Drug Detail:Conjupri (Levamlodipine [ lee-vam-loe-di-peen ])
Drug Class: Calcium channel blocking agents
Highlights of Prescribing Information
CONJUPRI ® (levamlodipine) tablets, for oral use.
Initial U.S. Approval: 2019
Indications and Usage for Conjupri
CONJUPRI ® is calcium channel blocker and may be used alone or in combination with other antihypertensive agents for the treatment of hypertension, to lower blood pressure. Lowering blood pressure reduces the risk of fatal and nonfatal cardiovascular events, primarily strokes and myocardial infarctions. (1)
Conjupri Dosage and Administration
- Adult recommended starting dose: 2.5 mg orally once daily with maximum dose 5 mg once daily. (
2.1)
- Small, fragile, or elderly patients, or patients with hepatic insufficiency may be started on 1.25 mg once daily. ( 2.1)
- Pediatric starting dose: 1.25 mg to 2.5 mg once daily. ( 2.2)
Important Limitation: Doses in excess of 2.5 mg daily have not been studied in pediatric patients. ( 2.2)
Dosage Forms and Strengths
- Tablets: 2.5 mg (functionally scored), and 5 mg, 2.5 mg tablets can be split for 1.25 mg dose. ( 3)
Contraindications
- Known sensitivity to amlodipine. ( 4)
Warnings and Precautions
- Symptomatic hypotension is possible, particularly in patients with severe aortic stenosis. However, acute hypotension is unlikely. ( 5.1)
- Worsening angina and acute myocardial infarction can develop after starting or increasing the dose of amlodipine, particularly in patients with severe obstructive coronary artery disease. ( 5.2)
- Titrate slowly in patients with severe hepatic impairment. ( 5.3)
Adverse Reactions/Side Effects
Most common adverse reactions to amlodipine is edema which occurred in a dose related manner. Other adverse experiences not dose related but reported with an incidence >1.0% are fatigue, nausea, abdominal pain and somnolence. ( 6)
To report SUSPECTED ADVERSE REACTIONS, call WraSer Pharmaceuticals LLC at 1-888-252-3901 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
- Do not exceed doses greater than 20 mg daily of simvastatin. ( 7.2)
Use In Specific Populations
- Pediatric: Effect on patients less than 6 years old is not known. ( 8.4).
- Geriatric: Start dosing at the low end of the dose range. ( 8.5)
See 17 for FDA-approved patient labeling.
Revised: 8/2021
Full Prescribing Information
1. Indications and Usage for Conjupri
1.1 Hypertension
CONJUPRI ® is indicated for the treatment of hypertension in adults and pediatric patients 6 years and older, to lower blood pressure. Lowering blood pressure reduces the risk of fatal and nonfatal cardiovascular events, primarily strokes and myocardial infarctions. These benefits have been seen in controlled trials of antihypertensive drugs from a wide variety of pharmacologic classes including levamlodipine.
Control of high blood pressure should be part of comprehensive cardiovascular risk management, including, as appropriate, lipid control, diabetes management, antithrombotic therapy, smoking cessation, exercise, and limited sodium intake. Many patients will require more than one drug to achieve blood pressure goals. For specific advice on goals and management, see published guidelines, such as those of the National High Blood Pressure Education Program's Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC).
Numerous antihypertensive drugs, from a variety of pharmacologic classes and with different mechanisms of action, have been shown in randomized controlled trials to reduce cardiovascular morbidity and mortality, and it can be concluded that it is blood pressure reduction, and not some other pharmacologic property of the drugs, that is largely responsible for those benefits. The largest and most consistent cardiovascular outcome benefit has been a reduction in the risk of stroke, but reductions in myocardial infarction and cardiovascular mortality also have been seen regularly.
Elevated systolic or diastolic pressure causes increased cardiovascular risk, and the absolute risk increase per mmHg is greater at higher blood pressures, so that even modest reductions of severe hypertension can provide substantial benefit. Relative risk reduction from blood pressure reduction is similar across populations with varying absolute risk, so the absolute benefit is greater in patients who are at higher risk independent of their hypertension (for example, patients with diabetes or hyperlipidemia), and such patients would be expected to benefit from more aggressive treatment to a lower blood pressure goal.
