Drug Detail:Covid-19 vaccine (janssen) (monograph) (Medically reviewed)
Drug Class:
| FULL EMERGENCY USE AUTHORIZATION (EUA) PRESCRIBING INFORMATION | |
| JANSSEN COVID-19 VACCINE | |
| FULL EMERGENCY USE AUTHORIZATION (EUA) PRESCRIBING INFORMATION: CONTENTS*
WARNING: THROMBOSIS WITH THROMBOCYTOPENIA SYNDROME (TTS)
|
|
1. Indications and Usage for Covid-19 Vaccine Janssen
Janssen COVID-19 vaccine is authorized for use under an Emergency Use Authorization (EUA) for active immunization to prevent coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in individuals 18 years of age and older for whom other FDA-authorized or approved COVID-19 vaccines are not accessible or clinically appropriate, and in individuals 18 years of age and older who elect to receive the Janssen COVID-19 Vaccine because they would otherwise not receive a COVID-19 vaccine.
2. Covid-19 Vaccine Janssen Dosage and Administration
For intramuscular injection only.
2.1 Preparation for Administration
- The Janssen COVID-19 Vaccine is a colorless to slightly yellow, clear to very opalescent suspension. Visually inspect the Janssen COVID-19 Vaccine vials for particulate matter and discoloration prior to administration. If either of these conditions exists, do not administer the vaccine.
- Before withdrawing each dose of vaccine, carefully mix the contents of the multi-dose vial by swirling gently in an upright position for 10 seconds. Do not shake.
- Each dose is 0.5 mL. Each vial contains five doses. Do not pool excess vaccine from multiple vials.
- The Janssen COVID-19 Vaccine does not contain a preservative. Record the date and time of first use on the Janssen COVID-19 Vaccine vial label. After the first dose has been withdrawn, hold the vial between 2° to 8°C (36° to 46°F) for up to 6 hours or at room temperature (maximally 25°C/77°F) for up to 2 hours. Discard if vaccine is not used within these times.
2.2 Administration
Visually inspect each dose in the dosing syringe prior to administration. The Janssen COVID-19 Vaccine is a colorless to slightly yellow, clear to very opalescent suspension. During the visual inspection,
- verify the final dosing volume of 0.5 mL.
- confirm there are no particulates and that no discoloration is observed.
- do not administer if vaccine is discolored or contains particulate matter.
Administer the Janssen COVID-19 Vaccine intramuscularly.
3. Dosage Forms and Strengths
Janssen COVID-19 Vaccine is a suspension for intramuscular injection. A single dose is 0.5 mL.
4. Contraindications
4.1 Severe Allergic Reactions
Do not administer the Janssen COVID-19 Vaccine to individuals with a known history of severe allergic reaction (e.g., anaphylaxis) to any component of the Janssen COVID-19 Vaccine [see Description (13)] .
4.2 Thrombosis with Thrombocytopenia
Do not administer the Janssen COVID-19 Vaccine to individuals with a history of thrombosis with thrombocytopenia following the Janssen COVID-19 Vaccine or any other adenovirus-vectored COVID-19 vaccine (e.g., AstraZeneca's COVID-19 vaccine which is not authorized or approved in the United States) [see Warnings and Precautions (5.2)] .
5. Warnings and Precautions
5.1 Management of Acute Allergic Reactions
Appropriate medical treatment used to manage immediate allergic reactions must be immediately available in the event an acute anaphylactic reaction occurs following administration of the Janssen COVID-19 Vaccine.
Monitor Janssen COVID-19 Vaccine recipients for the occurrence of immediate adverse reactions according to the Centers for Disease Control and Prevention guidelines ( https://www.cdc.gov/vaccines/covid-19/clinical-considerations/managing-anaphylaxis.html).
5.2 Thrombosis with Thrombocytopenia Syndrome (TTS)
Reports to the Vaccine Adverse Events Reporting System (VAERS), a passive surveillance system, provide evidence for an increased risk of thrombosis with thrombocytopenia syndrome (TTS) with onset of symptoms approximately one to two weeks after administration of the Janssen COVID-19 Vaccine.
An analysis of VAERS reports of TTS following the receipt of the Janssen COVID-19 Vaccine used the following case definition:
- a thrombosis in an unusual location for a thrombus (i.e., cerebral vein, visceral artery or vein, extremity artery, central artery or vein) and new-onset thrombocytopenia (i.e., platelet count <150,000/μL) occurring any time after vaccination;
or - new-onset thrombocytopenia (i.e., platelet count <150,000/μL), thrombosis in an extremity vein or pulmonary artery in the absence of thrombosis at an unusual location, and a positive anti-PF4 antibody ELISA test or functional HIT (heparin-induced thrombocytopenia) platelet test occurring any time after vaccination.
Cases of TTS following administration of the Janssen COVID-19 Vaccine have been reported in males and females, in a wide age range of individuals 18 years and older, with the highest reporting rate (approximately 8 cases per 1,000,000 doses administered) in females ages 30–49 years; overall, approximately 15% of TTS cases have been fatal. The clinical course of these events shares features with autoimmune heparin-induced thrombocytopenia. Specific risk factors for TTS following administration of the Janssen COVID-19 Vaccine and the level of potential excess risk due to vaccination are under investigation. Currently available evidence supports a causal relationship between TTS and the Janssen COVID-19 Vaccine.
Healthcare professionals should be alert to the signs and symptoms of TTS in individuals who receive the Janssen COVID-19 Vaccine. In individuals with suspected TTS following administration of the Janssen COVID-19 Vaccine, the use of heparin may be harmful and alternative treatments may be needed. Consultation with hematology specialists is strongly recommended.
The American Society of Hematology has published considerations relevant to the diagnosis and treatment of TTS following administration of the Janssen COVID-19 Vaccine ( https://www.hematology.org/covid-19/vaccine-induced-immune-thrombotic-thrombocytopenia).
