluated in a single-arm, open-label Phase 2 trial in antiretroviral treatment-naïve HIV-1-infected pediatric subjects 12 to less than 18 years of age and weighing at least 32 kg (TMC-C213). Thirty-six (36) subjects were enrolled with a median age of 14.5 years (range 12 to 17 years), and were 55.6% female, 88.9% Black, and 11.1% Asian. The majority of subjects (24/36) received RPV in combination with FTC and TDF. Of these 24 subjects, 20 had baseline HIV RNA ≤100,000 copies/mL. The baseline characteristics and efficacy outcomes at Week 48 are further described below for the 20 subjects.
The median baseline plasma HIV-1 RNA and CD4+ cell count were 49,550 (range 2060 to 92,600 copies/mL) and 437.5 cells/mm3 (range 123 to 983 cells/mm3), respectively. At Week 48, 80% (16/20) of the subjects had HIV RNA <50 copies/mL, 15% (3/20) had HIV RNA ≥50 copies/mL, and one subject discontinued therapy prior to Week 48 and before reaching virologic suppression (HIV RNA <50 copies/mL). At Week 48, the mean increase in CD4+ cell count from baseline was 225 cells/mm3.
16. How is Complera supplied
COMPLERA tablets are white, capsule shaped, film coated, and debossed with "GSI" on both sides. Each bottle contains 30 tablets, a silica gel desiccant, and a polyester fiber coil, and is closed with a child-resistant closure.
17. Patient Counseling Information
Advise the patient to read the approved patient labeling (Patient Information).
| This Patient Information has been approved by the U.S. Food and Drug Administration. | Revised: 11/2019 | ||
| Patient Information COMPLERA™ (kom-PLEH-rah) (emtricitabine, rilpivirine, tenofovir disoproxil fumarate) tablets |
|||
| Important: Ask your healthcare provider or pharmacist about medicines that should not be taken with COMPLERA. For more information, see the section "What should I tell my healthcare provider before taking COMPLERA?" | |||
| What is the most important information I should know about COMPLERA? COMPLERA can cause serious side effects, including: Worsening of Hepatitis B virus (HBV) infection. Your healthcare provider will test you for HBV before starting treatment with COMPLERA. If you have HBV infection and take COMPLERA, your HBV may get worse (flare-up) if you stop taking COMPLERA. A "flare-up" is when your HBV infection suddenly returns in a worse way than before.
|
|||
| What is COMPLERA?
COMPLERA is a prescription medicine that is used to treat Human Immunodeficiency Virus-1 (HIV-1) in people weighing at least 77 lb (35 kg) who:
COMPLERA contains 3 medicines (emtricitabine, rilpivirine, tenofovir disoproxil fumarate) combined in one tablet. Emtricitabine (EMTRIVA®) and tenofovir disoproxil fumarate (VIREAD®) are HIV-1 nucleoside analog reverse transcriptase inhibitors (NRTIs). Rilpivirine (Edurant®) is an HIV-1 non-nucleoside analog reverse transcriptase inhibitor (NNRTI). It is not known if COMPLERA is safe and effective in children less than 12 years of age or who weigh less than 77 lb (35 kg). |
|||
| Who should not take COMPLERA? Do not take COMPLERA if you also take:
|
|||
| What should I tell my healthcare provider before taking COMPLERA? Before taking COMPLERA, tell your healthcare provider about all your medical conditions, including if you:
Some medicines interact with COMPLERA. Keep a list of your medicines to show your healthcare provider and pharmacist when you get a new medicine.
|
|||
How should I take COMPLERA?
|
|||
| What are the possible side effects of COMPLERA? COMPLERA can cause serious side effects, including:
|
|||
|
|
||
|
|||
|
|
||
| These are not all the possible side effects of COMPLERA. Call your healthcare provider for medical advice about side effects. You may report side effects to US FDA at 1-800-FDA-1088. |
|||
How should I store COMPLERA?
|
|||
| General information about safe and effective use of COMPLERA
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use COMPLERA for a condition for which it was not prescribed. Do not give COMPLERA to other people, even if they have the same symptoms you have. It may harm them. You can ask your healthcare provider or pharmacist for information about COMPLERA that is written for health professionals. For more information, go to www.COMPLERA.com. |
|||
| What are the ingredients of COMPLERA? Active ingredients: emtricitabine, rilpivirine hydrochloride, and tenofovir disoproxil fumarate. Inactive ingredients: croscarmellose sodium, lactose monohydrate, magnesium stearate, microcrystalline cellulose, polysorbate 20, povidone, pregelatinized starch. The tablet film coating contains hypromellose, lactose monohydrate, polyethylene glycol, titanium dioxide, triacetin. Manufactured and distributed for: Gilead Sciences, Inc. Foster City, CA 94404 COMPLERA, EMTRIVA, and VIREAD are trademarks of Gilead Sciences, Inc., or its related companies. All other trademarks referenced herein are the property of their respective owners. © 2019 Gilead Sciences, Inc. All rights reserved. 202123-GS-013A |
|||
| COMPLERA ACCESS
emtricitabine, rilpivirine hydrochloride, and tenofovir disoproxil fumarate tablet, film coated |
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| Labeler - Gilead Sciences, Inc. (185049848) |