Some antihypertensive drugs have smaller blood pressure effects (as monotherapy) in black patients, and many antihypertensive drugs have additional approved indications and effects (e.g., on angina, heart failure, or diabetic kidney disease). These considerations may guide selection of therapy.
Levamlodipine may be used alone or in combination with other antihypertensive agents.
2. Conjupri Dosage and Administration
2.1 Adults
The usual initial antihypertensive oral dose of levamlodipine is 2.5 mg once daily, and the maximum dose is 5 mg once daily.
Small, fragile, or elderly patients, or patients with hepatic insufficiency may be started on 1.25 mg once daily and this dose may be used when adding levamlodipine to other antihypertensive therapy.
Adjust dosage according to blood pressure goals. In general, wait 7 to 14 days between titration steps. Titrate more rapidly, however, if clinically warranted, provided the patient is assessed frequently.
3. Dosage Forms and Strengths
Tablet, 2.5 mg is white to off-white, capsule shaped, flat-faced tablet with functional score on each side, engraved with "OE" on one side and "B47" on the other side.
Tablet of 2.5 mg can be split for 1.25 mg dose for medical conditions that need 1.25 mg dose.
Tablet, 5 mg is white to off-white, soap shaped, flat-faced tablet, engraved with “OE” on one side and “B48” on the other side.
4. Contraindications
Levamlodipine is contraindicated in patients with known sensitivity to amlodipine.
5. Warnings and Precautions
5.1 Hypotension
Symptomatic hypotension is possible, particularly in patients with severe aortic stenosis. Because of the gradual onset of action, acute hypotension is unlikely.
6. Adverse Reactions/Side Effects
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Amlodipine has been evaluated for safety in more than 11,000 patients in U.S. and foreign clinical trials. In general, treatment with amlodipine besylate was well-tolerated at doses up to 10 mg daily. Most adverse reactions reported during therapy with amlodipine were of mild or moderate severity. In controlled clinical trials directly comparing amlodipine besylate (N = 1730) at doses up to 10 mg to placebo (N = 1250), discontinuation of amlodipine besylate because of adverse reactions was required in only about 1.5% of patients and was not significantly different from placebo (about 1%). The most commonly reported side effects more frequent than placebo are reflected in the table below. The incidence (%) of side effects that occurred in a dose related manner are as follows:
| Amlodipine | Placebo | |||
|---|---|---|---|---|
| 2.5mg | 5mg | 10mg | ||
| N=275 | N=296 | N=268 | N=520 | |
| Edema | 1.8 | 3.0 | 10.8 | 0.6 |
| Dizziness | 1.1 | 3.4 | 3.4 | 1.5 |
| Flushing | 0.7 | 1.4 | 2.6 | 0.0 |
| Palpitation | 0.7 | 1.4 | 4.5 | 0.6 |
Other adverse reactions that were not clearly dose related but were reported with an incidence greater than 1.0% in placebo-controlled clinical trials include the following:
| Amlodipine (%)
(N=1730) | Placebo (%)
(N=1250) |
|
|---|---|---|
| Fatigue | 4.5 | 2.8 |
| Nausea | 2.9 | 1.9 |
| Abdominal Pain | 1.6 | 0.3 |
| Somnolence | 1.4 | 0.6 |
For several adverse experiences that appear to be drug and dose related, there was a greater incidence in women than men associated with amlodipine treatment as shown in the following table:
| Amlodipine (%) | Placebo (%) | |||
|---|---|---|---|---|
| Male = %
(N=1218) | Female = %
(N=512) | Male = %
(N=914) | Female = %
(N=336) |
|
| Edema | 5.6 | 14.6 | 1.4 | 5.1 |
| Flushing | 1.5 | 4.5 | 0.3 | 0.9 |
| Palpitations | 1.4 | 3.3 | 0.9 | 0.9 |
| Somnolence | 1.3 | 1.6 | 0.8 | 0.3 |
The following events occurred in <1% but >0.1% of patients in controlled clinical trials or under conditions of open trials or marketing experience where a causal relationship is uncertain; they are listed to alert the physician to a possible relationship:
Cardiovascular: arrhythmia (including ventricular tachycardia and atrial fibrillation), bradycardia, chest pain, peripheral ischemia, syncope, tachycardia, vasculitis.