Recipients of Janssen COVID-19 Vaccine should be instructed to seek immediate medical attention if they develop shortness of breath, chest pain, leg swelling, persistent abdominal pain, neurological symptoms (including severe or persistent headaches or blurred vision), or petechiae beyond the site of vaccination.
5.3 Immune Thrombocytopenia (ITP)
Reports of adverse events following use of the Janssen COVID-19 Vaccine under emergency use authorization suggest an increased risk of immune thrombocytopenia (ITP) during the 42 days following vaccination. Individuals with a history of ITP should discuss with their healthcare provider the risk of ITP and the potential need for platelet monitoring following vaccination with the Janssen COVID-19 Vaccine.
5.4 Guillain-Barré Syndrome
Reports of adverse events following use of the Janssen COVID-19 Vaccine under emergency use authorization suggest an increased risk of Guillain-Barré syndrome during the 42 days following vaccination.
5.5 Myocarditis and Pericarditis
Reports of adverse events following use of the Janssen COVID-19 Vaccine under emergency use authorization suggest increased risks of myocarditis and pericarditis, particularly within the period 0 through 7 days following vaccination.
The CDC has published considerations related to myocarditis and pericarditis after vaccination, including for vaccination of individuals with a history of myocarditis or pericarditis ( https://www.cdc.gov/vaccines/covid-19/clinical-considerations/interim-considerations-us.html#myocarditis-pericarditis).
5.6 Syncope
Syncope (fainting) may occur in association with administration of injectable vaccines. Procedures should be in place to avoid injury from fainting.
6. Adverse Reactions/Side Effects
It is MANDATORY for vaccination providers to report to the Vaccine Adverse Event Reporting System (VAERS) all vaccine administration errors, all serious adverse events, cases of myocarditis, cases of pericarditis, cases of Multisystem Inflammatory Syndrome (MIS) in adults, and hospitalized or fatal cases of COVID-19 following vaccination with the Janssen COVID-19 Vaccine. To the extent feasible, provide a copy of the VAERS form to Janssen Biotech, Inc. Please see the REQUIREMENTS AND INSTRUCTIONS FOR REPORTING ADVERSE EVENTS AND VACCINE ADMINISTRATION ERRORS section for details on reporting to VAERS or Janssen Biotech, Inc.
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Booster Dose Following Primary Vaccination with Janssen COVID-19 Vaccine
Overall, in 5 clinical studies conducted in Belgium, Brazil, Colombia, France, Germany, Japan, Netherlands, Philippines, South Africa, Spain, United Kingdom and United States, approximately 9,000 participants have received 2 doses of the Janssen COVID-19 Vaccine, administered at least 2 months apart and approximately 2,700 participants had at least 2 months of safety follow-up after the booster dose.
A randomized, double-blind, placebo-controlled Phase 2 study, COV2001 (NCT04535453) (Study 2), evaluated the frequency and severity of local and systemic adverse reactions within 7 days of administration of a booster dose of the Janssen COVID-19 Vaccine administered approximately 2 months after the primary vaccination in healthy adults 18 through 55 years of age and adults 65 years and older in good or stable health. A total of 141 individuals received at least one dose of the vaccine and 137 received both the primary vaccination and the booster dose at an interval of 2 months. The median age of individuals was 48 years, and 48 individuals (34%) were 65 years of age and older. Data on solicited adverse reactions after the primary vaccination and after a booster dose are shown in Tables 5–8.
Solicited adverse reactions
| Adverse Reactions | Primary Vaccination
N=93 n(%) | Booster Dose
N=89 n(%) |
|---|---|---|
|
||
| Injection Site Pain | ||
| Any | 58 (62.4%) | 53 (59.6%) |
| Grade 3 * | 0 | 1 (1.1%) |
| Injection Site Erythema | ||
| Any (≥25 mm) | 1 (1.1%) | 1 (1.1%) |
| Grade 3 † | 0 | 0 |
| Injection Site Swelling | ||
| Any (≥25 mm) | 1 (1.1%) | 0 |
| Grade 3 † | 0 | 0 |
| Adverse Reactions | Primary Vaccination
N=48 n(%) | Booster Dose
N=48 n(%) |
|---|---|---|
|
||
| Injection Site Pain | ||
| Any | 17 (35.4%) | 10 (20.8%) |
| Grade 3 * | 0 | 0 |
| Injection Site Erythema | ||
| Any (≥25 mm) | 0 | 0 |
| Grade 3 † | 0 | 0 |
| Injection Site Swelling | ||
| Any (≥25 mm) | 0 | 0 |
| Grade 3 † | 0 | 0 |
| Adverse Reactions | Primary Vaccination
N=93 n(%) | Booster Dose
N=89 n(%) |
|---|---|---|
|
||
| Headache | ||
| Any | 49 (52.7%) | 37 (41.6%) |
| Grade 3 * | 2 (2.2%) | 1 (1.1%) |
| Fatigue | ||
| Any | 55 (59.1%) | 46 (51.7%) |
| Grade 3 † | 1 (1.1%) | 0 |
| Myalgia | ||
| Any | 44 (47.3%) | 32 (36.0%) |
| Grade 3 † | 3 (3.2%) | 2 (2.2%) |
| Nausea | ||
| Any | 13 (14.0%) | 9 (10.1%) |
| Grade 3 † | 1 (1.1%) | 0 |
| Fever‡ | ||
| Any | 13 (14.0%) | 5 (5.6%) |
| Grade 3 | 1 (1.1%) | 0 |
| Adverse Reactions | Primary Vaccination
N=48 n(%) | Booster Dose
N=48 n(%) |
|---|---|---|
|
||
| Headache | ||
| Any | 9 (18.8%) | 13 (27.1%) |
| Grade 3 * | 0 | 0 |
| Fatigue | ||
| Any | 9 (18.8%) | 16 (33.3%) |
| Grade 3 † | 0 | 0 |
| Myalgia | ||
| Any | 4 (8.3%) | 5 (10.4%) |
| Grade 3 † | 0 | 0 |
| Nausea | ||
| Any | 0 | 1 (2.1%) |
| Grade 3 † | 0 | 0 |
| Fever‡ | ||
| Any | 1 (2.1%) | 0 |
| Grade 3 | 0 | 0 |
6.2 Post Authorization Experience
The following adverse reactions have been identified during post-authorization use of the Janssen COVID-19 Vaccine. Because these reactions are reported voluntarily, it is not always possible to reliably estimate their frequency or establish a causal relationship to vaccine exposure.
Blood and Lymphatic System Disorders: Thrombosis with thrombocytopenia, Lymphadenopathy, Immune thrombocytopenia.
Cardiac disorders: Myocarditis, Pericarditis.
Ear and labyrinth disorders: Tinnitus.
Gastrointestinal disorders: Diarrhea, Vomiting.
Immune System Disorders: Allergic reactions, including anaphylaxis.
Nervous System Disorders: Guillain-Barré syndrome, Syncope, Paresthesia, Hypoesthesia, Facial Paralysis (including Bell's Palsy).
Vascular Disorders: Capillary leak syndrome, Thrombosis with thrombocytopenia, Venous thromboembolism (with or without thrombocytopenia).
8 REQUIREMENTS AND INSTRUCTIONS FOR REPORTING ADVERSE EVENTS AND VACCINE ADMINISTRATION ERRORS
See Overall Safety Summary (Section 6) for additional information.
The vaccination provider enrolled in the federal COVID-19 Vaccination Program is responsible for MANDATORY reporting of the listed events following Janssen COVID-19 Vaccine administration to the Vaccine Adverse Event Reporting System (VAERS):
- Vaccine administration errors whether or not associated with an adverse event,
- Serious adverse events* (irrespective of attribution to vaccination),
- Cases of myocarditis,
- Cases of pericarditis,
- Cases of Multisystem Inflammatory Syndrome (MIS) in adults,
- Cases of COVID-19 that result in hospitalization or death.
* Serious Adverse Events are defined as:
- Death;
- A life-threatening adverse event;
- Inpatient hospitalization or prolongation of existing hospitalization;
- A persistent or significant incapacity or substantial disruption of the ability to conduct normal life functions;
- A congenital anomaly/birth defect;
- An important medical event that based on appropriate medical judgement may jeopardize the individual and may require medical or surgical intervention to prevent one of the outcomes listed above.
10. Drug Interactions
There are no data to assess the concomitant administration of the Janssen COVID-19 Vaccine with other vaccines.
11. Use In Specific Populations
11.3 Pediatric Use
Emergency Use Authorization of the Janssen COVID-19 Vaccine does not include use in individuals younger than 18 years of age.
11.4 Geriatric Use
Clinical studies of Janssen COVID-19 Vaccine included individuals 65 years of age and older and their data contributes to the overall assessment of safety and efficacy [see Overall Safety Summary (6.1) and Clinical Trial Results and Supporting Data for EUA (18)] . Of the 21,895 individuals who received a single-dose of the Janssen COVID-19 Vaccine in COV3001, 19.5% (n=4,259) were 65 years of age and older and 3.7% (n=809) were 75 years of age and older. No overall differences in safety or efficacy were observed between individuals 65 years of age and older and younger individuals.
13. Covid-19 Vaccine Janssen Description
The Janssen COVID-19 Vaccine is a colorless to slightly yellow, clear to very opalescent sterile suspension for intramuscular injection. It contains no visible particulates. The vaccine consists of a replication-incompetent recombinant adenovirus type 26 (Ad26) vector expressing the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) spike (S) protein in a stabilized conformation.
The Ad26 vector expressing the SARS-CoV-2 S protein is grown in PER.C6 TetR cells, in media containing amino acids and no animal-derived proteins. After propagation, the vaccine is processed through several purification steps, formulated with inactive ingredients and filled into vials.
Each 0.5 mL dose of Janssen COVID-19 Vaccine is formulated to contain 5×10 10 virus particles (VP) and the following inactive ingredients: citric acid monohydrate (0.14 mg), trisodium citrate dihydrate (2.02 mg), ethanol (2.04 mg), 2-hydroxypropyl-β-cyclodextrin (HBCD) (25.50 mg), polysorbate-80 (0.16 mg), sodium chloride (2.19 mg). Each dose may also contain residual amounts of host cell proteins (≤0.15 mcg) and/or host cell DNA (≤3 ng).
Janssen COVID-19 Vaccine does not contain a preservative.
The vial stoppers are not made with natural rubber latex.
14. Covid-19 Vaccine Janssen - Clinical Pharmacology
14.1 Mechanism of Action
The Janssen COVID-19 Vaccine is composed of a recombinant, replication-incompetent human adenovirus type 26 vector that, after entering human cells, expresses the SARS-CoV-2 spike (S) antigen without virus propagation. An immune response elicited to the S antigen protects against COVID-19.
18 CLINICAL TRIAL RESULTS AND SUPPORTING DATA FOR EUA
18.1 Efficacy of Primary Vaccination
A primary analysis (cut-off date January 22, 2021) of a multicenter, randomized, double-blind, placebo-controlled Phase 3 Study (Study 1) was conducted in the United States, South Africa, Brazil, Chile, Argentina, Colombia, Peru and Mexico to assess the efficacy, safety, and immunogenicity of a single-dose of the Janssen COVID-19 Vaccine for the prevention of COVID-19 in adults aged 18 years and older. Randomization was stratified by age (18–59 years, 60 years and older) and presence or absence of comorbidities associated with an increased risk of progression to severe COVID-19. The study allowed for the inclusion of individuals with stable pre-existing medical conditions, defined as disease not requiring significant change in therapy during the 3 months preceding vaccination, as well as individuals with stable human immunodeficiency virus (HIV) infection.
A total of 44,325 individuals were randomized equally to receive Janssen COVID-19 Vaccine or saline placebo. Individuals are planned to be followed for up to 24 months, for assessments of safety and efficacy against COVID-19.