Central and Peripheral Nervous System: hypoesthesia, neuropathy peripheral, paresthesia, tremor, vertigo.
Gastrointestinal: anorexia, constipation, dysphagia, diarrhea, flatulence, pancreatitis, vomiting, gingival hyperplasia.
General: allergic reaction, asthenia, 1 back pain, hot flushes, malaise, pain, rigors, weight gain, weight decrease.
Musculoskeletal System: arthralgia, arthrosis, muscle cramps, 1 myalgia.
Psychiatric: sexual dysfunction (male 1 and female), insomnia, nervousness, depression, abnormal dreams, anxiety, depersonalization.
Respiratory System: dyspnea, 1 epistaxis.
Skin and Appendages: angioedema, erythema multiforme, pruritus, 1 rash, 1 rash erythematous, rash maculopapular.
Special Senses: abnormal vision, conjunctivitis, diplopia, eye pain, tinnitus.
Urinary System: micturition frequency, micturition disorder, nocturia.
Autonomic Nervous System: dry mouth, sweating increased.
Metabolic and Nutritional: hyperglycemia, thirst.
Hemopoietic: leukopenia, purpura, thrombocytopenia.
Amlodipine therapy has not been associated with clinically significant changes in routine laboratory tests. No clinically relevant changes were noted in serum potassium, serum glucose, total triglycerides, total cholesterol, HDL cholesterol, uric acid, blood urea nitrogen, or creatinine.
In the CAMELOT and PREVENT studies of amlodipine in coronary artery disease, the adverse event profile was similar to that reported previously (see above), with the most common adverse event being peripheral edema.
- 1
- These events occurred in less than 1% in placebo-controlled trials, but the incidence of these side effects was between 1% and 2% in all multiple dose studies.
6.2 Postmarketing Experience
Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
The following postmarketing event has been reported infrequently where a causal relationship is uncertain: gynecomastia. In postmarketing experience, jaundice and hepatic enzyme elevations (mostly consistent with cholestasis or hepatitis), in some cases severe enough to require hospitalization, have been reported in association with use of amlodipine.
Postmarketing reporting has also revealed a possible association between extrapyramidal disorder and amlodipine.
Amlodipine has been used safely in patients with chronic obstructive pulmonary disease, well-compensated congestive heart failure, coronary artery disease, peripheral vascular disease, diabetes mellitus, and abnormal lipid profiles.
7. Drug Interactions
8. Use In Specific Populations
8.4 Pediatric Use
Levamlodipine (1.25 to 2.5 mg daily) is effective in lowering blood pressure in patients 6 to 17 years [see Clinical Studies (14.1)] . Effect of levamlodipine on blood pressure in patients less than 6 years of age is not known.
8.5 Geriatric Use
Clinical studies of amlodipine did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy. Elderly patients have decreased clearance of amlodipine with a resulting increase of AUC of approximately 40–60%, and a lower initial dose may be required [see Dosage and Administration (2.1)] .
10. Overdosage
Overdosage might be expected to cause excessive peripheral vasodilation with marked hypotension and possibly a reflex tachycardia. In humans, experience with intentional overdosage of amlodipine is limited.
Single oral doses of amlodipine equivalent to 40 mg amlodipine/kg and 100 mg amlodipine/kg in mice and rats, respectively, caused deaths. Single oral amlodipine doses equivalent to 4 or more mg amlodipine/kg or higher in dogs (11 or more times the maximum recommended human dose on a mg/m 2 basis) caused a marked peripheral vasodilation and hypotension.
If massive overdose should occur, initiate active cardiac and respiratory monitoring. Frequent blood pressure measurements are essential. Should hypotension occur, provide cardiovascular support including elevation of the extremities and the judicious administration of fluids. If hypotension remains unresponsive to these conservative measures, consider administration of vasopressors (such as phenylephrine) with attention to circulating volume and urine output. As amlodipine is highly protein bound, hemodialysis is not likely to be of benefit.
11. Conjupri Description
The active ingredient levamlodipine maleate is the maleate salt of levamlodipine, the pharmacologically active isomer of amlodipine, a long-acting calcium channel blocker.