The primary efficacy analysis population of 39,321 individuals (19,630 in the Janssen COVID-19 Vaccine group and 19,691 in the placebo group) included 38,059 SARS-CoV-2 seronegative individuals at baseline and 1,262 individuals with an unknown serostatus. Demographic and baseline characteristics were similar among individuals who received the Janssen COVID-19 Vaccine and those who received placebo (see Table 9).
| Janssen COVID-19 Vaccine
(N=19,630) n (%) | Placebo
(N=19,691) n (%) |
|
|---|---|---|
|
||
| Sex | ||
| Male | 10,924 (55.6) | 10,910 (55.4) |
| Female | 8,702 (44.3) | 8,777 (44.6) |
| Age (years) | ||
| Mean (SD) | 51.1 (15.0) | 51.2 (15.0) |
| Median | 52.0 | 53.0 |
| Min, max | (18; 100) | (18; 94) |
| Age group | ||
| ≥18 to 59 years of age | 12,830 (65.4) | 12,881 (65.4) |
| ≥60 years of age | 6,800 (34.6) | 6,810 (34.6) |
| ≥65 years of age | 3,984 (20.3) | 4,018 (20.4) |
| ≥75 years of age | 755 (3.8) | 693 (3.5) |
| Race* | ||
| White | 12,200 (62.1) | 12,216 (62.0) |
| Black or African American | 3,374 (17.2) | 3,390 (17.2) |
| Asian | 720 (3.7) | 663 (3.4) |
| American Indian/Alaska Native † | 1,643 (8.4) | 1,628 (8.3) |
| Native Hawaiian or other Pacific Islander | 54 (0.3) | 45 (0.2) |
| Multiple | 1,036 (5.3) | 1,087 (5.5) |
| Unknown | 262 (1.3) | 272 (1.4) |
| Not reported | 341 (1.7) | 390 (2.0) |
| Ethnicity | ||
| Hispanic or Latino | 8,793 (44.8) | 8,936 (45.4) |
| Not Hispanic or Latino | 10,344 (52.7) | 10,259 (52.1) |
| Unknown | 173 (0.9) | 162 (0.8) |
| Not reported | 319 (1.6) | 333 (1.7) |
| Region | ||
| Northern America (United States) | 9,185 (46.8) | 9,171 (46.6) |
| Latin America | 7,967 (40.6) | 8,014 (40.7) |
| Southern Africa (South Africa) | 2,478 (12.6) | 2,506 (12.7) |
| Comorbidities‡ | ||
| Yes | 7,830 (39.9) | 7,867 (40.0) |
| No | 11,800 (60.1) | 11,824 (60.0) |
Primary analysis
The median length of follow up for efficacy for individuals in the study was 8 weeks post-vaccination. Vaccine Efficacy (VE) for the co-primary endpoints against moderate to severe/critical COVID-19 in individuals who were seronegative or who had an unknown serostatus at baseline was 66.9% (95% CI: 59.0; 73.4) at least 14 days after vaccination and 66.1% (95% CI: 55.0; 74.8) at least 28 days after vaccination (see Table 10).
| Subgroup | Janssen COVID-19 Vaccine
N=19,630 | Placebo
N=19,691 | % Vaccine Efficacy
(95% CI) |
||
|---|---|---|---|---|---|
| COVID-19 Cases
(n) | Person-Years | COVID-19 Cases
(n) | Person-Years | ||
|
|||||
| 14 days post-vaccination | |||||
| All subjects* | 116 | 3116.6 | 348 | 3096.1 | 66.9
(59.0; 73.4) |
| 18 to 59 years of age | 95 | 2106.8 | 260 | 2095.0 | 63.7
(53.9; 71.6) |
| 60 years and older | 21 | 1009.8 | 88 | 1001.2 | 76.3
(61.6; 86.0) |
| 28 days post-vaccination | |||||
| All subjects* | 66 | 3102.0 | 193 | 3070.7 | 66.1
(55.0; 74.8) † |
| 18 to 59 years of age | 52 | 2097.6 | 152 | 2077.0 | 66.1
(53.3; 75.8) |
| 60 years and older | 14 | 1004.4 | 41 | 993.6 | 66.2
(36.7; 83.0) |
Vaccine efficacy against severe/critical COVID-19 at least 14 days after vaccination was 76.7% (95% CI: 54.6; 89.1) and 85.4% (95% CI: 54.2; 96.9) at least 28 days after vaccination (see Table 11).
| Subgroup | Janssen COVID-19 Vaccine
N=19,630 | Placebo
N=19,691 | % Vaccine Efficacy
(95% CI) |
||
|---|---|---|---|---|---|
| COVID-19 Cases
(n) | Person-Years | COVID-19 Cases
(n) | Person-Years | ||
|
|||||
| 14 days post-vaccination | |||||
| Severe/critical | 76.7 | ||||
| 14 | 3125.1 | 60 | 3122.0 | (54.6; 89.1) * | |
| 28 days post-vaccination | |||||
| Severe/critical | 85.4 | ||||
| 5 | 3106.2 | 34 | 3082.6 | (54.2; 96.9) * | |
Among all COVID-19 cases with onset at least 14 days post vaccination, including cases diagnosed by a positive PCR from a local laboratory and still awaiting confirmation at the central laboratory (as of January 22, 2021), there were 2 COVID-19 related hospitalizations in the vaccine group (with none after 28 days) and 29 in the placebo group (with 16 after 28 days).
As of the primary analysis cut-off date of January 22, 2021, there were no COVID-19-related deaths reported in Janssen COVID-19 Vaccine recipients compared to 5 COVID-19-related deaths reported in placebo recipients, who were SARS-CoV-2 PCR negative at baseline.
Janssen COVID-19 Vaccine Efficacy in Countries With Different Circulating SARS-CoV-2 Variants.
Exploratory subgroup analyses of vaccine efficacy against moderate to severe/critical COVID-19 and severe/critical COVID-19 for Brazil, South Africa, and the United States were conducted (see Table 12). For the subgroup analyses, all COVID-19 cases accrued up to the primary efficacy analysis data cut-off date, including cases confirmed by the central laboratory and cases with documented positive SARS-CoV-2 PCR from a local laboratory which are still awaiting confirmation by the central laboratory, were included. The concordance rate observed up to the data cut-off date between the PCR results from the local laboratory and the central laboratory was 90.3%.
| Onset | Severity | ||
|---|---|---|---|
| Moderate to Severe/Critical
Point estimate (95% CI) | Severe/Critical
Point estimate (95% CI) |
||
| US | at least 14 days after vaccination | 74.4% (65.0; 81.6) | 78.0% (33.1; 94.6) |
| at least 28 days after vaccination | 72.0% (58.2;81.7) | 85.9% (-9.4; 99.7) | |
| Brazil | at least 14 days after vaccination | 66.2% (51.0; 77.1) | 81.9% (17.0; 98.1) |
| at least 28 days after vaccination | 68.1% (48.8; 80.7) | 87.6% (7.8; 99.7) | |
| South Africa | at least 14 days after vaccination | 52.0% (30.3; 67.4) | 73.1% (40.0; 89.4) |
| at least 28 days after vaccination | 64.0% (41.2; 78.7) | 81.7% (46.2; 95.4) | |
Strain sequencing was conducted on available samples with sufficient viral load from centrally confirmed COVID-19 cases (one sequence per case). As of February 12, 2021, samples from 71.7% of central laboratory confirmed primary analysis cases had been sequenced [United States (73.5%), South Africa (66.9%) and Brazil (69.3%)]. In the United States, 96.4% of strains were identified as the Wuhan-H1 variant D614G; in South Africa, 94.5% of strains were identified as the 20H/501Y.V2 variant (B.1.351 lineage); in Brazil, 69.4% of strains were identified to be a variant of the P.2 lineage and 30.6% of strains were identified as the Wuhan-H1 variant D614G. As of February 12, 2021, SARS-CoV-2 variants from the B1.1.7 or P.1 lineages were not found in any of the sequenced samples.
19. How is Covid-19 Vaccine Janssen supplied
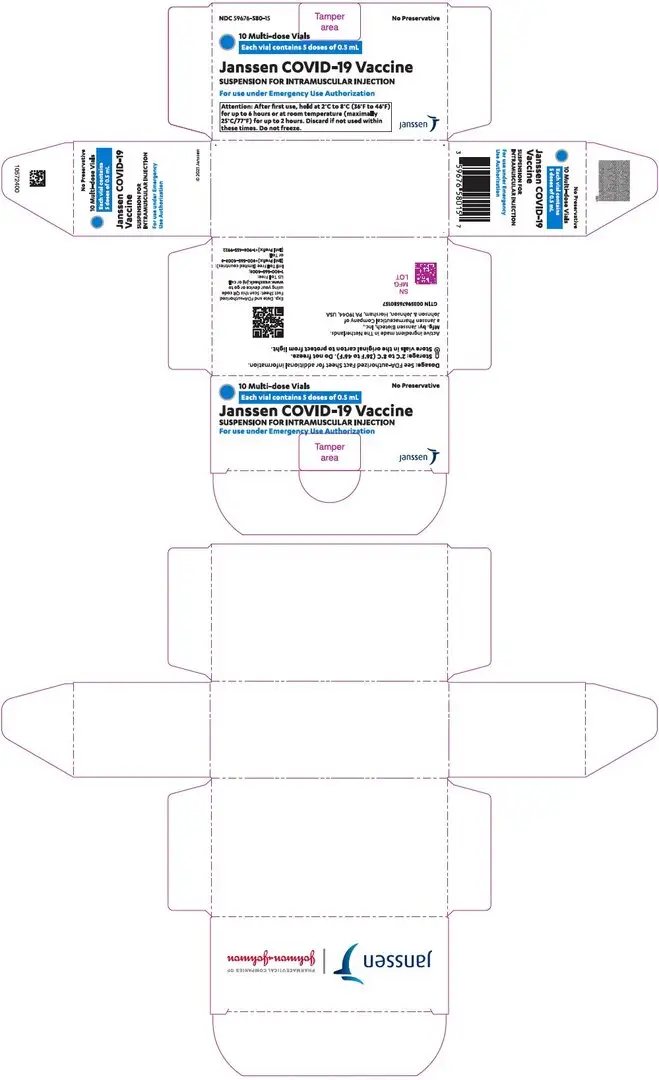
Janssen COVID-19 Vaccine is supplied in a carton of 10 multi-dose vials (NDC 59676-580-15). A maximum of 5 doses can be withdrawn from the multi-dose vial.
The storage and handling information in this Fact Sheet supersedes the storage and handling information on the carton and vial labels.
20 PATIENT COUNSELING INFORMATION
Advise the recipient or caregiver to read the Fact Sheet for Recipients and Caregivers.
Instruct Janssen COVID-19 Vaccine recipients to seek immediate medical attention for shortness of breath, chest pain, leg swelling, persistent abdominal pain, neurological symptoms (including severe or persistent headaches or blurred vision), or petechiae beyond the site of vaccination [see Warnings and Precautions (5.2)] .
In individuals with a history of ITP, discuss the risk of ITP and the potential need for platelet monitoring following vaccination with the Janssen COVID-19 Vaccine [see Warnings and Precautions (5.3)] .
The vaccination provider must include vaccination information in the state/local jurisdiction's Immunization Information System (IIS) or other designated system. Advise recipient or caregiver that more information about IISs can be found at: https://www.cdc.gov/vaccines/programs/iis/about.html.
21 CONTACT INFORMATION
For general questions or to access the most recent Janssen COVID-19 Vaccine Fact Sheets, scan the QR code using your device, visit www.janssencovid19vaccine.com or call the telephone numbers provided below.
| QR Code | Fact Sheets Website | Telephone numbers |
|---|---|---|
|
| www.janssencovid19vaccine.com. | US Toll Free: 1-800-565-4008
US Toll: 1-908-455-9922 |
This Full EUA Prescribing Information may have been updated. For the most recent Full EUA Prescribing Information, please see www.janssencovid19vaccine.com.