Levamlodipine maleate is chemically described as (S)3-ethyl-5-methyl-2-(2-aminoethoxymethyl)-4-(2-chlorophenyl)-1,4-dihydro-6-methyl-3,5-pyridinedicarboxylate maleate, and its structural formula is:
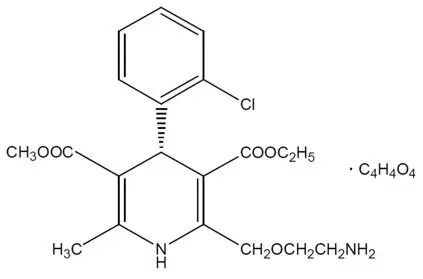
Levamlodipine maleate is an off-white to light yellow crystalline powder with a molecular weight of 524.95. It is slightly soluble in water and sparingly soluble in ethanol. CONJUPRI ® (levamlodipine) tablets are formulated as white to off-white tablets containing 2.5, and 5 mg of levamlodipine (equivalent to 3.2, and 6.4 mg of levamlodipine maleate respectively), for oral administration. In addition to the active ingredient, levamlodipine maleate, each tablet contains the following inactive ingredients: betadex, colloidal silicon dioxide, magnesium stearate, microcrystalline cellulose, and pregelatinized starch .
12. Conjupri - Clinical Pharmacology
12.1 Mechanism of Action
Amlodipine is a dihydropyridine calcium antagonist (calcium ion antagonist or slow-channel blocker) that inhibits the transmembrane influx of calcium ions into vascular smooth muscle and cardiac muscle. Experimental data suggest that amlodipine binds to both dihydropyridine and nondihydropyridine binding sites. The contractile processes of cardiac muscle and vascular smooth muscle are dependent upon the movement of extracellular calcium ions into these cells through specific ion channels. Amlodipine inhibits calcium ion influx across cell membranes selectively, with a greater effect on vascular smooth muscle cells than on cardiac muscle cells. Negative inotropic effects can be detected in vitro but such effects have not been seen in intact animals at therapeutic doses. Serum calcium concentration is not affected by amlodipine. Within the physiologic pH range, amlodipine is an ionized compound (pKa=8.6), and its kinetic interaction with the calcium channel receptor is characterized by a gradual rate of association and dissociation with the receptor binding site, resulting in a gradual onset of effect.
Amlodipine is a peripheral arterial vasodilator that acts directly on vascular smooth muscle to cause a reduction in peripheral vascular resistance and reduction in blood pressure.
Amlodipine is a 1:1 racemic mixture of levamlodipine and dextro amlodipine, it has been demonstrated that levamlodipine is the pharmacologically active, anti-hypertensive isomer.
12.3 Pharmacokinetics
The exposure (C max and AUC) of levamlodipine is similar between CONJUPRI ® 5 mg and Norvasc ® (amlodipine besylate) 10 mg under fasting condition.
12.4 Pediatric Patients
Sixty-two hypertensive patients aged 6 to 17 years received doses of amlodipine between 1.25 mg and 20 mg. Weight-adjusted clearance and volume of distribution were similar to values in adults.
Drug Interactions
In vitro data indicate that amlodipine has no effect on the human plasma protein binding of digoxin, phenytoin, warfarin, and indomethacin.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Rats and mice treated with amlodipine in the diet for up to two years, at concentrations calculated to provide daily dosage levels of 0.5, 1.25, and 2.5 amlodipine mg/kg/day, showed no evidence of a carcinogenic effect of the drug. For the mouse, the highest dose was, on a mg/m 2 basis, similar to the maximum recommended human dose of 10 mg amlodipine/day. 2 For the rat, the highest dose was, on a mg/m 2 basis, about twice the maximum recommended human dose. 2
Mutagenicity studies conducted with amlodipine revealed no drug related effects at either the gene or chromosome level.
There was no effect on the fertility of rats treated orally with amlodipine (males for 64 days and females for 14 days prior to mating) at doses up to 10 mg amlodipine/kg/day (8 times the maximum recommended human dose 2 of 10 mg/day on a mg/m 2 basis).
- 2
- Based on patient weight of 50 kg
| CONJUPRI
levamlodipine tablet |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| CONJUPRI
levamlodipine tablet |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - WraSer LLC (121828334) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| CSPC OUYI PHARMACEUTICAL CO., LTD | 421303775 | manufacture(66992-425, 66992-430) | |