Manufactured by:
Janssen Biotech, Inc.
a Janssen Pharmaceutical Company of Johnson & Johnson
Horsham, PA 19044, USA
Revised: March/13/2023
© 2021 Janssen Pharmaceutical Companies
Patient Counseling Information
EMERGENCY USE AUTHORIZATION (EUA) OF
THE JANSSEN COVID-19 VACCINE TO PREVENT CORONAVIRUS DISEASE 2019
(COVID-19)
The Janssen COVID-19 Vaccine is authorized for use in individuals 18 years of age and older for whom other FDA-authorized or approved COVID-19 vaccines are not accessible or clinically appropriate, and for individuals 18 years of age and older who elect to receive the Janssen COVID-19 Vaccine because they would otherwise not receive a COVID-19 vaccine. The Janssen COVID-19 Vaccine can cause blood clots with low levels of platelets (blood cells that help your body stop bleeding), which may be fatal.
You are being offered the Janssen COVID-19 Vaccine to prevent Coronavirus Disease 2019 (COVID-19) caused by SARS-CoV-2 because there is currently a pandemic of COVID-19.
This Fact Sheet contains information to help you understand the risks and benefits of receiving the Janssen COVID-19 Vaccine.
The Janssen COVID-19 Vaccine may prevent you from getting COVID-19.
Read this Fact Sheet for information about the Janssen COVID-19 Vaccine. Talk to the vaccination provider if you have questions.
The Janssen COVID-19 Vaccine has received EUA from FDA to provide the following doses in individuals 18 years of age and older for whom other FDA-authorized or approved COVID-19 vaccines are not accessible or clinically appropriate, and in individuals 18 years of age and older who elect to receive the Janssen COVID-19 Vaccine because they would otherwise not receive a COVID-19 vaccine:
- A single dose primary vaccination.
- A single booster dose after completing a primary vaccination with the Janssen COVID-19 Vaccine.
- A single booster dose after completing primary vaccination with a different authorized or approved COVID-19 vaccine.
The Janssen COVID-19 Vaccine may not protect everyone.
This Fact Sheet may have been updated. For the most recent Fact Sheet, please visit www.janssencovid19vaccine.com.
WHAT YOU NEED TO KNOW BEFORE YOU GET THIS VACCINE
WHAT IS COVID-19?
COVID-19 is caused by a coronavirus called SARS-CoV-2. This type of coronavirus has not been seen before. You can get COVID-19 through contact with another person who has the virus. It is predominantly a respiratory illness that can affect other organs. People with COVID-19 have had a wide range of symptoms reported, ranging from mild symptoms to severe illness. Symptoms may appear 2 to 14 days after exposure to the virus. Common symptoms may include: fever or chills; cough; shortness of breath; fatigue; muscle or body aches; headache; new loss of taste or smell; sore throat; congestion or runny nose; nausea or vomiting; diarrhea.
WHAT IS THE JANSSEN COVID-19 VACCINE?
The Janssen COVID-19 Vaccine is an unapproved vaccine that may prevent COVID-19.
Under an EUA, the FDA has authorized the emergency use of the Janssen COVID-19 Vaccine to prevent COVID-19 in individuals 18 years of age and older for whom other FDA-authorized or approved COVID-19 vaccines are not accessible or clinically appropriate, and in individuals 18 years of age and older who elect to receive the Janssen COVID-19 Vaccine because they would otherwise not receive a COVID-19 vaccine.
For more information on EUA, see the " What is an Emergency Use Authorization (EUA)?" section at the end of this Fact Sheet.
WHAT SHOULD YOU MENTION TO YOUR VACCINATION PROVIDER BEFORE YOU GET THE JANSSEN COVID-19 VACCINE?
Tell the vaccination provider about all of your medical conditions, including if you:
- have any allergies,
- have a fever,
- have a bleeding disorder or are on a blood thinner,
- have ever had a low level of platelets (blood cells that help your body stop bleeding),
- are immunocompromised or are on a medicine that affects your immune system,
- are pregnant or plan to become pregnant,
- are breastfeeding,
- have received another COVID-19 vaccine,
- have ever fainted in association with an injection.
WHO SHOULD GET THE JANSSEN COVID-19 VACCINE?
FDA has authorized the emergency use of the Janssen COVID-19 Vaccine in individuals 18 years of age and older for whom other FDA-authorized or approved COVID-19 vaccines are not accessible or clinically appropriate, and in individuals 18 years of age and older who elect to receive the Janssen COVID-19 Vaccine because they would otherwise not receive a COVID-19 vaccine.
WHO SHOULD NOT GET THE JANSSEN COVID-19 VACCINE?
You should not get the Janssen COVID-19 Vaccine if you:
- had a severe allergic reaction after a previous dose of this vaccine.
- had a severe allergic reaction to any ingredient of this vaccine.
- had a blood clot along with a low level of platelets (blood cells that help your body stop bleeding) following Janssen COVID-19 Vaccine or following AstraZeneca's COVID-19 vaccine (not authorized or approved in the United States).
WHAT ARE THE INGREDIENTS IN THE JANSSEN COVID-19 VACCINE?
The Janssen COVID-19 Vaccine includes the following ingredients: recombinant, replication-incompetent adenovirus type 26 expressing the SARS-CoV-2 spike protein, citric acid monohydrate, trisodium citrate dihydrate, ethanol, 2-hydroxypropyl-β-cyclodextrin (HBCD), polysorbate-80, sodium chloride.
HOW IS THE JANSSEN COVID -19 VACCINE GIVEN?
The Janssen COVID-19 Vaccine will be given to you as an injection into the muscle.
Primary Vaccination
The Janssen COVID-19 Vaccine is administered as a single dose.
Booster Dose
- A single booster dose of the Janssen COVID-19 Vaccine may be administered at least two months after primary vaccination with the Janssen COVID-19 Vaccine.
- A single booster dose of the Janssen COVID-19 Vaccine may be administered after completing primary vaccination with a different authorized or approved COVID-19 vaccine. Please check with your health care provider regarding timing of the booster dose.
HAS THE JANSSEN COVID-19 VACCINE BEEN USED BEFORE?
The Janssen COVID-19 Vaccine is an unapproved vaccine. In clinical trials, more than 61,000 individuals 18 years of age and older have received the Janssen COVID-19 Vaccine. Millions of individuals have received the vaccine under EUA since February 27, 2021.
WHAT ARE THE BENEFITS OF THE JANSSEN COVID-19 VACCINE?
The Janssen COVID-19 Vaccine has been shown to prevent COVID-19. The duration of protection against COVID-19 is currently unknown.
WHAT ARE THE RISKS OF THE JANSSEN COVID-19 VACCINE?
Side effects that have been reported with the Janssen COVID-19 Vaccine include:
- Injection site reactions: pain, redness of the skin and swelling.
- General side effects: headache, feeling very tired, muscle aches, nausea, and fever.
- Swollen lymph nodes.
- Blood clots.
- Unusual feeling in the skin (such as tingling or a crawling feeling) (paresthesia), decreased feeling or sensitivity, especially in the skin (hypoesthesia).
- Persistent ringing in the ears (tinnitus).
- Diarrhea, vomiting.
Severe Allergic Reactions
There is a remote chance that the Janssen COVID-19 Vaccine could cause a severe allergic reaction. A severe allergic reaction would usually occur within a few minutes to one hour after getting a dose of the Janssen COVID-19 Vaccine. For this reason, your vaccination provider may ask you to stay at the place where you received your vaccine for monitoring after vaccination. Signs of a severe allergic reaction can include:
- Difficulty breathing,
- Swelling of your face and throat,
- A fast heartbeat,
- A bad rash all over your body,
- Dizziness and weakness.
Blood Clots with Low Levels of Platelets
Blood clots involving blood vessels in the brain, lungs, abdomen, and legs along with low levels of platelets (blood cells that help your body stop bleeding), have occurred in some people who have received the Janssen COVID-19 Vaccine. In people who developed these blood clots and low levels of platelets, symptoms began approximately one to two weeks after vaccination. Blood clots with low levels of platelets following the Janssen COVID-19 Vaccine have been reported in males and females, across a wide age range of individuals 18 years and older; reporting has been highest in females ages 30 through 49 years (about 8 cases for every 1,000,000 vaccine doses administered), and about 1 out of every 7 cases has been fatal. You should seek medical attention right away if you have any of the following symptoms after receiving the Janssen COVID-19 Vaccine:
- Shortness of breath,
- Chest pain,
- Leg swelling,
- Persistent abdominal pain,
- Severe or persistent headaches or blurred vision,
- Easy bruising or tiny blood spots under the skin beyond the site of the injection.
Immune Thrombocytopenia (ITP)
Immune Thrombocytopenia (ITP) is a disorder that can cause easy or excessive bruising and bleeding due to very low levels of platelets. ITP has occurred in some people who have received the Janssen COVID-19 Vaccine. In most of these people, symptoms began within 42 days following receipt of the Janssen COVID-19 Vaccine. The chance of having this occur is very low. If you have ever had a diagnosis of ITP, talk to your vaccination provider before you get the Janssen COVID-19 Vaccine. You should seek medical attention right away if you develop any of the following symptoms after receiving the Janssen COVID-19 Vaccine:
- Easy or excessive bruising or tiny blood spots under the skin beyond the site of the injection,
- Unusual or excessive bleeding.
Guillain Barré Syndrome
Guillain Barré syndrome (a neurological disorder in which the body's immune system damages nerve cells, causing muscle weakness and sometimes paralysis) has occurred in some people who have received the Janssen COVID-19 Vaccine. In most of these people, symptoms began within 42 days following receipt of the Janssen COVID-19 Vaccine. The chance of having this occur is very low. You should seek medical attention right away if you develop any of the following symptoms after receiving the Janssen COVID-19 Vaccine:
- Weakness or tingling sensations, especially in the legs or arms, that's worsening and spreading to other parts of the body.
- Difficulty walking.
- Difficulty with facial movements, including speaking, chewing, or swallowing.
- Double vision or inability to move eyes.
- Difficulty with bladder control or bowel function.
These may not be all the possible side effects of the Janssen COVID-19 Vaccine. Serious and unexpected effects may occur. The Janssen COVID-19 Vaccine is still being studied in clinical trials.
WHAT SHOULD I DO ABOUT SIDE EFFECTS?
If you experience a severe allergic reaction, call 9-1-1, or go to the nearest hospital.
Call the vaccination provider or your healthcare provider if you have any side effects that bother you or do not go away.
Report vaccine side effects to FDA/CDC Vaccine Adverse Event Reporting System (VAERS). The VAERS toll-free number is 1-800-822-7967 or report online to https://vaers.hhs.gov/reportevent.html. Please include "Janssen COVID-19 Vaccine EUA" in the first line of box #18 of the report form.
In addition, you can report side effects to Janssen Biotech, Inc. at the contact information provided below.
| Fax number | Telephone numbers | |
|---|---|---|
| [email protected] | 215-293-9955 | US Toll Free: 1-800-565-4008
US Toll: (908) 455-9922 |
You may also be given an option to enroll in v-safe. V-safe is a new voluntary smartphone-based tool that uses text messaging and web surveys to check in with people who have been vaccinated to identify potential side effects after COVID-19 vaccination. V-safe asks questions that help CDC monitor the safety of COVID-19 vaccines. V-safe also provides live telephone follow-up by CDC if participants report a significant health impact following COVID-19 vaccination. For more information on how to sign up, visit: www.cdc.gov/vsafe.
WHAT IF I DECIDE NOT TO GET THE JANSSEN COVID-19 VACCINE?
Under the EUA, there is an option to accept or refuse receiving the vaccine. Should you decide not to receive the Janssen COVID-19 Vaccine, it will not change your standard medical care.
ARE OTHER VACCINES AVAILABLE FOR PREVENTING COVID-19 BESIDES JANSSEN COVID-19 VACCINE?
COMIRNATY and SPIKEVAX are FDA-approved COVID-19 vaccines. Other vaccines to prevent COVID-19 may be available under EUA. The Janssen COVID-19 Vaccine is only authorized if other COVID-19 vaccines are not accessible or clinically appropriate, and for individuals who elect to receive the Janssen COVID-19 Vaccine because they would otherwise not receive a COVID-19 vaccine.
CAN I RECEIVE THE JANSSEN COVID-19 VACCINE AT THE SAME TIME AS OTHER VACCINES?
Data have not yet been submitted to FDA on administration of the Janssen COVID-19 Vaccine at the same time as other vaccines. If you are considering receiving the Janssen COVID-19 Vaccine with other vaccines, discuss your options with your healthcare provider.
WHAT IF I AM PREGNANT OR BREASTFEEDING?
If you are pregnant or breastfeeding, discuss your options with your healthcare provider.
WILL THE JANSSEN COVID-19 VACCINE GIVE ME COVID-19?
No. The Janssen COVID-19 Vaccine does not contain SARS-CoV-2 and cannot give you COVID-19.
KEEP YOUR VACCINATION CARD
When you receive the Janssen COVID-19 Vaccine, you will get a vaccination card to document the name of the vaccine and date of when you received the vaccine.
ADDITIONAL INFORMATION
If you have questions or to access the most recent Janssen COVID-19 Vaccine Fact Sheets, scan the QR code using your device, visit the website or call the telephone numbers provided below.
| QR Code | Fact Sheets Website | Telephone numbers |
|---|---|---|
|
| www.janssencovid19vaccine.com. | US Toll Free: 1-800-565-4008
US Toll: (908) 455-9922 |
HOW CAN I LEARN MORE?
- Ask the vaccination provider.
- Visit CDC at https://www.cdc.gov/coronavirus/2019-ncov/index.html.
- Visit FDA at https://www.fda.gov/emergency-preparedness-and-response/mcm-legal-regulatory-and-policy-framework/emergency-use-authorization.
Contact your local or state public health department.
WHERE WILL MY VACCINATION INFORMATION BE RECORDED?
The vaccination provider may include your vaccination information in your state/local jurisdiction's Immunization Information System (IIS) or other designated system. For more information about IISs visit: https://www.cdc.gov/vaccines/programs/iis/about.html.
CAN I BE CHARGED AN ADMINISTRATION FEE FOR RECEIPT OF THE COVID-19 VACCINE?
No. At this time, the provider cannot charge you for a vaccine dose and you cannot be charged an out-of-pocket vaccine administration fee or any other fee if only receiving a COVID-19 vaccination. However, vaccination providers may seek appropriate reimbursement from a program or plan that covers COVID-19 vaccine administration fees for the vaccine recipient (private insurance, Medicare, Medicaid, HRSA COVID-19 Uninsured Program for non-insured recipients).
WHERE CAN I REPORT CASES OF SUSPECTED FRAUD?
Individuals becoming aware of any potential violations of the CDC COVID-19 Vaccination Program requirements are encouraged to report them to the Office of the Inspector General, U.S. Department of Health and Human Services, at 1-800-HHS-TIPS or TIPS.HHS.GOV.
WHAT IS THE COUNTERMEASURE INJURY COMPENSATION PROGRAM?
The Countermeasures Injury Compensation Program (CICP) is a federal program that may help pay for costs of medical care and other specific expenses for certain people who have been seriously injured by certain medicines or vaccines, including this vaccine. Generally, a claim must be submitted to the CICP within one (1) year from the date of receiving the vaccine. To learn more about this program, visit www.hrsa.gov/cicp or call 1-855-266-2427.
WHAT IS AN EMERGENCY USE AUTHORIZATION (EUA)?
The United States FDA has made the Janssen COVID-19 Vaccine available under an emergency access mechanism called an EUA. The EUA is supported by a Secretary of Health and Human Services (HHS) declaration that circumstances exist to justify the emergency use of drugs and biological products during the COVID-19 pandemic.
The Janssen COVID-19 Vaccine has not undergone the same type of review as an FDA-approved or cleared product. FDA may issue an EUA when certain criteria are met, which includes that there are no adequate, approved, and available alternatives. In addition, the FDA decision is based on the totality of scientific evidence available showing that the product may be effective to prevent COVID-19 during the COVID-19 pandemic and that the known and potential benefits of the product outweigh the known and potential risks of the product. All of these criteria must be met to allow for the product to be used during the COVID-19 pandemic.
The EUA for the Janssen COVID-19 Vaccine is in effect for the duration of the COVID-19 declaration justifying emergency use of these products, unless terminated or revoked (after which the products may no longer be used).
Manufactured by:
Janssen Biotech, Inc.
a Janssen Pharmaceutical Company of Johnson & Johnson
Horsham, PA 19044, USA
© 2021 Janssen Pharmaceutical Companies
For more information, call US Toll Free: 1-800-565-4008, US Toll: (908) 455-9922 or go to www.janssencovid19vaccine.com
Revised: May/05/2022

| JANSSEN COVID-19 VACCINE
ad26.cov2.s injection, suspension |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - Janssen Products, LP (804684207) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Janssen | 415960780 | api manufacture(59676-580) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Jannsen Biologics B.V. | 409612918 | api manufacture(59676-580) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Grand River Aseptic Manufacturing, Inc. | 005593490 | manufacture(59676-580) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Emergent Manufacturing Operations Baltimore LLC | 968316831 | manufacture(59676-580) , analysis(59676-580) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Packaging Coordinators, LLC | 078525133 | pack(59676-580) , label(59676-580) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Catalent Indiana, LLC | 172209277 | manufacture(59676-580) , pack(59676-580) , analysis(59676-580) | |